Abstract
The purpose of this retrospective study was to develop a pre-treatment laboratory prognostic index (LPI) based on laboratory results that might serve as an extension to clinicopathological parameters for prognosis and treatment in patients with oral squamous cell carcinoma (OSCC). Pre-treatment LPI was calculated from C-reactive protein (CRP), hemoglobin (Hb) levels, and count of white blood cells (WBCs) due to significant (P < 0.05) association with locoregional recurrence measured for each parameter by receiver operating characteristic (ROC) curves in 187 patients with OSCC. Positive predictive values (+PV, precision rate) and negative predictive values (−PV) of LPI were measured. Likelihood ratios (LRs) were used to assess how good the pre-treatment LPI diagnostic test is to determine locoregional recurrence of the disease. CRP expression by cancer cells was confirmed by immunocytochemistry and FACS analysis. ROC analysis determined cutoff values for CRP levels, Hb levels, and WBC count and showed significant differences between nonrecurrent and recurrent group of OSCC. On univariate analysis, patients with high pre-treatment LPI (LPI ≥ 2, hazard ratio (HR) = 3.8670, 95% confidence interval (CI) = 2.2518–6.6407, P < 0.0001) had a significant poorer prognosis. Multivariate analysis showed that the most important independent prognostic factor was high pre-treatment LPI (LPI ≥ 2, HR = 3.6450, 95% CI = 2.3964–5.5441, P < 0.0001). Moreover, pre-treatment LPI ≥ 2 showed high probability that locoregional recurrence will be present later (+PV, LPI ≥ 2, 86.4%, 95% CI = 65.1–97.1). High +LR gave an excellent indication for a good quality of the test (LR+, LPI ≥ 2, 12.77, 95% CI = 8.8–18.6). Immunohistochemistry and FACS analysis confirmed inflammatory CRP expression by cancer cells. This study highlights the combination of inflammatory CRP levels, Hb levels, and WBC count as the most important independent prognostic factor in predicting disease recurrence of patients with OSCC. LPI can be used as a pre-treatment inflammatory biomarker that may identify OSCC with a more aggressive biological phenotype of the disease and might be helpful for guiding further post-operative treatment in OSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several clinicopathological parameters (CP) have been implicated in prognosis, recurrence, and survival following oral squamous cell carcinoma (OSCC). The purpose of this retrospective study was to develop a pre-treatment laboratory prognostic index (LPI) based on laboratory results that might serve as an extension to CP for prognosis and treatment in patients with OSCC.
The presence of a systemic inflammatory response has been thought to indicate poor prognosis in OSCC, based on the assumption that the processes underlying such a response play important roles in the progression of OSCC. In 1863, Virchow hypothesized that the origin of cancer was at sites of chronic inflammation, in part based on his hypothesis that some classes of irritants, together with the tissue injury and ensuing inflammation they cause, enhance cell proliferation [1]. Basic scientists have clearly demonstrated the importance of molecular and cellular pathways linking cancer and inflammation [1–3].
OSCC frequently metastasizes to the lymph nodes, which represents the most important predictor of patient survival rates. For about 50% of patients with OSCC have detectable lymph node involvement at presentation. Less than 40% of patients with lymph node metastasis at presentation survive 5 years, compared to 90% of patients without metastasis [4–6], meaning that the survival rate decreases by approximately 50% when nodal metastasis is present. Therefore, identification of poor prognostic factors in association with inflammation has become an important issue in the management of OSCC [7]. Poor prognosis is well known in advanced International Union Against Cancer (UICC) stages of the disease [8]. Other prognostic factors have also been proposed in the literature. In the pathogenesis of OSCC, pre-treatment measurement of elevated serum C-reactive protein (CRP) levels [9–14] and low hemoglobin (Hb) levels [15–20] has been identified to be promising for adverse prognosis. Increased count of white blood cells (WBCs) is associated with adverse prognosis in other cancer entities [21–24] but has not been identified for OSCC [25]. However, in the pathogenesis of OSCC, there are no studies regarding these risk factors together in a clinical context. Moreover, cutoff values for each parameter have not been determined for predicting survival of OSCC as yet. Therefore, the aims of this study were to measure pre-treatment cut-off values for laboratory parameters, to investigate relevant prognostic factors and to analyze survival rates in patients with OSCC in a larger patient cohort. Moreover, we elucidated a parameter that identifies a more aggressive biological phenotype of the disease, which might be helpful for guiding further post-operative treatment in OSCC.
Materials and methods
Patients and tumor specimen
Out of 484 patients with OSCC, we retrospectively reviewed the records of 187 patients after primary radical R0 tumor resection in our department between 1997 and 2010. Patients with nonresectable disease, inadequate follow-up data, and patients with preoperative antineoplastic therapies (chemoradiation/chemotherapy) were excluded from the study. The material was archival formalin-fixed, paraffin-embedded tissue from routine histopathologic work-up. The material had been stored with permission of the local ethics committee, after informed consent obtained from the patients prior to surgical resection.
Follow-up data were obtained from our local tumor registry of Middle Frankonia/Germany and was complete (100%) for all patients. The last follow-up was recorded from the last outpatient visit or the date of death. Tumor and patient characteristics are summarized in Table 1.
Histopathologic analysis and tumor staging
Tumor blocks of paraffin-embedded tissue were selected by experienced pathologists, evaluating the routine H.E.-stained sections. Sections from all available tumors underwent intensive histopathologic assessment, blinded to the prior histopathology report. Serial tissue sections (2 μm thickness) were cut from formalin-fixed paraffin-embedded (FFPE) blocks on a microtome and mounted from warm water onto adhesive microscope slides. Tumor staging was performed according to the 6th edition of the TNM staging system by the UICC/AJCC of 2002 [26]. Grading was performed according to WHO criteria [27]. Tumor characteristics (UICC stage, pT-categories, pN-categories, cM-categories, infiltrated lymph nodes, residual tumor status, tumor size, site distribution, grading, microvascular invasion, and lymphatic vessel involvement), treatment characteristics (surgery alone, surgery and radiotherapy with/without chemotherapy, or brachytherapy) patient characteristics (gender, age, personal history, habitual history) were collected in a database (EXCEL, Microsoft). Surgical margin status was determined on final histopathologic evaluation. Close margins were deemed positive in all analyses, whereas negative margins were considered greater than or equal to 10 mm from resection margin after tissue fixation.
Measurement of CRP levels, Hb levels, and WBC count
Standard laboratory techniques were used to determine pre-treatment CRP levels, Hb levels, and WBCs in 187 patients with OSCC.
Cell culture and immunocytochemistry
We analyzed inflammatory CRP expression in cells (1 × 104) from the squamous cell carcinoma (SCC) cell line SCC-4 (American Type Culture Collection, ATCC) in cytospins as a positive control of CRP expression by cancer cells. Cytospins of the SCC-4 cell line were fixed in acetone and dried for 10 min. Slides were then incubated in normal serum (2%) and bovine serum albumin (BSA) (0.5%) at room temperature for 20 min to block nonspecific binding. Subsequently, slides were incubated with the horseradish peroxidase (HRP)-conjugated primary antibody (Ab) or control antibody overnight at 4°C in a humidified chamber, followed by 5 min of incubation with DAB (Biogenex) and counterstained with hematoxylin. Slides were analyzed using a Zeiss camera (Jena, Germany). The photographed images were imported into the Microsoft Office Picture Manager.
Flow cytometric analysis of CRP expression by SCC-4 cells
Cells (5 × 106) derived from SCC-4 cancer cell line were analyzed on a flow cytometer (Beckman Coulter, Krefeld, Germany) with an appropriate software package (Coulter, Epics XL-MCL, System II). In each sample, we analyzed a constant cluster of tumor cells. Fluorescein isothiocyanate (FITC)-conjugated CRP Ab was purchased from HyTest Ltd (Turku, Finland), and FITC-conjugated isotype control Ab was purchased from Beckman Coulter (Krefeld, Germany). The total suspension of cells was pelleted and resuspended in PBS. For intracellular staining, we used the Intraprep-Kit (Beckman Coulter, Krefeld). Cells were incubated with CRP or control Ab for 20 min. After washing, cells were analyzed by FACS, equipped with the FITC emission signal detector FL1 (488 nm, green).
Statistical analysis
Statistical analysis was performed with MedCalc Software, version 11.6.1 (Mariakerke, Belgium). Disease-free survival (DFS) was calculated from the time of tumor resection until obvious locoregional recurrence or tumor conditional death, respectively. The DFS times were estimated using the Kaplan–Meier method [28] and were compared by using the log-rank test [29]. Multivariate analyses were performed using the Cox proportional hazards model [30]. All parameters that were found significant on univariate analysis were included. Hazard ratios for variables that may influence survival status in univariate and multivariate analysis were provided with 95% CI. Chi-square test (χ2) and Fisher’s exact test were used to investigate the relation between two categorical variables.
Receiver operating characteristic curves (ROC) were plotted to determine the best cutoff ranges for OSCC recurrence group screening for each value, and the relevant sensitivities and specificities were calculated [31]. Area under the curve (AUC) analysis was determined for quality measurement of the classifier (CRP, Hb, WBC, LPI). The cutoff point was determined as the value corresponding with the highest diagnostic average of sensitivity and specificity (highest diagnostic accuracy). These values were graphical displayed in a plot versus criterion values and in an interactive dot diagram to study the accuracy of each diagnostic test. Based on resulting sensitivity and specificity, the likelihood ratios (LRs) were calculated +LR = sensitivity/(1 − specificity) and −LR = (1 − sensitivity)/specificity). LRs were used to assess how good the single values and pre-treatment LPI diagnostic test are to determine locoregional recurrence of OSCC. LR is the ratio between the probability of a positive test (positive likelihood ratio, +LR) or a negative test (negative likelihood ratio, −LR) result given the presence of disease recurrence and the probability of a positive or negative test result given the absence of disease recurrence. The predictive value (PV) is the probability that disease recurrence is present (positive predictive value, +PV, precision rate) when the test is positive or absent (negative predictive value, −PV) when the test is negative (expressed as a percentage). For the calculation of predictive values, prevalence of disease recurrence has been stated to be 33.2% among subjects with OSCC (Table 1). All P values presented were 2-sided, and P < 0.05 was considered statistically significant.
Results
Demographic data of all patients
Out of 484 with OSCC, 187 patients were identified with complete pre-treatment laboratory parameters (CRP levels, Hb levels, and WBCs). There were 123 men (65.8%) and 64 women (34.2%). Mean age was 62 years (95% CI for the mean 60.5–63.9 years). The most common primary tumor site was floor of the mouth (26.7%) followed by alveolar ridge (38.5%), tongue (19.8%), buccal mucosa (7.5%), lips (4.8%), and palate (2.7%). Mean tumor thickness was 11.8 mm (95% CI for the mean 9.3–14.2 mm). Distant metastases were present in 1.6% of OSCC patients (n = 3). Average follow-up period was 26.4 ± 21.3 months. On histopathologic evaluation, tumors were regarded as well differentiated (15.5%, G1), moderately differentiated (46.5%, G2), poorly differentiated (36.9%, G3), or undifferentiated (2%, G4). Comparing the depth of invasion, 84 (44.9%) tumors were pT1, 38 (20.3%) tumors were pT2, 16 (8.6%) tumors were pT3, and 49 (26.2%) tumors were pT4. Regarding cervical lymph node assessment, 52 patients (27.8%) had nodal metastases confirmed by pathologic examinations. Lymphatic vessel invasion (L+) was positive in 125 patients (66.8%), and microvascular invasion (V+) was observed in 22 cases (11.8%). With regard to pathologic tumor stage, 74 patients (39.6%) had stage I disease, 27 patients (14.4%) had stage II disease, 23 patients (12.3%) had stage III disease, and 63 patients (33.7%) had stage IV disease. Table 1 presents the results in detail.
Concerning the clinicopathological characteristics and prognostic factors that may influence survival status (Table 1), all patients were categorized into two groups based on whether they had locoregional recurrence or had no locoregional recurrence. Comparison of the clinicopathologic characteristics between the nonrecurrent group (n = 125) and recurrent group (n = 62) disclosed no significant difference in age (P = 0.6062), gender (P = 0.9268), site distribution (P = 0.9559), and treatment modalities (P = 0.0782). Statistically significant difference between the nonrecurrent group and recurrent group was observed in histological grading (P < 0.0001), depth of invasion (P < 0.0001), cervical lymph node metastasis (P = 0.0013), lymphatic vessel invasion (P = 0.0028), microvascular invasion (P = 0.005), and tumor stage (P < 0.0001). A comparison of variables between the two groups is presented in Table 1.
Evaluation of the prognostic impact of single laboratory tests (CRP/Hb/WBC) and calculated Pre-treatment laboratory prognostic index (LPI) for the screening of OSCC disease recurrence
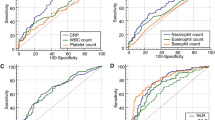
Similar to a previous work, a prognostic index was calculated from elevated inflammatory CRP and WBC [32]. In addition, low Hb levels were integrated in the calculation due to the suggested inflammatory condition in cancer patients. Subsequently, patients were categorized into four different groups (Table 2). ROC analysis determined cutoff values for CRP, Hb, and WBC in OSCC recurrent versus nonrecurrent group (Figs. 1a–f, 2). LPI was calculated from pre-treatment CRP levels, Hb levels, and WBCs due to significant associations with locoregional recurrence (Fig. 1g–i; Table 1) measured for each parameter by ROC analysis (Fig. 2). LPI was calculated because of low diagnostic efficiency of each value (CRP, Hb, and WBCs) alone (Table 3). Comparing Laboratory Prognostic index, 48 (25.7%) patients had LPI 0, 71 (38%) patients had LPI 1, 46 (24.5%) patients had LPI 2, and 22 (11.8%) patients had LPI 3. Table 1 presents the results in detail. Statistically significant difference between the nonrecurrent group and recurrent group was observed for LPI (P < 0.0001).
Plot versus criterion values (a–c), interactive dot diagrams (d–f), and univariate disease-free survival (DFS) Kaplan–Meier curves (g–i) for CRP, hemoglobin (Hb), and white blood cell count (WBC) in patients of nonrecurrent and recurrent group with OSCC. In Plot versus criterion values (part of ROC curve analysis), the sensitivity and specificity are plotted against the different criterion values for identifying best cutoff values: CRP (a), Hb (b), and WBC (c). In the interactive dot diagrams (part of ROC curve analysis), the data of the nonrecurrent and recurrent group are displayed as dots on two vertical axes. The horizontal line indicates the cutoff points with the best separation/highest accuracy (minimal false negative and false positive results) between nonrecurrent and recurrent group. The corresponding test characteristics sensitivity and specificity are shown at the right side of the display: CRP (d), Hb (e), and WBC (f). Kaplan–Meier survival curves for DFS stratified by measured cutoff points with highest accuracy of CRP (g), Hb (h), and WBC (i) show significant differences in univariate analysis
Receiver operating characteristic curves (ROC) for CRP, hemoglobin (Hb), and white blood cell count (WBC) in patients with OSCC. The true positive rates (sensitivity) are plotted in function of the false positive rate (100-specificity) for different cutoff points: ROC analysis for the diagnosis of recurrent group shows calculated cutoff values with highest diagnostic accuracy (arrows) of CRP (1), Hb (2), and WBC (3)
The cutoff points of CRP level, Hb level, WBC count, as well as LPI that gave the best sensitivity and specificity for the diagnosis of disease recurrent group were evaluated using area AUC analysis (Fig. 2). Additionally, values were graphical displayed in a plot versus criterion value diagram and in an interactive dot diagram to show and to control the highest diagnostic accuracy of each diagnostic test (Fig. 1a–f). A comparison of variables, calculated sensitivities, and specificities between the parameters is presented in Table 3. The LRs and PVs of CRP (calculated cutoff value of 1.1 mg/dl) were +LR 4.03, −LR 0.46 and +PV 66.7%, −PV 81.5%, respectively. AUC was 0.781 (P < 0.0001, Table 3). Analysis of Hb (calculated cutoff value of 12.7 g/dl) showed +LR 2.02, −LR 0.50 and +PV 50.8%, −PV 80.0%, respectively. AUC was 0.701 (P < 0.0001, Table 3). Analysis of WBC (calculated cutoff value of 9.6 count/nl) showed +LR 2.65, −LR 0.70 and +PV 56.8%, −PV 74.1%, respectively. AUC was 0.598 (P = 0.0335, Table 3). Finally, LRs and PVs of LPI (calculated cutoff value of LPI 2) were identified as +LR 12.77, −LR 0.71 and +PV 86.4%, −PV 73.9%, respectively. AUC was 0.767 (P < 0.0001, Table 3).
Table 4 shows that LPI is associated with clinicopathological characteristics and prognostic factors in patients with OSCC. Statistically significant association between LPI and clinicopathological characteristics was observed in histological grading (P = 0.0363, Table 4), depth of invasion (P = 0.0002, Table 4), and tumor stage (P = 0.0002, Table 4).
Univariate analysis
Based on Kaplan–Meier survival analysis, the disease-free 1-, 3-, and 5-year survival rates in all surgically treated patients were 89, 76, and 65%, respectively. Kaplan–Meier survival curves for CRP, Hb, and WBCs are shown in Fig. 1g–i. Log-rank test analysis of Kaplan–Meier survival curves identified CRP (P < 0.0001, Fig. 1g), Hb (P < 0.0001, Fig. 1h), and WBCs (P < 0.0001, Fig. 1i), histological grading (P < 0.0001, Table 5), depth of invasion (P < 0.0001, Table 5), cervical lymph node metastasis (P < 0.0008, Table 5), lymphatic vessel invasion (P < 0.0045, Table 5), microvascular invasion (P < 0.0001, Table 5), and high LPI (LPI ≥ 2, P < 0.0001, Table 5; Fig. 3a) as significantly affecting survival. In addition, there was a significant difference in the 5-year DFS rate regarding these clinicopathologic characteristics and prognostic factors (Table 1). Conversely, there was no significant difference in the 5-year DFS rate in age (P = 0.1051, Table 1) and gender (P = 0.9160, Table 1).
Kaplan–Meier (a) and Cox-regression (b) survival curves for disease-free survival (DFS) stratified by low (LPI 0/1) and high (LPI 2/3) pre-treatment laboratory prognostic index (LPI). DFS 1-, 3-, and 5-year survival rates (LPI 0/1 vs. LPI 2/3) in univariate Kaplan–Meier survival curve (a) are 95% versus 77%, 83% versus 40%, and 67% versus 18%, respectively. DFS 1-, 3-, and 5-year survival rates (LPI 0/1 versus LPI 2/3) in multivariate Cox-regression (b) survival curve were 94% versus 84%, 83% versus 55%, and 67% versus 29%, respectively
Multivariate analysis
Multivariate analysis using the Cox proportional hazards model demonstrate depth of invasion (pT3/4, P = 0.0249, Table 6), microvascular invasion (V+, P = 0.0053, Table 6), and high LPI (LPI ≥ 2, P = 0.0001, Table 6; Fig. 3b) as independent prognostic factors in all (n = 187) OSCCs. Cervical lymph node metastasis (LN+, P = 0.2289, Table 6), histological grading (G3/4, P = 0.3543, Table 6), and lymphatic vessel invasion (L+, P = 0.4918, Table 6) were not found to be independent prognostic factors.
Flow cytometric analysis and immunocytochemistry of CRP expression by SCC-4 cells
FACS analysis and immunocytochemistry were performed to show expression of inflammatory CRP by cancer cells. CRP expression of the cancer cell line SCC-4 measured by FACS analysis (Fig. 4a, b) was confirmed by immunocytochemistry (Fig. 4c, d).
Immunocytochemistry staining and FACS analysis of CRP expression from the SCC-4 cancer cell line. Immunocytochemistry IgG control (a). CRP staining (cytoplasmic staining pattern, brown) in cytospins serve as positive control of CRP expression by cancer cells and shows 80–90% positive cells. Original magnification: ×200-fold. FACS IgG control (c). FACS analysis confirms immunocytochemistry CRP expression (d) by SCC-4 cancer cells
Discussion
In this hospital-based study conducted in Middle Frankonia/Germany, 187 patients with oral cavity squamous cell carcinoma treated at a single institution by primary surgical resection and factors affecting survival were analyzed. This is the first study focusing on the development of a pre-treatment laboratory prognostic index (LPI) based on laboratory results that might serve as an extension to clinicopathological parameters (CP) for prognosis and treatment in a representative patient cohort with oral squamous cell carcinoma (OSCC).
Although Khandavalli [10] has analyzed elevated CRP levels as an independent risk factor for OSCC, others do not support a positive association between pre-operative CRP levels and prognosis of OSCC [11]. Several possible mechanisms have been proposed for the relationship between CRP and cancer. First, tumor growth can cause tissue inflammation and hence increase CRP levels [33, 34]. Second, CRP could be an indicator of an immune response to tumor antigens [35]. Third, there is evidence that cancer cells can increase the production of inflammatory proteins, which could explain the high CRP concentrations in patients with cancer [35]. Some cancerous cells have been shown to express CRP [36, 37], and cancer cell lines have been shown to secrete Interleukin-6 (IL-6) and IL-8, which in turn induce the production of CRP [38, 39]. These mechanisms imply that increased CRP is a response to the neoplastic process and that CRP concentrations could thus provide a marker for identifying people with cancer at an early stage when treatment might be more effective. Our data support this suggestion although the cutoff value has been determined at a low level.
Similarly, anemia is associated with poor cancer control [20, 40]. Pre-treatment Hb values of <14.5 g/dl have already been identified with decreased local tumor control rate [41, 42]. Our data are well in line with these previous results. In anemic patients, hypoxia is more pronounced [43]. Tumor hypoxia, mostly resulting from poor perfusion and anemia, is one of the key factors in inducing the development of cell clones with an aggressive and treatment-resistant phenotype that leads to rapid progression and poor prognosis. Hypoxic conditions lead to the elaboration of proangiogenic and growth-stimulating proteins and provide some measure of therapeutic resistance [42].
The effect of tumor development on circulating leukocyte number has not been clarified for OSCC. Elevated WBC count was found to be an independent prognostic factor, associated with reduced survival in human metastatic melanoma, pancreatic carcinoma, and renal carcinoma [21–24, 44–46]. Our results support these findings of other cancer entities. The infiltrating inflammatory cells within the tumor are associated with an increased circulating leukocyte number (WBC count) and have pleiotropic functions: inhibition of tumor growth by nonspecific cytotoxic mechanisms and induction of cell lysis, or conversely, promotion of tumor development through the release of inflammatory mediators [47]. Therefore, measurement of CRP level, Hb level, and WBC count is useful and cheap baseline inflammation markers regarding evaluation of prognosis in OSCC. Moreover, a cutoff value for the association with locoregional recurrence measured for each parameter by receiver operating characteristic (ROC) curves has not been determined as yet. Indeed, single parameters were associated with significant reduced survival in univariate analysis. However, each value alone (CRP level, Hb level, and WBC count) failed to be relevant in predicting locoregional disease recurrence. Therefore, we measured a combination of these pre-treatment laboratory parameters (LPI). Multivariate analysis showed that the most important independent prognostic factor was high pre-treatment LPI (LPI ≥ 2). Moreover, high pre-treatment showed high probability that locoregional recurrence will be present later. High +LR gave an excellent indication for a good quality of the test.
In the present study, we focused on the putative role of inflammatory parameters in the pathogenesis of OSCC. Others stated that the amount of extrahepatic synthesis of CRP in neurons, monocytes, lymphocytes, and tumor cells as a local inflammatory response is too little to influence serum CRP [37, 48]. However, our results of CRP expression by cancer cells measured by immunocytochemistry and FACS analysis might be in accordance with a ‘smouldering’ inflammation in OSCC suggested by the low CRP cutoff value.
Incorporated into a LPI, CRP levels, Hb levels, and WBC count identified 4 groups that were summarized into 2 practical subgroups (LPI 0/1 ‘low’ and LPI 2/3 ‘high’) of OSCC patients with distinct risks of disease progression and death. This information might be pertinent to treatment decision-making and might also influence a revised staging system. If the objective is prognostic stratification, then using anatomic features alone for staging is no longer sufficient.
Conclusions
In conclusion, this is the first study that has analyzed a pre-treatment LPI by measuring a cutoff value for laboratory results by ROC analysis in patients with OSCC. It highlights the combination of inflammatory CRP, low Hb levels, and elevated WBCs as the most important independent prognostic factor in predicting disease recurrence of patients with OSCC. LPI can be used as a pre-treatment inflammatory biomarker that may identify recurrent group with a more aggressive biological phenotype of the disease. LPI might be helpful for guiding further post-operative treatment in OSCC. Therefore, high LPI is a new developed prognostic feature for OSCC patients with subsequent therapeutic implications.
Abbreviations
- OSCC:
-
Oral squamous cell carcinoma
- LPI:
-
Pre-treatment laboratory prognostic index
- CP:
-
Clinicopathological parameters
- CRP:
-
C-reactive protein
- Hb:
-
Hemoglobin
- WBCs:
-
White blood cells
- ROC:
-
Receiver operating characteristic
- +PV:
-
Positive predictive value
- −PV:
-
Negative predictive value
- +LR:
-
Positive likelihood ratio
- −LR:
-
Negative likelihood ratio
References
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45.
Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431(7007):405–6.
Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68(11):4026–30. doi:10.1158/0008-5472.CAN-08-0427.
Grandi C, Alloisio M, Moglia D, Podrecca S, Sala L, Salvatori P, Molinari R. Prognostic significance of lymphatic spread in head and neck carcinomas: therapeutic implications. Head Neck Surg. 1985;8(2):67–73.
Greenberg JS, Fowler R, Gomez J, Mo V, Roberts D, El Naggar AK, Myers JN. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer. 2003;97(6):1464–70. doi:10.1002/cncr.11202.
Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26(3–4):645–62. doi:10.1007/s10555-007-9082-y.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi:10.1038/nature01322.
Kreppel M, Eich HT, Kubler A, Zoller JE, Scheer M. Prognostic value of the sixth edition of the UICC’s TNM classification and stage grouping for oral cancer. J Surg Oncol. 2010;102(5):443–9. doi:10.1002/jso.21547.
Tartour E, Deneux L, Mosseri V, Jaulerry C, Brunin F, Point D, Validire P, Dubray B, Fridman WH, Rodriguez J. Soluble interleukin-2 receptor serum level as a predictor of locoregional control and survival for patients with head and neck carcinoma: results of a multivariate prospective study. Cancer. 1997;79(7):1401–8. doi:10.1002/(SICI)1097-0142(19970401)79:7<1401:AID-CNCR18>3.0.CO;2-3.
Khandavilli SD, Ceallaigh PO, Lloyd CJ, Whitaker R. Serum C-reactive protein as a prognostic indicator in patients with oral squamous cell carcinoma. Oral Oncol. 2009;45(10):912–4. doi:10.1016/j.oraloncology.2009.03.015.
Kruse AL, Luebbers HT, Gratz KW. C-reactive protein levels: a prognostic marker for patients with head and neck cancer? Head Neck Oncol. 2010;2:21. doi:10.1186/1758-3284-2-21.
Jablonska E, Piotrowski L, Grabowska Z. Serum levels of IL-1b, IL-6, TNF-a, sTNF-RI and CRP in patients with oral cavity cancer. Pathol Oncol Res. 1997;3(2):126–9.
Gallo O, Gori AM, Attanasio M, Martini F, Giusti B, Brunelli T, Gallina E. Interleukin-6 and acute-phase proteins in head and neck cancer. Eur Arch Otorhinolaryngol. 1995;252(3):159–62.
Chen HH, Chen IH, Liao CT, Wei FC, Lee LY, Huang SF. Preoperative circulating C-reactive protein levels predict pathological aggressiveness in oral squamous cell carcinoma: a retrospective clinical study. Clin Otolaryngol. 2011;36(2):147–53. doi:10.1111/j.1749-4486.2011.02274.x.
Gibson AD, Crawford J, Jain VK. Impact of correction of anemia on clinical outcome in patients with cancer: recent data from head and neck and breast cancer trials. Support Cancer Ther. 2004;1(3):145–9. doi:A1U14R0MK9712G53.
Hu K, Harrison LB. Impact of anemia in patients with head and neck cancer treated with radiation therapy. Curr Treat Options Oncol. 2005;6(1):31–45.
Dubray B, Mosseri V, Brunin F, Jaulerry C, Poncet P, Rodriguez J, Brugere J, Point D, Giraud P, Cosset JM. Anemia is associated with lower local-regional control and survival after radiation therapy for head and neck cancer: a prospective study. Radiology. 1996;201(2):553–8.
Lee WR, Berkey B, Marcial V, Fu KK, Cooper JS, Vikram B, Coia LR, Rotman M, Ortiz H. Anemia is associated with decreased survival and increased locoregional failure in patients with locally advanced head and neck carcinoma: a secondary analysis of RTOG 85–27. Int J Radiat Oncol Biol Phys. 1998;42(5):1069–75. doi:S0360-3016(98)00348-4.
Prosnitz RG, Yao B, Farrell CL, Clough R, Brizel DM. Pretreatment anemia is correlated with the reduced effectiveness of radiation and concurrent chemotherapy in advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(4):1087–95. doi:10.1016/j.ijrobp.2004.07.710.
Kumar P. Impact of anemia in patients with head and neck cancer. Oncologist. 2000;5(Suppl 2):13–8.
Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–50. doi:10.1038/nrc1388.
Lee YJ, Lee HR, Nam CM, Hwang UK, Jee SH. White blood cell count and the risk of colon cancer. Yonsei Med J. 2006;47(5):646–56. doi:200610646.
Grimm RH Jr, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA. 1985;254(14):1932–7.
Shankar A, Mitchell P, Rochtchina E, Tan J, Wang JJ. Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Am J Epidemiol. 2007;165(4):375–82. doi:10.1093/aje/kwk022.
Kruse AL, Luebbers HT, Gratz KW. Evaluation of white blood cell count as a possible prognostic marker for oral cancer. Head Neck Oncol. 2011;3:13. doi:10.1186/1758-3284-3-13.
Sobin LH, Ch W. UICC. TNM classification of malignant tumors. 6th ed. Berlin: Springer; 2002.
Hamilton SR, Aaltonen LA. Pathology and genetics. Tumours of the digestive system. 3rd ed. Lyon: IARC Press; 2000.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;75:457–87.
Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–70.
Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:1987–2001.
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–77.
Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15(2):425–30. doi:10.1158/1078-0432.CCR-08-0149.
Basso D, Fabris C, Meani A, Del Favero G, Vianello D, Angonese C, Meggiato T, Bellinvia S, Fogar P, Petrin P, et al. C reactive protein in pancreatic cancer and chronic pancreatitis. Ann Clin Res. 1988;20(6):414–6.
O’Hanlon DM, Lynch J, Cormican M, Given HF. The acute phase response in breast carcinoma. Anticancer Res. 2002;22(2B):1289–93.
Wang CS, Sun CF. C-reactive protein and malignancy: clinico-pathological association and therapeutic implication. Chang Gung Med J. 2009;32(5):471–82. doi:3205/320501.
Nozoe T, Korenaga D, Futatsugi M, Saeki H, Maehara Y, Sugimachi K. Immunohistochemical expression of C-reactive protein in squamous cell carcinoma of the esophagus—significance as a tumor marker. Cancer Lett. 2003;192(1):89–95. doi:S0304383502006304.
Jabs WJ, Busse M, Kruger S, Jocham D, Steinhoff J, Doehn C. Expression of C-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue. Kidney Int. 2005;68(5):2103–10. doi:10.1111/j.1523-1755.2005.00666.x.
O’Riordain MG, Falconer JS, Maingay J, Fearon KC, Ross JA. Peripheral blood cells from weight-losing cancer patients control the hepatic acute phase response by a primarily interleukin-6 dependent mechanism. Int J Oncol. 1999;15(4):823–7.
Wigmore SJ, Fearon KC, Sangster K, Maingay JP, Garden OJ, Ross JA. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int J Oncol. 2002;21(4):881–6.
Bhattathiri VN. Relation of erythrocyte and iron indices to oral cancer growth. Radiother Oncol. 2001;59(2):221–6. doi:S0167814001003267.
Overgaard J, Hansen HS, Andersen AP, Hjelm-Hansen M, Jorgensen K, Sandberg E, Berthelsen A, Hammer R, Pedersen M. Misonidazole combined with split-course radiotherapy in the treatment of invasive carcinoma of larynx and pharynx: report from the DAHANCA 2 study. Int J Radiat Oncol Biol Phys. 1989;16(4):1065–8.
Vaupel P. Hypoxia and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist. 2008;13(Suppl 3):21–6. doi:10.1634/theoncologist.13-S3-21.
Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9(8):1221–35. doi:10.1089/ars.2007.1628.
Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93(3):273–8. doi:10.1038/sj.bjc.6602702.
Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, Navaglia F, Zambon CF, Pasquali C, Venza E, Pedrazzoli S, Plebani M. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32(1):22–8. doi:00006676-200601000-00004.
Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24(13):1997–2005. doi:10.1200/JCO.2005.03.9594.
Al-Sarireh B, Eremin O. Tumour-associated macrophages (TAMS): disordered function, immune suppression and progressive tumour growth. J R Coll Surg Edinb. 2000;45(1):1–16.
Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279(47):48487–90. doi:10.1074/jbc.R400025200.
Acknowledgments
The authors thank the assistance of Mrs. Corinna Buettner for her technical support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimm, M., Lazariotou, M. Clinical relevance of a new pre-treatment laboratory prognostic index in patients with oral squamous cell carcinoma. Med Oncol 29, 1435–1447 (2012). https://doi.org/10.1007/s12032-011-0045-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0045-3