Abstract
Despite multimodal treatment, patients with astrocytoma still face a poor survival, and identification of valuable prognostic factors is crucial to yield effective individual therapy strategies. The aim of this study was to investigate progranulin (PGRN) expression in astrocytomas and explore its association with tumor grade and overall patient survival by scoring the PGRN immunoreactivity of both tumor cells and blood vessels. About 210 astrocytoma samples with different WHO grades and 14 normal brain tissues were studied by immunohistochemistry for PGRN. Semi-quantitative RT-PCR and Western blot were carried out to confirm its expression in 35 tumor specimens. Serum levels of PGRN in glioblastoma were examined by enzyme immunometric assay. PGRN expression was almost undetectable in the normal brain tissues by immunohistochemistry but increased in both astrocytoma cells and tumor blood vessels with pathological grading. Sera in glioblastoma were significantly higher than in healthy control. In grade II astrocytoma, strong vascular PGRN expression was closely related to tumor recurrence. In glioblastoma, high total PGRN expression, strong vascular PGRN expression, and strong tumor cellular PGRN expression all correlated with decreased patient survival in univariate analysis. However, only total PGRN expression as well as vascular PGRN expression status was independently associated with patient’s survival in the multivariate analysis. These results suggest that PGRN, involved in astrocytoma progression, may serve as a prognostic biomarker for glioblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytomas are the most common primary malignant brain tumors, accounting for approximately 75% of all types of glioma. They are classified into four malignancy grades (Grade I–IV) according to the World Health Organization (WHO) criterion. The overall prognosis for patients with astrocytomas is poor and closely related to WHO grade. Glioblastoma (Grade IV), the most prevalent and aggressive form of astrocytomas, has the poorest prognosis with median survival of less than 1 year despite multidisciplinary therapeutic approaches [1]. Early diagnosis and personalized clinical intervention based on genetic profiles may be of paramount value in the treatment of astrocytomas. Thus, understanding the molecular basis of astrocytoma progression and identification of prognostic markers are needed to devise effective individual targeted therapy.
Astrocytomas, particularly high-grade types, are characterized by aggressive growth and intense vascularity, which are associated with their ability to produce growth factors such as endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and platelet-derived growth factor (PDGF) [2]. Progranulin (PGRN), also known as PC-cell-derived growth factor, proepithelin, or granulin–epithelin precursor, is an 88-KD secreted glycoprotein that belongs to a novel class of growth factors. Constitutive expression of PGRN at high levels occurs during remodeling processes such as embryonic development, tissue repair, inflammation, and tumorigenesis [3]. Previous studies have suggested that PGRN regulates a wide range of biological processes involved in tumor development and progression, such as enhanced cell proliferation, survival, migration, invasion, and angiogenesis [4–8]. These tumor-promoting functions of PGRN are known to be associated with activation of growth factor-related signaling system including PI3 K/Akt, MAPK/ERK, and FAK pathways [5, 8]. Moreover, PGRN has been found to be overexpressed in several human tumor types, including glioblastoma, breast cancer, ovarian cancer, hepatocellular carcinoma, prostate cancer as well as multiple myeloma [3, 9–13].
Despite that PGRN mRNA expression has been reported to be highly expressed in glioblastomas [9], the expression pattern and clinical significance of PGRN has not been described previously in various forms of human astrocytomas. In this study, we investigated the expression profile of PGRN protein and its possible prognostic relevance in a larger collective of patients with astrocytoma.
Materials and methods
Patients
A total of 210 samples were obtained from operated astrocytoma patients at the Department of Neurosurgery, Qilu Hospital of Shandong University from 2005 to 2009. None of the patients had received radiotherapy or chemotherapy prior to surgery. About 14 normal brain tissue samples were collected from patients who underwent surgery for reasons other than brain tumors such as cerebral trauma. The relevant demographic and clinicopathological information was obtained from medical records and described in detail in Table 1. This study was approved by the Institutional Review Board of Shandong University.
Immunohistochemistry
Tumor-containing sections of 5-μm thickness were baked at 60°C for 50 min, deparaffinized in xylene, and rehydrated in graded concentrations of ethanol. Antigen retrieval was achieved by microwaving in 10 mmol/l of sodium citrate buffer at pH 6 for 20 min. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide in distilled water. Immunostaining involved sequential applications of primary antibody (PGRN at 1:150, R&D System; CD31 at 1:100, Santa Cruz Biotechnology) for overnight at 4°C, followed by biotinylated secondary antibodies (R&D System) at 37°C for 1 h and strepto–avidin–biotin complex (R&D System) at 37°C for 30 min. Diamino benzidine was used as the enzyme substrate to observe the specific antibody localization, and hematoxylin was used as a nuclear counterstain.
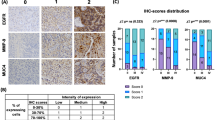
Immunostaining for PGRN was scored separately for the tumor cells and the tumor blood vessels. PGRN staining in tumor cells was scored for intensity (0, no signal; 1, weak; 2, moderate; and 3, marked) and the percentage of PGRN positive cells (0, negative; 1, <30%; 2, 30–60%; 3, >60%). The scores of each tumor specimen were summed to give a final score of 0–6, and the PGRN expression in tumor cells was finally determined as weak expression (score 0–4) and strong expression (score 5–6). The percentage of positive tumor blood vessels and the blood vessel staining intensity were assessed in the same way. The PGRN staining score in tumor cells and blood vessels were summed to reach a total tumor score with a maximum score of 12 points. Tumor samples scored 9–12 were considered overexpression (high expression). CD31 was used as a blood vessel marker.
Enzyme immunometric assay
The serum samples were isolated through centrifugation from peripheral venous blood and stored at −80°C until use. Seventeen patients with glioblastoma were available for evaluation of serum levels of PGRN. Age-matched control sera were obtained from eight healthy volunteers. Levels of PGRN in the sera were measured with ELISA methods according to the manufacturer’s instructions (R&D System).
Semi-quantitative RT-PCR
The mRNA level of PGRN in 35 astrocytoma samples was examined by semi-quantitative RT-PCR as described previously [1]. The primers used in this study are as follows: β-actin: sense, 5-ATC ATG TTT GAG ACC TTA AA-3; antisense 5-CAT CTC TTG CTC GAA GTC CA-3; PGRN [6]: sense, 5-AAT GTG ACA TGG AGG TGA GC-3 antisense, 5-AGC AGG TCT GGT TAT CAT GG-3. The cycling conditions used for PCR was as follows: 5 min at 95°C, 32 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s. β-actin was used for each RNA sample as an internal control.
Western blot analysis
The protein level of PGRN in 35 tumor samples was also measured by Western blotting according to a standard method as described previously [1]. The following primary antibodies were used: goat anti-PGRN (1:1,200; R&D) and rat anti-β-actin (1:700; Santa Cruz).
Statistical analysis
The statistical package SPSS 13.0 (SPSS, Chicago, IL, USA) was used for all analyses. PGRN expression levels in specimens of different pathological grades were compared using ANOVA with Bonferroni correction. Chi-square test was used to analyze the relationship between PGRN expression and the clinical parameters. Survival curves were plotted using the Kaplan–Meier method and compared by the log-rank test. Survival data were evaluated using univariate and multivariate Cox regression analyses. A P value <0.05 was considered statistically significant.
Results
Expression of PGRN in astrocytomas
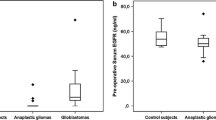
On immunohistochemistry assay, PGRN expression in normal brain tissues was almost undetectable in our conditions (Fig. 2a); however, PGRN positive tumor cells were detected in 96.2% of astrocytoma samples, and PGRN positive blood vessels were detected in 82.4% of the tumors. PGRN immunoreactivity was mostly detected in the cytoplasm of astrocytoma cells. Meanwhile, a minority of tumor cells stained in the nucleus, which tended to become more common in Grade III–IV astrocytoma cells. PGRN staining of tumor blood vessels was found to be localized in the cytoplasm of endothelial cells as well as on the blood vessel wall (Fig. 2c). As shown in Fig. 4, both tumor cellular and vascular PGRN expression increased with tumor grade. In glioblastoma, PGRN positive tumor cells showed a clear cytoplasmatic staining in both the smaller fusiform and stellate-shaped tumor cells as well as in the multi-nucleated giant cells (Fig. 3a–d). PGRN positive blood vessels were detected in 91.1% glioblastomas, and 51.1% samples exhibited strong vascular PGRN expression. As shown in Fig. 3g–h, intense immunostaining of PGRN was often detected in the pseudopalisading cells as well as neighboring vessels. Although strong PGRN staining blood vessels are generally closely related to strong staining tumor cells (Fig. 3d), most immunoreactivity could be observed in tumor blood vessels whereas relatively weak staining in tumor cells (Fig. 3e–f). Semiquantitative RT-PCR and Western blot have further analyzed the PGRN expression. As shown in Fig. 5a, PGRN mRNA was not detected in normal brain tissues, while expressed in all tumor samples. Western bolt analysis showed that PGRN was highly expressed in astrocytomas, whereas it was very weakly detected in normal brain tissues (Fig. 5b–c). We also evaluated serum levels of PGRN in the patients with glioblastoma; Fig. 1 showed significantly higher levels of PGRN in glioblastoma patients than healthy control.
Immunohistochemical staining of PGRN in GradeI–III astrocytomas and normal brain tissues. a Normal brain tissues with negative PGRN expression; b gradeIastrocytoma with few weak PGRN expression cells; c the blood vessels were strongly immunopositive and the tumor cells weakly positive in a Grade II astrocytoma, and the endothelium was marked by CD31 in the same tumor d; e and f expression of PGRN in both tumor cells and blood vessels of a Grade III astrocytoma [original magnification, ×200 (e); ×400 (f)]
Immunohistochemical staining of PGRN in glioblastomas. PGRN expression was detected in the cytoplasm of both the smaller fusiform and stellate-shaped tumor cells as well as in the multinucleated giant cells (a–d). Vascular PGRN staining varied between tumors as some tumor blood vessels showed no or weak expression (a, c), whereas other tumor showed marked PGRN staining in the cytoplasm of endothelial cells and blood vessel wall (d, e). Serial sections of glioblastoma sample were stained with PGRN and the endothelial cell marker CD31 (e, f). (g, h) High PGRN immunostaining was generally detected in pseudopalisading cells and neighboring vessels [original magnification, ×200 (g); ×400 (h)]
Correlation of PGRN expression with clinicopathological parameters
Progranulin immunostaining in tumor cells and blood vessels were scored separately (Table 2). As shown in Fig. 4, the tumor cellular PGRN scores, the blood vessels PGRN scores, and total PGRN scores all increased with tumor grade. We did not find significant associations between PGRN expression and age, gender, KPS scores, tumor locations, or image status. However, strong vascular PGRN expression was significantly related to tumor recurrence in Grade II astrocytomas (6/8 vs. 4/21, P = 0.005).
PGRN expression is up-regulated in astrocytomas. a RT-PCR analysis of PGRN mRNA levels in the astrocytoma specimens and normal control brain tissues, and β-actin was used as an internal control. b Expression of PGRN protein in astrocytoma samples and normal brain tissues by Western blot analysis. c Relative expression of PGRN in 5 normal brain tissues and 35 surgical astrocytoma samples. The expression levels are presented as a ratio between PGRN and loading control β-actin. *P<0.05, compared to astrocytomas
Correlation of PGRN expression with overall survival
Figure 6 showed the Kaplan–Meier overall survival curves for glioblastoma patients according to PGRN expression. Univariate analysis revealed that high total PGRN expression, strong tumor cellar PGRN expression, and strong vascular PGRN expression all associated with decreased overall survival of glioblastoma patients (Table 3). In multivariate Cox regression model adjusting for age, gender, and KPS score, only total PGRN expression and vascular PGRN expression were independently prognostic factors for overall survival of patients with glioblastoma (Table 3). For Grade I–III astrocytomas, we did not find any significant correlation between the PGRN expression and overall patient survival (data not show), which may be due to smaller sample size and shorter follow-up time.
Discussion
Astrocytomas are the most frequent malignant primary brain tumors, and prognosis for patients with high-grade astrocytoma is poor. Multiple aggressive treatment approaches, such as wide local excision and postoperative radiation therapy as well as chemotherapy, have been used clinically. However, these approaches do not benefit all patients equally. Adverse effects of these approaches such as brain radiation necrosis even severely affect patients’ quality of life and functional outcome. Therefore, identification of new molecular prognostic factors may contribute to better assessment of the survival probability, consequently, the tailoring of treatment for each individual patient.
High-grade astrocytomas are characterized by rapid growth, marked angiogenesis, and extensive local invasion. These properties of astrocytomas are directly related to their genetic aberrations in growth factor-regulated signaling pathways. To date, multiple growth factors, such as HGF, PDGF, and VEGF, have been suggested to be overexpressed in astrocytomas and play crucial role in their malignant progression [2]. Pharmaceutical agents targeting these factors are being intensively tested in preclinical and clinical studies, with some offering promising results. PGRN is a pluripotent growth factor secreted by rapidly proliferating cells such as skin cells, deep crypts of the gastrointestinal tract, and immune cells. In addition to its normal regulatory role in wound repair, cellular mitosis, and inflammatory response, previous studies have highlighted its multiple roles in tumorigenesis [3]. It has been shown that PGRN stimulates tumor cell growth and invasion, confers resistance to apoptosis, and adversely impacts therapeutic responses [4–8, 14, 15]. Ho et al. [16] have recently reported that anti-PGRN mAb can inhibit liver cancer cell proliferation and reduce tumor angiogenesis in vivo, suggesting that PGRN may be a promising therapeutic target. In addition, PGRN has been recognized as an important modulator of tumor-stromal communication. In an animal model of breast cancer, PGRN could induce resident tissue fibroblasts to express genes that promoted malignant tumor progression [17]. In addition, the expression of PGRN has also been investigated in several types of human cancers and was found to correlate closely with some clinicopathological parameters that increase tumor aggressiveness. In a previous report with breast carcinomas, high PGRN expression was frequently found in invasive ductal carcinomas and significantly associated with high tumor grade, proliferation index, and p53 expression [13]. In an analysis of 110 liver cancers, 46 patients that expressed higher PGRN protein exhibited significantly large tumor size, venous infiltration, and early intrahepatic recurrence [10]. In esophageal squamous cell carcinoma, PGRN expression correlated with the depth of tumor invasion and lymph node metastasis [18]. Recently, Tkaczuk et al. [19] found that circulating PGRN in serum of breast cancer patients is also at an increased level when compared to healthy volunteers.
Although many tumors have been shown to express PGRN at high level, the expression profile of PGRN protein in most form of astrocytomas remains unknown. Liau et al. [9] previously reported that highest expression of PGRN occurred in glioblastomas using cDNA microarray analysis. Consistent with this finding, we observed that astrocytoma cells exhibited abundant PGRN expression in the cytoplasm in immunohistochemical assay, in contrast to normal brain tissues that displayed absence or weak expression in scattered neuron cells. Moreover, our results showed that PGRN expression positively correlated with pathological grading, with highest in glioblastomas. Similarly, Western blot analysis also demonstrated an increased trend of PGRN protein level from grade I to grade IV astrocytomas. In addition, we also found the serum levels of PGRN in patients with glioblastoma are significantly higher than healthy controls. Take together, our data suggests that increased PGRN levels may be closely associated with the pathogenesis and progression of astrocytoma.
In the present study, we also detected strong PGRN immunoreactivity in tumor blood vessels, even in those samples with weak tumor cellular immunostaining. By scoring PGRN staining in tumor cells and blood vessels respectively, we found that both the vascular and tumor cellular PGRN expressions increased with tumor grade. These findings indicated that vascular PGRN expression may contribute to creating a permissive tumor microenvironment that promotes malignant progression. We further evaluated the correlation between PGRN expression and clinicopathological parameters including patient prognosis. We found that strong vascular PGRN expression was significantly correlated with tumor recurrence in Grade II astrocytomas (P = 0.005). However, the microvessel density in these tumors was also significantly higher than those with weak vascular expression (data not shown). This may be consistent with the previous findings that Grade II astrocytomas have a heterogeneous clinical course, and angiogenic potential is of prognostic importance in these tumors [20]. In glioblastoma, survival analysis demonstrated that high tumor cellular, vascular, and total PGRN expressions are all significantly associated with poor prognosis (P = 0.001; P = 0.000; P = 0.000, respectively). In multivariate analysis, both total PGRN staining score and vascular PGRN expression are independent prognostic factors for overall survival of patients with glioblastoma. In addition, we also investigated the microvessel density in the glioblastomas, and no differences were found between glioblastomas receiving a low versus a high PGRN vascular staining scores (data not show).Our results suggest that PGRN might be a reliable prognostic indicator in glioblastoma patients.
In conclusion, we have shown that PGRN expression was up-regulated in astrocytomas and positively correlated with pathological grading. Moreover, low PGRN expression in glioblastoma patients is significantly associated with a longer overall survival. This suggests that PGRN can serve as a potential prognostic biomarker for glioblastoma. For increased PGRN levels can be detected in the sera of glioblastoma patients, further study will focus on the correlation between its serum level and tumor tissue expression as well as patient prognosis.
References
Liu Q, Li G, Li R, Shen J, et al. IL-6 promotion of glioblastoma cell invasion and angiogenesis in U251 and T98G cell lines. J Neurooncol. 2010;100:165–76.
Arrieta O, Garcia E, Guevara P, et al. Hepatocyte growth factor is associated with poor prognosis of malignant gliomas and is a predictor for recurrence of meningioma. Cancer. 2002;94:3210–8.
He Z, Bateman A. Progranulin (granulin–epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–12.
Tangkeangsirisin W, Serrero G. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis. 2004;25:1587–92.
He Z, Ismail A, Kriazhev L, et al. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res. 2002;62:5590–6.
Liu Y, Xi L, Liao G, et al. Inhibition of PC cell-derived growth factor (PCDGF)/granulin–epithelin precursor (GEP) decreased cell proliferation and invasion through downregulation of cyclin D and CDK4 and inactivation of MMP-2. BMC Cancer. 2007;7:22.
Kamrava M, Simpkins F, Alejandro E, et al. Lysophosphatidic acid and endothelin-induced proliferation of ovarian cancer cell lines is mitigated by neutralization of granulin-epithelin precursor (GEP), a prosurvival factor for ovarian cancer. Oncogene. 2005;24:7084–93.
Monami G, Emiliozzi V, Bitto A, et al. Proepithelin regulates prostate cancer cell biology by promoting cell growth, migration, and anchorage-independent growth. Am J Pathol. 2009;174:1037–47.
Liau LM, Lallone RL, Seitz RS, et al. Identification of a human glioma-associated growth factor gene, granulin, using differential immuno-absorption. Cancer Res. 2000;60:1353–60.
Cheung ST, Wong SY, Leung KL, et al. Granulin-epithelin precursor over-expression promotes growth and invasion of hepatocellular carcinoma. Clin Cancer Res. 2004;10:7629–36.
Pan CX, Kinch MS, Kiener PA, et al. PC cell-derived growth factor expression in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Clin Cancer Res. 2004;10:1333–7.
Davidson B, Alejandro E, Florenes VA, et al. Granulin-epithelin precursor is a novel prognostic marker in epithelial ovarian carcinoma. Cancer. 2004;100:2139–47.
Serrero G, Ioffe OB. Expression of PC-cell-derived growth factor in benign and malignant human breast epithelium. Hum Pathol. 2003;34:1148–54.
Cheung ST, Cheung PF, Cheng CK, et al. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC) B5 regulate liver cancer cell chemoresistance. Gastroenterology. 2011;140:344–55.
Kim WE and Serrero G. PC cell-derived growth factor stimulates proliferation and confers Trastuzumab resistance to Her-2-overexpressing breast cancer cells. Clin Cancer Res. 2006;12:4192–9.
Ho JC, Ip YC, Cheung ST, et al. Granulin-epithelin precursor as a therapeutic target for hepatocellular carcinoma. Hepatology. 2008;47:1524–32.
Elkabets M, Gifford AM, Scheel C, Nilsson B, et al. Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest. 2011;121:784–99.
Chen XY, Li JS, Liang QP, et al. Expression of PC cell-derived growth factor and vascular endothelial growth factor in esophageal squamous cell carcinoma and their clinicopathologic significance. Chin Med J (Engl). 2008;121:881–6.
Tkaczuk KR, Yue B, Zhan M, et al. Increased circulating level of the survival factor gp88 (progranulin) in the serum of breast cancer patients when compared to healthy subjects. Breast Cancer (Auckl). 2011;5:155–62.
Dhermain F, Saliou G, Parker F, et al. Microvascular leakage and contrast enhancement as prognostic factors for recurrence in unfavorable low-grade gliomas. J Neurooncol. 2010;97:81–8.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 30872645) and the Natural Science Foundation of Shandong Province of China (No. Y2008C57).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Li, G., Yin, J. et al. Progranulin overexpression predicts overall survival in patients with glioblastoma. Med Oncol 29, 2423–2431 (2012). https://doi.org/10.1007/s12032-011-0131-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0131-6