Abstract
Previous studies have suggested that insulin-like growth factor binding protein-3 (IGFBP-3) acts as a tumor suppressor in human esophageal squamous cell carcinoma (ESCC). The present study was designed to investigate the clinical and prognostic significance of IGFBP-3 in ESCC patients. In this study, IGFBP-3 was detected by immunohistochemistry (IHC) in paraffin-embedded tissues from 110 ESCC patients, of which 110 were from primary cancer sites and 56 from matched adjacent non-malignant sites. Differences in IGFBP-3 expression and clinical characteristics were compared by χ2 test. Correlations between prognostic outcomes and with IGFBP-3 expression were investigated using Kaplan–Meier analysis and the Cox proportional hazards model. Among adjacent non-malignant tissues, 83.9% of individual tissue staining was scored as either high for IGFBP-3. However, among ESCC cases, only 51.8% of the cancer tissues were scored as high IGFBP-3 expression. In addition, IGFBP-3 expression inversely correlated with pathological classification (P < 0.05 for T, N, and M classifications) and clinical staging (P = 0.006). Furthermore, patients with higher levels of IGFBP-3 had prolonged overall survival (P < 0.001). In conclusion, reduced IGFBP-3 expression may be a risk factor for advanced clinicopathological classification and poor patient survival. These findings suggest that IGFBP-3 may serve as a useful marker for the prognostic evaluation of ESCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal squamous cell carcinoma (ESCC) is a common human malignancy worldwide with an unfavorable prognosis [1]. The main histological type in East Asia is squamous cell carcinoma (SCC) [2]. Despite the great advances achieved in surgery and in chemoradiotherapy technology recently, the overall 5-year survival rate of ESCC patient remains less than 30% and the high probability of recurrence is still the main cause of the associated poor quality of life and of death [3, 4]. The reason for this is most likely the inherent heterogeneity in the biology of tumors [5]. Factors that determine the outcome of ESCC are complicated and include residual disease, latent metastasis, and resistance to therapy, among others. At present, there is still no specific and/or sensitive marker for the accurate prediction of ESCC prognosis following surgery and/or chemoradiotherapy. Therefore, it is necessary to develop novel and improved markers with increased specificity and/or sensitivity for the clinical evaluation of ESCC prognosis [6–8].
Recently, insulin-like growth factor binding protein-3 (IGFBP-3), a member of the IGFBP family, has been shown to inhibit cell proliferation and to activate pro-apoptotic factors in cell lines derived from breast, lung, and prostate cancers, among others, and to reduce tumor growth [9–12]. A recent study found that IGFBP-3 mRNA and protein expression were increased in primary and immortalized human esophageal cells overexpressing epidermal growth factor receptor (EGFR) and, further, IGFBP-3 appears to negatively regulate the proliferation of esophageal cancer cells [13]. Other reports suggested that IGFBP-3 expression level correlates with radiosensitivity in ESCC cells [14] and is a marker for glioblastoma chemosensitivity to Semustine [15]. To date, however, little is known about the significance of IGFBP-3 expression and ESCC prognosis.
The present study was performed to investigate the clinical and prognostic implications of IGFBP-3 expression in patients with ESCC. We used immunohistochemistry (IHC) to examine the expression of IGFBP-3 protein in 110 biopsy specimens from primary ESCC tumors. The correlation between IGFBP-3 expression and patient clinical and prognostic factors was evaluated to determine whether expression of IGFBP-3 could predict ESCC patient survival.
Materials and methods
Patients and tissue specimens
Paraffin-embedded, archived samples were obtained from 110 patients that were diagnosed with ESCC at the Cancer Center, Sun Yat-Sen University, between January 2002 and December 2008. The original clinical assessment records were available, and tissue samples were assessed histologically. Clinical staging was assessed according to the 2002 TNM (tumor-node-metastasis) staging of International Union Against Cancer. Of the 110 ESCC samples, 56 matched adjacent non-malignant tissues were available as controls. Cases were selected based on the availability of biopsy specimens and follow-up data. Patients with distant metastases (except in supraclavicular or celiac lymph nodes) and those with previous treatments were excluded. All of the samples used in this study were endoscopic biopsy specimens obtained before treatment. Prior informed consent from the patients and approval from the Institute Research Ethics Committee of the Cancer Center, Sun Yat-Sen University were obtained.
Immunohistochemistry (IHC)
IHC staining was performed on 5-μm tissue sections rehydrated through graded alcohols, as previously described [16]. Endogenous peroxidase activity was blocked by incubation with 0.3% hydrogen peroxide for 15 min. For antigen retrieval, tissue slides were boiled in 10 mM citrate buffer (pH 6.0) in a pressure cooker for 10 min. Non-specific antibody binding was blocked with 10% normal rabbit serum for 20 min. Tissue sections were incubated with a 1:50 dilution of anti-IGFBP-3 polyclonal antibody (directed to amino acids 113–210 of human IGFBP-3; Santa Cruz Biotechnology, Inc., USA) for 1 h at 37°C in a moist chamber. Sections were then incubated with 1:100 dilution biotinylated rabbit anti-mouse immunoglobulin for 30 min at 37°C, treated with a streptavidin–peroxidase conjugate for 30 min at 37°C, then 3′,3′-diaminobenzidine (DAB) was used as a chromogenic substrate using standard methodology. All sections were stained in DAB for the same duration of time. Nuclei were counterstained with Meyer’s hematoxylin. The primary antibody was replaced normal murine IgG in negative controls. Immuno-positive tissue sections were used as positive controls.
Measurements of IGFBP-3 expression by IHC assay
Five random fields were selected for scoring from each slide, and the mean score for each slide was used for the final analyses. Positive staining was assessed using a five-point scoring system: 0 (no positive cells), 1 (<10% positive cells), 2 (10–35% positive cells), 3 (36–70% positive cells), and 4 (>70% positive cells). To maximize objectivity, the intensity of positive staining was also used in a four-point scoring system: 0 (negative staining), 1 (weak, light yellow staining), 2 (moderate, yellow–brown staining), and 3 (strong, brown staining). The IGFBP-3 expression index was calculated as follows: expression index = (intensity score) × (positive score). The cut-off value for high and low levels of expression was based on a measurement of heterogeneity, and statistical analysis of overall survival was done using a log-rank test. Optimal cut-off values for this assessment system were identified as follows: high expression of IGFBP-3 was defined as an expression index score of ≥4, and low expression of IGFBP-3 was defined as an expression index score of <4. Two independent pathologists (D. Xie and M.Y. Cai), blinded with respect to the clinicopathological information, performed the scoring.
Western blotting analysis
Total protein was isolated from 11 pairs of fresh endoscopic biopsy specimens of ESCC tissue and adjacent non-malignant esophagus tissue using TRIzol buffer (Invitrogen, Carlsbad, CA) [17]. Equal amounts of whole cell and tissue lysates were resolved by SDS–polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene difluoride (PVDF) membrane (Pall Corp., Port Washington, NY, USA). Blots were incubated with primary mouse monoclonal antibodies against human anti-IGFBP-3 (Santa Cruz Biotechnology, with 1:200 dilution), and immunoreactivity was detected with enhanced chemiluminescence kit (Amersham Biosciences, Uppsala, Sweden). This detailed procedure was performed as previous described [14]. All procedures were conducted in accordance with the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed with SPSS software (SPSS Standard version 13.0, SPSS, Chicago, IL, USA). As IGFBP-3 protein expression did not follow a normal distribution, statistical evaluation was performed using non-parametric tests. The χ2 test was used to evaluate differences in IGFBP-3 expression between two categories of tissues. The χ2 test or the Fisher’s exact test was used to assess a correlation between IGFBP-3 expression and clinicopathological characteristics. Relative risks (RRs) of death associated with IGFBP-3 expression and other variables were estimated using univariate and multivariate Cox proportional hazards models. In our analyses, we defined a RR of 1.000 as a baseline for factors such as age (≤55 years), T1, N0, M0, clinical stage I and high IGFBP-3 expression. Survival curves were plotted using Kaplan–Meier survival analyses and compared using a log-rank test. Survival time was calculated from the date of ESCC diagnosis to the date of death for any cause. Further analyses of survival curves were based on stratifying TNM classifications and clinical stages. In all cases, differences with a P value of <0.05 were considered statistically significant.
Results
Decreased expression of IGFBP-3 in archival ESCC samples
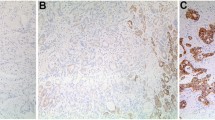
The IHC analysis showed that the IGFBP-3 expression was significantly decreased in primary cancer tissue compared with matched adjacent non-malignant tissue (χ2 = 17.562, P = 0.001). As shown in Fig. 1, among the non-malignant tissues, as many as 83.9% (47 of 56) of the individual tissue samples stained highly for IGFBP-3 (Fig. 1a). Among ESCC cases, however, only 51.8% (57 of 110) of cancer tissues were defined as having a high expression of IGFBP-3 (Fig. 1b), and the remaining ESCC cases (48.2%, 53 of 110) were scored as having no or low IGFBP-3 expression (Fig. 1c, d).
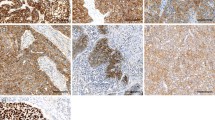
Immunohistochemical analysis of IGFBP-3 staining. a IGFBP-3 shows strong cytoplasm staining in matched normal esophageal tissues (×200 magnification). b An ESCC case (case 11) shows a high level of IGFBP-3 expression, in which more than 70% of ESCC cells stain positively for IGFBP-3 protein in the cytoplasm (×200 magnification). c An ESCC case (case 15) showing negative staining for IGFBP-3 (×200 magnification). d Low expression of IGFBP-3 was detected in an ESCC case (case 92), in which less than 20% ESCC cells showed positive staining of IGFBP-3 protein in the cytoplasm (×200 magnification). e Low expression of IGFBP-3 protein was detected by Western blotting in 5/5 ESCC cases (T1–5) compared with adjacent non-malignant esophageal tissue (N1–5)
Downregulation of IGFBP-3 protein analysis in fresh ESCC samples
We further examined the status of IGFBP-3 protein expression in 11 pairs of fresh ESCC tissue and adjacent non-malignant esophageal specimens by Western blotting. We found that a total of 5/11 (45.4%) ESCCs had reduced levels of IGFBP-3 protein expression compared with adjacent non-malignant esophageal tissue (Fig. 1e).
Decreased expression of IGFBP-3 correlates with clinical aggressiveness of ESCC
We further examined possible correlations between IGFBP-3 expression and clinical aggressiveness of ESCC. As shown in Table 1, analysis of 110 ESCC cases indicated that the expression of IGFBP-3 was strongly correlated with both clinicopathological classifications T, N, and M and clinical staging (χ2 test, P < 0.05 for all categories). These observations suggest a correlation between decreased expression of IGFBP-3 and clinical progression in ESCC patients.
Decreased IGFBP-3 expression is associated with poor prognosis in ESCC patients
Having demonstrated the correlation between IGFBP-3 expression and clinicopathological features, we next examined the relationship between IGFBP-3 expression and patient survival. Our analyses indicated a correlation between IGFBP-3 expression and the survival status of ESCC patients (P = 0.001; Table 1). Kaplan–Meier curves showed that in the primary ESCC category, patients expressing higher levels of IGFBP-3 had a longer overall survival time (median of 68.3 months), whereas patients expressing lower levels of IGFBP-3 had a much shorter survival time (median, 26.5 months; log-rank test, P < 0.001; Fig. 2a). The cumulative five-year survival rate was 45.6% for patients with high levels of IGFBP-3 (26 of 57). Surprisingly, the survival rate dramatically dropped to 15.0% for patients with reduced levels of IGFBP-3 (8 of 53). In light of the involvement of IGFBP-3 in the development of ESCC, we further analyzed patient survival in terms of IGFBP-3 expression in adjacent non-malignant tissues. As shown in Fig. 2b, examining adjacent non-malignant tissues demonstrated that the overall survival time in patients expressing high basal levels of IGFBP-3 (median of 66.3 months) was much longer than in patients expressing low levels of IGFBP-3 (median of 28.7 months; log-rank test, P = 0.001). Furthermore, IGFBP-3 expression still strongly correlated with patient survival even after stratifying patients on the basis of their clinicopathological classification (Fig. 3a–h).
IGFBP-3 expression influences overall survival. a Kaplan–Meier curves show that patients with low IGFBP-3 expression have poor overall survival (analysis of 110 primary ESCC tissues; P < 0.001). b Patients with high IGFBP-3 expression show better overall survival (analysis of 56 matched adjacent non-malignant ESCC tissues; P < 0.001)
Overall survival curves stratified by IGFBP-3 levels according to T, N, M classifications and clinical staging. a, b Differences in survival curves according to IGFBP-3 expression were seen in the T2 + T3 classification panel (P = 0.001) and the T4 classification panel (P = 0.016). c In the N0 classification panel, patients with high IGFBP-3 expression show much better overall survival (P < 0.001) than those with low expression. d In the N1 classification panel, overall patient survival is also significantly different between the low and high IGFBP-3 expression groups (P < 0.001). e, f Survival time is longer in patients with high IGFBP-3 expression regardless of distant lymph node metastases (P < 0.001 and P = 0.003, respectively). g, h Patients with high IGFBP-3 expression show better overall survival at different clinical stages (P < 0.001) than those with low expression
IGFBP-3 expression is a prognostic factor in ESCC patients
To determine whether IGFBP-3 could serve as a prognostic factor for ESCC, we examined overall survival using Cox regression proportional hazard analysis. As shown in Table 2, univariate Cox regression analysis revealed that reduced levels of IGFBP-3 protein expression was associated with a significantly increased risk of cancer-related death (P < 0.001) in ESCC patients. The RRs indicated that tumor size, distant lymph node metastasis, and clinical staging are also predictors of poor prognosis.
After adjusting for potential confounding factors, a reduced expression of IGFBP-3 in ESCC was found to predict poor survival in an independent manner (P < 0.001; Table 3). Analyses using multivariate Cox regression model for clinicopathological diagnoses (Table 3) gave the following results: clinicopathological classifications T, N, distant lymph node metastasis, and clinical staging predict poor overall survival.
Discussion
Since ESCC patients with the same clinicopathological classifications often display considerable variability in disease progression and survival, the traditional grading system may have reached its limits in providing critical information regarding patient prognosis and influencing treatment strategies. Thus, novel diagnostics and risk assessment strategies are urgently needed. IGFBP-3, a major carrier protein for insulin-like growth factors (IGF)-I and IGF-II in circulating blood [18], is known to regulate the bioavailability of insulin and insulin-like growth factors by modulating their interactions with signaling receptors [19, 20]. There is now evidence to suggest that IGFBP-3 is also involved in the induction of apoptosis [21]. For example, IGFBP-3 has been shown to increase ceramide-induced apoptosis in breast cancer and to enhance both p53-dependent and p53-independent apoptosis in various cancer cell lines [22, 23]. The observation that overexpression of IGFBP-3 increases apoptosis suggests that it may act as a tumor suppressor. Further indirect evidence for this is provided by reports that aberrant IGFBP-3 promoter hypermethylation and gene silencing is observed in various cancers and that this is associated with cancer progression [24–27]. However, in contrast, a previous study found that IGFBP-3 could stimulate cell proliferation under certain circumstances [28]. To the best of our knowledge, no study has yet been performed to investigate the status of IGFBP-3 and its potential impact in ESCC tumorigenesis.
Gene expression is modulated by both genetic and epigenetic mechanisms, and it is increasingly being recognized that epigenetic changes play an important role in carcinogenesis [29]. Epigenetic silencing of IGFBP-3 by promoter methylation has been reported in a number of solid tumors [27, 30–32]. In our present study, a majority (83.9%) of matched non-malignant tissues stained highly for cytoplasmic IGFBP-3, whereas only 51.8% of our cases showed high cytoplasmic IGFBP-3 staining in primary ESCC tissues. These observations echo those of a previous report [13]. Furthermore, we found that IGFBP-3 expression is inversely correlated with clinicopathological classifications of ESCC, which, to the best of our knowledge, has not been reported thus far.
The most important finding of our current study was the prognostic significance of IGFBP-3 expression in ESCC. Indeed, further analyses of primary cancer tissues have revealed for the first time that ESCC patients with low levels of IGFBP-3 expression had a reduced overall survival compared with those expressing higher IGFBP-3 levels, indicating a clinical value for IGFBP-3 in assessing the prognosis of ESCC patients. It should be emphasized that poor survival has been further observed in patients with low IGFBP-3 expression in normal esophageal tissue, making a stronger argument for the usefulness of IGFBP-3 protein expression for the prognosis of ESCC. These observations lead us to propose that IGFBP-3 may play an important role in the aggressiveness of ESCC. In the present study, with respect to patient survival, we also demonstrated a significantly increased risk of cancer-related death in patients with reduced IGFBP-3 expression. Our findings strongly suggest that IGFBP-3 may be a novel and important prognostic marker for ESCC.
Clinically, the current prognosis of ESCC mainly relies on T classification, lymph node status, distant metastasis, and clinical staging [33]. In our tests, univariate Cox proportional hazard analysis revealed that T, N, and M classifications could be high risk factors for cancer-related death (Table 2). In this respect, detailed investigations of phenotypic heterogeneity in ESCC may be important for revealing the underlying mechanism(s) for IGFBP-3 involvement in the development and progression of ESCC.
Having demonstrated the significance of IGFBP-3 expression levels for the prognosis of overall survival in ESCC patients, our next focus will be to investigate the potential value IGFBP-3 protein expression levels for predicting survival in other types of cancers and/or in disease-free survival. It may also be worthwhile investigating a possible correlation between IGFBP-3 expression levels and the outcome following chemotherapy and radiotherapy. Nevertheless, although further investigations are still needed, we suggest that IGFBP-3 expression could be useful in the prognostic assessment in patients with ESCC.
In summary, the present study demonstrates that low IGFBP-3 expression correlates with both clinicopathological classifications and poor overall survival in ESCC patients. Examination of IGFBP-3 expression by IHC could be used as an additional effective tool in identifying those ESCC patients at increased risk of tumor invasion and/or progression. Our findings suggest that IGFBP-3 may be a novel marker useful for prognostic assessment in patients with ESCC.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107.
Suntharalingam M. Definitive chemoradiation in the management of locally advanced esophageal cancer. Semin Radiat Oncol. 2007;17(1):22–8. doi:10.1016/j.semradonc.2006.09.008.
Rohatgi PR, Swisher SG, Correa AM, Wu TT, Liao Z, Komaki R et al. Failure patterns correlate with the proportion of residual carcinoma after preoperative chemoradiotherapy for carcinoma of the esophagus. Cancer. 2005;104(7):1349–55. doi:10.1002/cncr.21346.
Di Fiore F, Lecleire S, Rigal O, Galais MP, Ben Soussan E, David I, et al. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12(26):4185–90.
Luthra R, Wu TT, Luthra MG, Izzo J, Lopez-Alvarez E, Zhang L et al. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol. 2006;24(2):259–67. doi:10.1200/JCO.2005.03.3688.
He LR, Liu MZ, Li BK, Rao HL, Liao YJ, Guan XY et al. Prognostic impact of H3K27me3 expression on locoregional progression after chemoradiotherapy in esophageal squamous cell carcinoma. BMC Cancer. 2009;9:461. doi:10.1186/1471-2407-9-461.
He LR, Liu MZ, Li BK, Jia WH, Zhang Y, Liao YJ et al. High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int J Cancer. 2010;127(1):138–47. doi:10.1002/ijc.25031.
He LR, Liu MZ, Li BK, Rao HL, Liao YJ, Zhang LJ et al. Clusterin as a predictor for chemoradiotherapy sensitivity and patient survival in esophageal squamous cell carcinoma. Cancer Sci. 2009;100(12):2354–60. doi:10.1111/j.1349-7006.2009.01349.x.
Hollowood AD, Lai T, Perks CM, Newcomb PV, Alderson D, Holly JM. IGFBP-3 prolongs the p53 response and enhances apoptosis following UV irradiation. Int J Cancer. 2000;88(3):336–41. doi:10.1002/1097-0215(20001101)88:3<336::AID-IJC3>3.0.CO;2-A.
Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20(6):761–87.
Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. J Biol Chem. 1997;272(18):12181–8.
Williams AC, Collard TJ, Perks CM, Newcomb P, Moorghen M, Holly JM, et al. Increased p53-dependent apoptosis by the insulin-like growth factor binding protein IGFBP-3 in human colonic adenoma-derived cells. Cancer Res. 2000;60(1):22–7.
Takaoka M, Harada H, Andl CD, Oyama K, Naomoto Y, Dempsey KL et al. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 2004;64(21):7711–23. doi:10.1158/0008-5472.CAN-04-0715.
Yoshino K, Motoyama S, Koyota S, Shibuya K, Usami S, Maruyama K et al. IGFBP3 and BAG1 enhance radiation-induced apoptosis in squamous esophageal cancer cells. Biochem Biophys Res Commun. 2011;404(4):1070–5. doi:10.1016/j.bbrc.2010.12.115.
Zhao Z, Liu Y, He H, Chen X, Chen J, Lu YC. Candidate genes influencing sensitivity and resistance of human glioblastoma to Semustine. Brain Res Bull. 2011;86(3-4):189–94. doi:10.1016/j.brainresbull.2011.07.010.
Guo BH, Zhang X, Zhang HZ, Lin HL, Feng Y, Shao JY et al. Low expression of Mel-18 predicts poor prognosis in patients with breast cancer. Ann Oncol. 2010;21(12):2361–9. doi:10.1093/annonc/mdq241.
Xiong J, Yang Q, Kang J, Sun Y, Zhang T, Margaret G et al. Simultaneous isolation of DNA, RNA, and protein from Medicago truncatula L. Electrophoresis. 2010. doi:10.1002/elps.201000425.
Butt AJ, Firth SM, King MA, Baxter RC. Insulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cells. J Biol Chem. 2000;275(50):39174–81. doi:10.1074/jbc.M908888199M908888199.
Baxter RC, Butt AJ, Schedlich LJ, Martin JL. Antiproliferative and pro-apoptotic activities of insulin-like growth factor-binding protein-3. Growth Horm IGF Res. 2000;10 Suppl A:S10–1.
Oh Y, Muller HL, Lamson G, Rosenfeld RG. Insulin-like growth factor (IGF)-independent action of IGF-binding protein-3 in Hs578T human breast cancer cells. Cell surface binding and growth inhibition. J Biol Chem. 1993;268(20):14964–71.
Butt AJ, Williams AC. IGFBP-3 and apoptosis—a license to kill? Apoptosis. 2001;6(3):199–205.
Gill ZP, Perks CM, Newcomb PV, Holly JM. Insulin-like growth factor-binding protein (IGFBP-3) predisposes breast cancer cells to programmed cell death in a non-IGF-dependent manner. J Biol Chem. 1997;272(41):25602–7.
Collard TJ, Guy M, Butt AJ, Perks CM, Holly JM, Paraskeva C, et al. Transcriptional upregulation of the insulin-like growth factor binding protein IGFBP-3 by sodium butyrate increases IGF-independent apoptosis in human colonic adenoma-derived epithelial cells. Carcinogenesis. 2003;24(3):393–401.
Torng PL, Lee YC, Huang CY, Ye JH, Lin YS, Chu YW et al. Insulin-like growth factor binding protein-3 (IGFBP-3) acts as an invasion-metastasis suppressor in ovarian endometrioid carcinoma. Oncogene. 2008;27(15):2137–47. doi:10.1038/sj.onc.1210864.
Torng PL, Lin CW, Chan MW, Yang HW, Huang SC, Lin CT. Promoter methylation of IGFBP-3 and p53 expression in ovarian endometrioid carcinoma. Mol Cancer. 2009;8:120. doi:10.1186/1476-4598-8-120.
Chang YS, Wang L, Liu D, Mao L, Hong WK, Khuri FR, et al. Correlation between insulin-like growth factor-binding protein-3 promoter methylation and prognosis of patients with stage I non-small cell lung cancer. Clin Cancer Res. 2002;8(12):3669–75.
Tomii K, Tsukuda K, Toyooka S, Dote H, Hanafusa T, Asano H et al. Aberrant promoter methylation of insulin-like growth factor binding protein-3 gene in human cancers. Int J Cancer. 2007;120(3):566–73. doi:10.1002/ijc.22341.
Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23(6):824–54.
Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16(4):168–74. doi:S0168-9525(99)01971-X.
Hanafusa T, Yumoto Y, Nouso K, Nakatsukasa H, Onishi T, Fujikawa T et al. Reduced expression of insulin-like growth factor binding protein-3 and its promoter hypermethylation in human hepatocellular carcinoma. Cancer Lett. 2002;176(2):149–58. doi:S0304383501007364.
Ibanez de Caceres I, Dulaimi E, Hoffman AM, Al-Saleem T, Uzzo RG, Cairns P. Identification of novel target genes by an epigenetic reactivation screen of renal cancer. Cancer Res. 2006;66(10):5021–8. doi:10.1158/0008-5472.CAN-05-3365.
Wiley A, Katsaros D, Chen H, Rigault de la Longrais IA, Beeghly A, Puopolo M et al. Aberrant promoter methylation of multiple genes in malignant ovarian tumors and in ovarian tumors with low malignant potential. Cancer. 2006;107(2):299–308. doi:10.1002/cncr.21992.
Ikeda G, Isaji S, Chandra B, Watanabe M, Kawarada Y. Prognostic significance of biologic factors in squamous cell carcinoma of the esophagus. Cancer. 1999;86(8):1396–405. doi:10.1002/(SICI)1097-0142(19991015)86:8<1396::AID-CNCR3>3.0.CO;2-H.
Acknowledgments
This work was supported by grants from the Major State Basic Research Program (973 project) of China (2010CB912802 and 2010CB529401). We would like to thank Dr. Qiao-Qiao Li for her valuable comments during the design of this study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, L., He, LR., Zhang, R. et al. Low expression of IGFBP-3 predicts poor prognosis in patients with esophageal squamous cell carcinoma. Med Oncol 29, 2669–2676 (2012). https://doi.org/10.1007/s12032-011-0133-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0133-4