Abstract
The efficacy of bevacizumab combined with infusional 5-fluorouracil/leucovorin (5-FU/LV) plus irinotecan (FOLFIRI) as the second-line treatment for metastatic colorectal cancer (mCRC) has not been fully clarified, although bevacizumab combined with infusional 5-FU/LV plus oxaliplatin (FOLFOX) in the second-line setting has demonstrated a survival benefit. We investigated the efficacy of bevacizumab plus FOLFIRI in mCRC patients who failed oxaliplatin-containing regimens without bevacizumab. Patients who received bevacizumab plus FOLFIRI or bevacizumab plus FOLFOX as second-line chemotherapy between July 2007 and March 2008 were registered (trial registration: UMIN000001547). Patient background data and progression-free survival (PFS), overall survival (OS), response, and bevacizumab-related adverse events were prospectively collected every 6 months. A total of 195 patients were enrolled from 26 institutions. Among them, 115 patients received bevacizumab plus FOLFIRI after failure of oxaliplatin and fluoropyrimidine (FOLFIRI+BV after OX/FU group), and 45 patients received bevacizumab plus FOLFOX after failure of irinotecan and fluoropyrimidine (FOLFOX+BV after IRI/FU group). Median PFS was 8.3 months (95% confidence interval [CI], 6.7–9.9) for the FOLFIRI+BV after OX/FU group and 7.8 months (95% CI, 5.8–9.7) for the FOLFOX+BV after IRI/FU group. Median OS was 21.6 months (95% CI, 17.6–25.6) and 16.5 months (95% CI, 11.8–21.2), respectively. Overall response rates were 25 and 29%, respectively. The most common grade ≥3 bevacizumab-related adverse events were hypertension (5.0%) and bleeding (3.8%). FOLFIRI+BV after OX/FU showed comparable efficacy to FOLFOX+BV after IRI/FU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A regimen of infusional 5-fluorouracil/leucovorin (5-FU/LV) plus irinotecan (FOLFIRI) followed by infusional 5-FU/LV plus oxaliplatin (FOLFOX) or the reverse sequence has, until recently, been the standard treatment strategy for metastatic colorectal cancer (mCRC) patients. However, in a ramdomized phase III trial conducted by the Groupe Coopérateur Multidisciplinaire en Oncologie (GERCOR), FOLFIRI resulted in a response rate of 4% and median progression-free survival (PFS) of 2.5 months in patients previously treated with FOLFOX [1]: these efficacy results were unsatisfactory. Recently, bevacizumab (a humanized monoclonal antibody that inhibits vascular endothelial growth factor [VEGF], a key mediator of angiogenesis) in combination with oxaliplatin- or irinotecan-containing regimens has become the standard of care for untreated patients with mCRC [2–5]. In the Eastern Cooperative Oncology Group (ECOG) E3200 study, bevacizumab plus FOLFOX showed a higher response rate (22.7% vs. 8.6%), longer PFS (median, 7.3 vs. 4.7 months; hazard ratio = 0.61; P < .0001), and longer overall survival (OS) (median, 12.9 vs. 10.8 months; hazard ratio = 0.75; P = .0011) compared with FOLFOX alone in the patients previously treated with combination irinotecan and fluoropyrimidine chemotherapy [6]. However, the additive benefit of bevacizumab to FOLFIRI after failure of oxaliplatin-containing therapy remains unclear.
The aim of the present study was to evaluate the activity of bevacizumab plus FOLFIRI after failure of oxaliplatin and fluoropyrimidine in the clinical practice setting.
Patients and methods
After bevacizumab was approved in Japan in June 2007, we initiated an observational cohort study to evaluate the efficacy and safety of bevacizumab treatment combined with FOLFIRI or FOLFOX after failure of chemotherapy in bevacizumab-naïve mCRC patients. Patient registration was controlled centrally by the Tsukuba Cancer Clinical Trial Group (TCTG).
Patients were included in this study if they (1) were ≥20 years old, (2) had a pathologic diagnosis of colorectal adenocarcinoma, (3) either had (a) prior chemotherapy stopped due to disease progression or unacceptable toxicity or (b) relapsed during or within 6 months following the last administration of preoperative or postoperative chemotherapy, and (4) had then received bevacizumab plus FOLFIRI or bevacizumab plus FOLFOX starting between June 2007 and March 2008. Patients all had an ECOG performance status (PS) of ≤2 and adequate organ function. The exclusion criteria were: first-line chemotherapy including bevacizumab, bevacizumab plus FOLFIRI administered after failure of irinotecan-containing therapy, or bevacizumab plus FOLFOX administered after failure of oxaliplatin-containing therapy.
This study was conducted according to the Japanese Ethical Guidelines for Epidemiological Research [7] and was approved by the Ethics Committee of each participating hospital. This study was registered with the University Hospital Medical Information Network (No. UMIN 000001547).
Chemotherapy
Bevacizumab was administered at a dose of 5 or 10 mg/kg every 2 weeks in combination with chemotherapy, based on physician choice. FOLFIRI consisted of infusional 5-FU/LV combined with irinotecan every 2 weeks, while FOLFOX consisted of infusional 5-FU/LV combined with oxaliplatin every 2 weeks. These chemotherapy regimens were continued until disease progression, unacceptable toxicity, or patient refusal. Dose reductions, delays, and discontinuation of chemotherapy were allowed at the discretion of the treating physician(s).
Assessments
Baseline data included patient background, prior chemotherapy regimens (fluoropyrimidine-, oxaliplatin-, irinotecan-containing, or other), and initial doses of bevacizumab and irinotecan or oxaliplatin. In addition, whether an epidermal growth factor receptor (EGFR) inhibitor was administered after discontinuation of bevacizumab combination chemotherapy was noted. Patient responses to chemotherapy, severe adverse events (grade ≥ 3), bevacizumab discontinuations due to bevacizumab-related adverse events, date of recorded progression, and death were collected every 6 months until either death, loss to follow-up, or study closure on August 31, 2010. Investigators assessed tumor response and disease progression according to the Response Evaluation Criteria In Solid Tumors (RECIST, version 1.0) every 2 or 3 months. Adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) at every hospital visit. Reported adverse events included bevacizumab-related toxicities and any other adverse events of grade ≥3.
Statistical analysis
OS was calculated as the period from the first day of bevacizumab treatment to death from any cause or to the date of last follow-up (censored). PFS was calculated as the period from the first day of bevacizumab treatment to either the date of recorded tumor progression, death from any cause, or last follow-up without disease progression (censored). OS and PFS were analyzed using the Kaplan–Meier method, with a confidence interval (CI) of 95%. Statistical analysis was performed using SPSS software, version 18.0 (IBM Japan, Tokyo).
Results
Patients
A total of 195 patients who failed with bevacizumab-naïve chemotherapy were enrolled in this observational cohort study, and 160 of these were evaluated in the present study (Fig. 1). Of the evaluated patients, 115 patients received bevacizumab plus FOLFIRI after failure of oxaliplatin and fluoropyrimidine (FOLFIRI+BV after OX/FU group), and 45 received bevacizumab plus FOLFOX after failure of irinotecan and fluoropyrimidine (FOLFOX+BV after IRI/FU group). The median follow-up time was 18.4 months (range, 0.6–36.8 months).
The patient characteristics of each group were similar (Table 1). Most patients had an ECOG PS of ≤1. Seven patients (6.1%) in the FOLFIRI+BV after OX/FU group had previously received FOLFOX in the adjuvant setting, and two patients (4.4%) in the FOLFOX+BV after IRI/FU group had previously received FOLFIRI in the adjuvant setting. A bevacizumab dose of 10 mg/kg every 2 weeks was used in 9 patients (7.8%) in the FOLFIRI+BV after OX/FU group and in 7 patients (16%) in the FOLFOX+BV after IRI/FU group. After bevacizumab combination chemotherapy, an EGFR inhibitor was administered to 39 patients (34%) in the FOLFIRI+BV after OX/FU group and 14 patients (31%) in the FOLFOX+BV after IRI/FU group. The KRAS status of these patients is not known.
The major reasons for discontinuation of bevacizumab combination chemotherapy were disease progression (72% in the FOLFIRI+BV after OX/FU group and 73% in the FOLFOX+BV after IRI/FU group) and toxicity (20% in the FOLFIRI+BV after OX/FU group and 22% in the FOLFOX+BV after IRI/FU group).
Efficacy
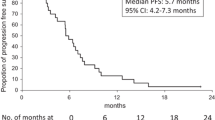
The number of reported PFS events was 103 (90%) in the FOLFIRI+BV after OX/FU group and 43 (96%) in the FOLFOX+BV after IRI/FU group. Median PFS was 8.3 months (95% CI, 6.7–9.9) for the FOLFIRI+BV after OX/FU group and 7.8 months (95% CI, 5.8–9.7) for the FOLFOX+BV after IRI/FU group (Fig. 2a). The number of reported deaths was 83 (72%) and 34 (76%), respectively. Median OS was 21.6 months (95% CI, 17.6–25.6) and 16.5 months (95% CI, 11.8–21.2), respectively (Fig. 2b).
The 7 patients in the FOLFIRI+BV after OX/FU group who had failed adjuvant FOLFOX experienced a median PFS of 12.5 months (95% CI, 3.8–29.0) and a median OS of 28.6 months (95% CI, 14.5–not reached).
The number of patients with measurable lesions was 103 (90%) in the FOLFIRI+BV after OX/FU group and 41 (91%) in the FOLFOX+BV after IRI/FU group. No patient in either group experienced a complete response. The overall response rate was 25% in the FOLFIRI+BV after OX/FU group and 29% in the FOLFOX+BV after IRI/FU group.
Safety
Grade ≥3 adverse events are listed in Table 2. The overall incidence of grade ≥3 bevacizumab-related adverse events was 11% in the FOLFIRI+BV after OX/FU group and 13% in the FOLFOX+BV after IRI/FU group. The most common events were hypertension (5.0%) and bleeding (3.8%). No gastrointestinal perforation was reported. Reported bevacizumab discontinuations due to bevacizumab-related adverse events were 15 (13%) in the FOLFIRI+BV after OX/FU group and 4 (9%) in the FOLFOX+BV after IRI/FU group. The major reason for discontinuation was bleeding episodes that occurred in six (grade 3) and five (grade 2). In all cases, bleeding episodes ceased after discontinuation of bevacizumab.
Grade ≥3 adverse events not related to bevacizumab occurred in 55 patients (48%) in the FOLFIRI+BV after OX/FU group and in 26 patients (58%) in the FOLFOX+BV after IRI/FU group. Neutropenia was the main toxicity in both groups. The grade ≥3 non-hematological toxicity rate was <10%, except for peripheral neuropathy (16%) in the FOLFOX+BV after IRI/FU group.
Four patients (3.5%) in the FOLFIRI+BV after OX/FU group and one (2.2%) in the FOLFOX+BV after IRI/FU group died within 30 days of the last treatment. These included three treatment-related deaths (2 due to interstitial pneumonitis and one due to pulmonary embolism).
Discussion
The benefit of adding bevacizumab to FOLFOX in previously treated patients with mCRC was shown in the E3200 study [6]. However, the efficacy of FOLFIRI+BV after OX/FU has remained unclear. In the present study, the efficacy results for FOLFOX+BV after IRI/FU (29% response rate, 7.8 months of median PFS) were comparable to those of FOLFOX+BV in the E3200 study (23% response rate, 7.3 months of median PFS), and FOLFIRI+BV after OX/FU also showed similar favorable outcomes (25% response rate, 8.3 months of median PFS). These results appear to be better than those of the second-line FOLFOX without bevacizumab or FOLFIRI without bevacizumab, which were reported in the GERCOR trial [1]. Taken together, the present study demonstrates that the addition of bevacizumab may provide a substantial benefit to second-line chemotherapy with FOLFIRI after failure of an oxaliplatin-containing regimen.
In general, bevacizumab is dosed at 5 mg/kg every 2 weeks when combined with first-line chemotherapy for mCRC; however, it was administered at 10 mg/kg every 2 weeks in the E3200 study, because it was used in the second-line setting, which is associated with a greater tumor burden. The optimal dose of bevacizumab (5 or 10 mg/kg/every 2 weeks) for the second-line chemotherapy setting for mCRC remains controversial. In the present study, the majority of patients were treated with bevacizumab 5 mg/kg/every 2 weeks, and our results suggest that this dose is sufficient, even within the second-line setting.
This study has some biases because it was an observational cohort study, so the present results must be confirmed in a randomized controlled study. However, most patients with mCRC are currently treated with bevacizumab plus FOLFOX or bevacizumab plus FOLFIRI as the standard first-line chemotherapy; thus, it is challenging to conduct a prospective trial to assess the benefit of bevacizumab added to second-line chemotherapy in bevacizumab-naïve patients. Thus, the results of the present study seem quite relevant. In particular, the present results in patients who received FOLFIRI+BV after OX/FU can be applied to the selection of the optimal second-line treatment regimen when adjuvant chemotherapy has failed. The standard adjuvant chemotherapy strategy for Stage III colon cancer is oxaliplatin-containing chemotherapy (FOLFOX4 in the MOSAIC trial, bolus 5-fluorouracil/leucovorin plus oxaliplatin [FLOX] in the NSABP C-07 trial, and capecitabine plus oxaliplatin [XELOX] in the XELOXA trial) [8–10]. Based on the results of the present study, if recurrence occurs during or within 6 months after adjuvant chemotherapy, bevacizumab plus FOLFIRI may be recommended.
In a randomized phase III trial, the anti-EGFR antibody panitumumab combined with FOLFIRI as a second-line treatment resulted in a median PFS of 5.9 months and a median OS of 14.5 months in patients harboring wild-type KRAS [11]. Patients who received panitumumab plus FOLFIRI had received prior oxaliplatin (67%) and bevacizumab (18%). These results are similar to those observed in patients who received FOLFIRI+BV after OX/FU in the present study. It has been suggested that patients with wild-type KRAS mCRC can elect to receive panitumumab plus FOLFIRI after oxaliplatin-containing chemotherapy. However, it is well known that the magnitude of the survival benefit of bevacizumab appears to be larger within first-line therapy than in second- or third-line therapy, and is not influenced by tumor KRAS status. In contrast, panitumumab or cetuximab after failure of 5-FU, oxaliplatin, and irinotecan has been shown to improve survival in the third-line setting [12, 13]. Thus, these findings suggest that bevacizumab plus FOLFIRI could be recommended rather than anti-EGFR antibodies to bevacizumab-naïve patients as earlier lines of treatment.
FOLFIRI+BV after OX/FU was well tolerated. In previous clinical trials and large multinational observational studies, the most common grade ≥3 bevacizumab-related adverse events were hypertension (5–20%), bleeding (3–4%), and thromboembolism (3–19%) in patients receiving either first- or second-line bevacizumab combination chemotherapy [6, 14–17], and treatment discontinuation due to any adverse event ranged from 23 to 30%. The safety profiles and toxicity severity observed in these previous reports are comparable to the observations in patients who received FOLFIRI+BV after OX/FU in the present study.
Conclusion
FOLFIRI+BV after OX/FU showed an adequately acceptable toxicity and efficacy similar to that for FOLFOX+BV after IRI/FU in clinical practice.
Abbreviations
- 5-FU/LV:
-
5-Flurouracil/leucovorin
- FOLFIRI:
-
Infusional 5-fluorouracil/leucovorin plus irinotecan
- FOLFOX:
-
Infusional 5-fluorouracil/leucovorin plus oxaliplatin
- mCRC:
-
Metastatic colorectal cancer
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- TCTG:
-
Tsukuba Cancer Clinical Trial Group
- ECOG:
-
Eastern Cooperative Oncology Group
- PS:
-
Performance status
- UMIN:
-
University Hospital Medical Information Network
- EGFR:
-
Epidermal growth factor receptor
- CI:
-
Confidence interval
- FOLFIRI+BV after OX/FU group:
-
Patients who received bevacizumab plus FOLFIRI after failure of oxaliplatin and fluoropyrimidine
- FOLFOX+BV after IRI/FU group:
-
Patients who received bevacizumab plus FOLFOX after failure of irinotecan and fluoropyrimidine
- FLOX:
-
Bolus 5-fluorouracil/leucovorin plus oxaliplatin
- XELOX:
-
Capecitabine plus oxaliplatin
References
Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37.
Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113–9.
Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol. 2007;25:4779–86.
Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44.
ETHICAL guidelines for epidemiological research. http://www.niph.go.jp/wadai/ekigakurinri/guidelines.pdf.
Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–204.
Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–71.
Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51.
Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13.
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65.
Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34.
Yildiz R, Buyukberber S, Uner A, et al. Bevacizumab plus irinotecan-based therapy in metastatic colorectal cancer patients previously treated with oxaliplatin-based regimens. Cancer Invest. 2010;28:33–7.
Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842–7.
Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist. 2009;14:862–70.
Bekaii-Saab TS, Bendell JC, Cohn AL, et al. Bevacizumab (BV) plus chemotherapy (CT) in second-line metastatic colorectal cancer (mCRC): Initial results from ARIES, a second BV observational cohort study (OCS). J Clin Oncol 2010;28:15s (suppl; abstr 3495).
Acknowledgments
The authors would like to thank all of the investigators at the 26 institutions and the patients who participated in this study. Study investigators: Chiba Cancer Center: T. Denda; Dongo Hospital: M. Matsuoka; Hokkaido University Hospital: Y. Komatsu, I. Iwanaga; Ibaraki Prefectural Central Hospital and Cancer Center: K. Amagai, M. Ozeki; Iwate prefectural Central Hospital: S. Kato; Kanagawa Cancer Center: S. Motomura, C. Hashimoto; Kinki University School of Medicine: T. Satoh, S. Fumita; Kitazato University East Hospital: W. Koizumi, T. Sasaki; Kobe University Hospital: T. Okuno, Y. Fujishima; Kushiro City General Hospital: T. Abe; Kyushu University Hospital: E. Baba; Minoh City Hospital: K. Kato, Y. Miyake; Mito Medical Center: T. Yamaguchi, S. Yoshida; Nagoya Memorial Hospital: K. Ina, R. Furuta; National Cancer Center Hospital: H. Yamada, A. Takashima; National Cancer Center Hospital East: T. Yoshino, H. Bando; National Kyushu Cancer Center: T. Esaki, M. Ohta; Osaka City General Hospital: S. Tokunaga, M. Hattori; Ritsurin Hospital: S. Indo, A. Teramoto; Saitama Medical University International Medical Center: K. Yamashita; Sakai Municipal Hospital: M. Fukunaga, H. Takemoto; Shikoku Cancer Center: T. Nishina, T. Kajiwara; Shizuoka Cancer Center: K. Yamazaki; Suita Municipal Hospital: K. Murata, S. Tanaka; Tokyo Medical University: K. Katsumata, Y. Mori; Tsukuba University Hospital: I. Hyodo, T. Moriwaki. This work was supported by the NPO Tsukuba Cancer Clinical Trial Group (TCTG). Portions of this study data have been previously presented at the 35th European Society for Medical Oncology Congress in 2010.
Conflict of interest
Dr. Hyodo received funds in an advisory role from Yakult Honsha and Chugai Pharmaceuticals. All other authors state that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moriwaki, T., Bando, H., Takashima, A. et al. Bevacizumab in combination with irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) in patients with metastatic colorectal cancer who were previously treated with oxaliplatin-containing regimens: a multicenter observational cohort study (TCTG 2nd-BV study). Med Oncol 29, 2842–2848 (2012). https://doi.org/10.1007/s12032-011-0151-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0151-2