Abstract
A gene expression profile analysis using an Affymetrix HG-U133 Plus 2.0 microarray with probes for 38,500 human full-length cDNAs was performed on a primary papillary thyroid carcinoma (PTC) and a nodular goiter (NG). ZCCHC12 was the gene with the most significant differential expression between PTC and NG, and this was verified using fluorescent quantitative PCR (FQ-PCR). A total of 9,485 genes were detected with a difference in transcription levels between PTC and NG. Of these, 2,098 were up-regulated with a signal log ratio (SLR) ≥ 1 and 1,714 were down-regulated with an SLR ≤ −1. Among these up-regulated and down-regulated genes, 12 genes were significantly up-regulated (SLR ≥ 5.0) and 6 genes were significantly down-regulated (SLR ≤ −5.0). The SLR of the ZCCHC12 gene was 8.8. The results of FQ-PCR showed that the medians of the log (ZCCHC12 RNA/GAPDH RNA) in PTC and NG were 0.73 and −1.68, respectively, and the difference between them was significant (P < 0.05). There were no significant correlations between the RNA levels of the ZCCHC12 gene and the clinicopathological and biochemical parameters of PTC in our pilot study. This study showed that a number of differentially expressed genes were discovered between PTC and NG. Significantly, the number of transcript copies of the ZCCHC12 gene in PTC was higher than in NG. The verified results of FQ-PCR were consistent with the microarray screening results. The ZCCHC12 gene may be a novel diagnostic molecular marker of PTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid nodules are a very common problem in clinical practice. In the United States, 4–7% of the adult population has a palpable thyroid nodule [1]. The prevalence of thyroid nodules is much greater when nodules that are detected by ultrasonography or at autopsy are included [2]. High-resolution ultrasound (US) can detect thyroid nodules in 19–67% of randomly selected individuals, with higher frequencies in women and the elderly [3]. Regardless of the level of iodine in the diet, the prevalence of thyroid nodules in adults in Northern China is more than 10% by thyroid ultrasonography [4]. Today, the most common lesion of benign thyroid nodules is nodular goiter (NG), and the most common lesion of thyroid malignancy is papillary thyroid carcinoma (PTC); only approximately 1 in 20 clinically identified nodules is malignant [5]. In the United States, approximately 44,670 new cases of thyroid cancer were diagnosed in 2010, and thyroid carcinomas in women ranked as the fifth most common type of cancer [6]. The management of a solitary thyroid nodule remains controversial, and distinguishing between benign and malignant thyroid nodules is a common diagnostic dilemma. The introduction of fine needle aspiration cytology (FNAC) in the 1970s simplified the evaluation of thyroid nodules and decreased the likelihood of surgery for a thyroid nodule by approximately 50% [7]. Despite the ability to identify PTC with some reliability by FNAC, clinicians must frequently make a decision regarding the management of patients with thyroid nodules on the basis of equivocal information.
Many attempts have been made to identify molecular markers for thyroid carcinoma that can preoperatively distinguish between benign and malignant lesions. None of the markers have proven to be ideal, including LGALS3 (galectin-3), KRT19 (keratin 19), FN1 (fibronectin 1), BRAF, RET/PTC, RAS, HBME-1, MET (met pro-oncogene), DPP4 (dipeptidyl-peptidase 4), SERPINA1 (serpin peptidase inhibitor, clade A, member 1), MUC1 (mucin 1), and TIMP1 (tissue inhibitor of metallopeptidase 1) [8–16]. Microarray technology has become a powerful tool for analyzing the gene expression profiles of tens of thousands of genes simultaneously. Parallel analysis of gene expression profiles using the microarray technique offers a large-scale platform for screening novel markers for potential clinical applications. In general, the two main foci of microarray investigations are the improvement in the understanding of the molecular etiology of thyroid neoplasms and the development of improved techniques in the detection of genetic markers that could improve the differential diagnosis of thyroid nodules.
In the present study, we first screened the differential gene expression profiles of papillary thyroid carcinoma and nodular goiter with an oligonucleotide microarray. Next, we used FQ-PCR to validate the most significantly differentially expressed gene (ZCCHC12). Finally, we analyzed the correlation between the ZCCHC12 transcription levels and the clinicopathological and biochemical parameters of PTC.
Materials and methods
Tissue specimens
Two thyroid tissue samples, including one PTC and one NG, were obtained intraoperatively from two patients during primary thyroidectomy. The tissue specimens were placed in tissue tubes with RNA preservation solution and stored at −80°C. The histopathologic diagnosis was confirmed according to the 2004 World Health Organization (WHO) criteria [17].
RNA extraction
The total RNA of the tissue specimens was isolated using TRIzol (Invitrogen, Carlsbad, CA) and was purified with the RNeasy Kit (QIAGEN, Valencia, CA) according to the manufacturer’s recommendations.
Oligonucleotide expression microarray analysis
The Affymetrix HG-U133 Plus 2.0 microarray (Affymetrix, Inc., Santa Clara, CA) was used for microarray analysis; this system includes 38,500 definite cDNA probes and more than 47,000 transcripts and variants. The samples were processed following the Affymetrix protocol. The procedure included the extraction and purification of RNA using TRIzol and QIAGEN’s RNeasy Mini Kit; the synthesis and purification of cDNA; the synthesis of biotin-labeled cRNA; and the hybridization, washing, staining, and scanning of the chip using GeneChip Operating Software (GCOS) (Affymetrix, Inc., Sana Clara, CA) to read and manage data.
Fluorescent quantitative polymerase chain reaction (FQ-PCR)
The most significantly differentially expressed gene, ZCCHC12, was selected for validation by FQ-PCR (Table 1).
In total, 52 thyroid tissue samples were acquired intraoperatively from 52 patients during primary thyroidectomy. According to the 2004 WHO criteria, PTC was diagnosed in 28 patients and NG was diagnosed in 24 patients. The tissue specimens were collected in EP tubes with RNA preservation solution within half an hour after the tissue was removed from the body. The specimens were stored at −80°C until use.
Among the 28 PTC patients, there were 15 females and 13 males, aged 12–76 years. The patients were euthyroid before surgery. The classic variant of PTC was diagnosed in 21 cases, the follicular variant in 6 cases, and the column cell variant in 1 case. Regional lymph node metastases were diagnosed during primary surgery in 27 patients.
The primer and probe sequence data were attained from the Genbank sequence data bank. The probe of H-ZCCHC12 was FAM-ACCTCTGTCCTTGCTCCTTCTCCCTGC-TAMRA, the sense primer was GGATACCAGCACATTGGAGGG, and the antisense primer was TATACCACTTTCACAAAGAATAAAGCTG. The FQ-PCR procedure included the extraction of tissue RNA using Trizol (Invitrogen, Carlsbad, CA), identification of the RNA sample, reverse transcription of the RNA, preparation of a positive standard template, and FQ-PCR. The ZCCHC12 gene transcript was analyzed by real-time reverse transcription-PCR (ABI PRISM 7900 Sequence Detector apparatus, Applied Biosystems). First-strand cDNA was synthesized. Reactions were performed by using SYBR Green (SYBR Green PCR Core Reagent, Perkin-Elmer), and quantification was performed using ABI PRISM 7900 SDS software. The quantification of each gene’s expression was calibrated using a standard reference curve obtained by serial dilutions of PCR product prepared from a mixture of cDNAs from the tissue samples that were handled separately but concomitantly with the clinical samples.
Ethical standards
The study had been approved by the ethics committee of Sun Yat-sen University Cancer Center and had therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The patients gave their informed consent prior to their inclusion in the study.
Statistical methods
The data were analyzed with the SPSS 13.0 software. A Mann–Whitney U rank sum test was used to compare the parameters between the two groups because of the skewed distribution of the data and the heterogeneity of variance.
Results
Microarray study
The differentially expressed genes between PTC and NG
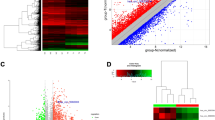
There were 9,485 differentially expressed genes between PTC and NG. A total of 2,098 genes were up-regulated in PTC with an SLR ≥ 1, whereas 1,714 genes were down-regulated with an SLR ≤ −1 in PTC. Among the total number of differentially expressed genes between PTC and NG, 12 genes were significantly up-regulated with an SLR ≥ 5.0 (Table 2) and 6 genes were significantly down-regulated with an SLR ≤ −5.0 (Table 3). The ZCCHC12 gene was the most significantly differentially expressed gene between PTC and NG, with an SLR of 8.8.
The FQ-PCR of the ZCCHC12 gene in PTC and NG
The amplification of the ZCCHC12 gene in PTC and NG
The amplification of the ZCCHC12 gene in PTC and NG was shown in Figs. 1 and 2.
The amplification of the GAPDH gene (internal reference) in PTC and NG
The amplification of the GAPDH gene in PTC and NG was shown in Figs. 3 and 4.
A comparison of the expression levels of the ZCCHC12 gene in PTC and NG
The number of amplification copies of the ZCCHC12 gene (RNA) in PTC and NG
The number of amplification copies of the ZCCHC12 gene in PTC was higher than that in NG, but the values in Table 4 should not be compared directly; the numbers of amplification copies of the ZCCHC12 gene from the different tissues should be normalized to the internal reference gene first (Table 4).
A comparison of the ZCCHC12 gene expression level in PTC and NG
After the numbers of amplification copies of the ZCCHC12 gene were compared with the numbers of copies of the internal reference gene (GAPDH), a series of ratio values were obtained. Because these ratio values showed a significantly skewed distribution, the common logarithm (log) of these ratios was calculated. The common logarithm of the ratios also showed a skewed distribution; so, they could not be analyzed statistically with a t test or ANOVA (analysis of variance). Therefore, the Mann–Whitney U rank sum test was chosen.
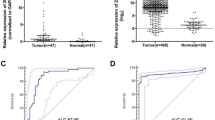
As shown in Table 5 and Fig. 5, the FQ-PCR showed that the medians of the log (ZCCHC12 RNA/GAPDH RNA) for PTC and NG were 0.73 and −1.68, respectively, and the difference between them was significant (P < 0.05). Therefore, the number of amplification copies of the ZCCHC12 gene in PTC was significantly higher than in NG.
The correlation between the amplification copy of the ZCCHC12 gene and the clinicopathological and biochemical parameters of PTC
We further analyzed the correlation between the numbers of amplification copies of the ZCCHC12 gene and the clinicopathological and biochemical parameters of PTC.
From Table 6, we conclude that the number of amplification copies of the ZCCHC12 gene in PTC had no correlation with the common clinicopathological and biochemical parameters of PTC (P > 0.05).
Discussion
Thyroid nodules are a very common problem in clinical practice. Most thyroid nodules are benign, and approximately 5% of all thyroid nodules are malignant. The key for the management of thyroid nodules is to discriminate malignant nodules from benign nodules. PTC accounts for approximately 80% of all incidences of thyroid cancer in the USA.
There have been many attempts to find a sensitive and specific molecular marker that can readily discriminate benign from malignant thyroid lesions, but the resultant markers have all fallen short in some way. Two main genetic alternations have been found in PTC (RET/PTC rearrangement and BRAF mutations) [12, 13]. Many molecular markers have been investigated, and some were considered to be associated with PTC (such as LGALS3, FN1, MET, DPP4, SERPINA1, KRT19, MUC1, TIMP1, CITED1, and DUSP6), but none of them has proven to be sensitive enough or specific enough to be translated into a clinically ideal marker [18].
The first array investigation of PTC was performed by Huang et al. [14] using the Affymetrix U95A array that contains more than 12,000 transcripts. In their study of 8 PTC tissues, which were compared with the surrounding normal tissue from the same eight patients, Huang et al. specified fifty genes with the most distinct gene expression changes. Some of the genes were already known to be differentially expressed in PTC. These researchers also identified a number of additional PTC specific genes, and many of them associated with the cell cycle or mitogenic control. In subsequent studies, many genes identified in the study conducted by Huang et al. were confirmed to distinguish between PTC and normal or benign thyroid tissue, independent of the microarray platform or the analysis algorithms used [16, 19–22].
The Affymetrix Human Genome U133 Plus 2.0 microarray was used, which provides comprehensive coverage of the transcribed human genome on a single array covering over 47,000 transcripts represented by more than 1 million distinct oligonucleotide entities. Our study demonstrates that there are many differentially expressed genes between PTC and NG. A comparison of the PTC samples with the NG samples using the Affymetrix HG-U133 Plus 2.0 whole genome microarray produced a list of 9,485 genes that were differentially expressed. Although we used only two microarrays to screen for differentially expressed genes, we not only obtained several genes known to be related to PTC (such as CITED1, SERPINA1, MET, TIMP1, TFF3, DPP4, FN1, LGALS3, TPO, EPS8, PROS1, and DIO1), but we also identified some new PTC-related genes (such as ZCCHC12 and WIF1). To validate the microarray results, we selected the most significantly differentially expressed gene between PTC and NG, the ZCCHC12 gene, for confirmation by FQ-PCR. This gene had not been implicated in thyroid carcinoma previously and was confirmed to be preferentially expressed in PTC by FQ-PCR. The results of the microarray and FQ-PCR were consistent and indicated that the ZCCHC12 gene may be a potential diagnostic molecular marker for PTC. The role of the ZCCHC12 gene in PTC requires further investigation. The preliminary analysis showed that the amplification copy number of the ZCCHC12 gene did not have a significant correlation with the clinicopathological and biochemical parameters of PTC.
Mouse ZCCHC12, or Sizn1 (Smad-interacting zinc finger protein 1), is a transcriptional coactivator in the bone morphogenic protein (BMP) signaling pathway, encoding a 402-amino acid protein with an N-terminal paraneoplastic antigen (MA)-homologous region, a single CCHC zinc finger motif, and a putative nuclear localization sequence [23]. Sizn1 positively modulates the BMP signal by interacting with Smad family members and the cAMP-responsive element-binding protein (CREB). Human ZCCHC12 shares 74% sequence identity with mouse ZCCHC12. Both mouse and human ZCCHC12 are located on chromosome X, contain a CCHC zinc finger motif at their C-terminus and have two putative nuclear localization signals (NLS). Four different sequence variants in human ZCCHC12 were characterized in 11 individuals with non-syndromic, X-linked mental retardation (NS-XLMR), implicating ZCCHC12 as a novel candidate gene for XLMR [24]. Cho et al. [25] reported that Sizn1 localized to promyelocytic leukemia protein nuclear bodies (PML-NBs) and showed that two SUMO interaction motifs (SIMs) in Sizn1 could bind to SUMO and govern SUMO conjugation to Sizn1 in the absence of the consensus motif for SUMO attachment. Li et al. [26] showed that human ZCCHC12 is a nuclear protein that is specifically expressed in human brain and mouse embryonic brain, functions as a positive modulator of activator protein 1 (AP-1) and the CREB signal and interacts with c-jun. Moreover, a novel nuclear localization signal that is necessary for the nuclear localization and punctate distribution was found in human ZCCHC12.
In conclusion, in the present study, we have demonstrated that a number of differentially expressed genes exist between PTC and NG. The number of transcript copies of the ZCCHC12 gene in PTC was significantly higher than in NG. The results verified by FQ-PCR were consistent with the microarray screening results. The ZCCHC12 gene may be a novel diagnostic molecular marker of PTC.
References
Singer PA, Cooper DS, Daniels GH, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. Arch Intern Med. 1996;156:2165–72.
Hegedüs L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102–32.
Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–31.
Teng W, Shan Zh, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–93.
Wong CKM, Wheeler MH. Thyroid nodules: rational management. World J Surg. 2000;24:934–41.
Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:1–24.
Hamberger B, Gharib H, Melton LJ III, et al. Fine-needle aspiration biopsy of thyroid nodules. Impact on thyroid practice and cost of care. Am J Med. 1982;73:381–4.
Coli A, Bigotti G, Zucchetti F, et al. Galectin-3, a marker of well-differentiated thyroid carcinoma, is expressed in thyroid nodules with cytological atypia. Histopathology. 2002;40:80–7.
Martins L, Matsuo SE, Ebina KN, et al. Galectin-3 messenger ribonucleic acid and protein are expressed in benign thyroid tumors. J Clin Endocrinol Metab. 2002;87:4806–10.
Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–7.
Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7.
Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–7.
Huang Y, Prasad M, Lemon WJ, et al. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci USA. 2001;98:15044–9.
Finley DJ, Zhu B, Barden CB, et al. Discrimination of benign and malignant thyroid nodules by molecular profiling. Ann Surg. 2004;240:425–36.
Jarzab B, Wiench M, Fujarewicz K, et al. Gene expression profile of papillary thyroid cancer: sources of variability and diagnostic implications. Cancer Res. 2005;65:1587–97.
DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. World health organization classification of tumors, pathology and genetics of tumors of endocrine organs. Lyon: IARC Press; 2004.
Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214.
Mazzanti C, Zeiger MA, Costouros NG, et al. Using gene expression profiling to differentiate benign versus malignant thyroid tumors. Cancer Res. 2004;64:2898–903.
Aldred MA, Huang Y, Liyanarachchi S, et al. Papillary and follicular thyroid carcinomas show distinctly different microarray expression profiles and can be distinguished by a minimum of five genes. J Clin Oncol. 2004;22:3531–9.
Finley DJ, Arora N, Zhu B, et al. Molecular profiling distinguishes papillary carcinoma from benign thyroid nodules. J Clin Endocrinol Metab. 2004;89:3214–23.
Giordano TJ, Kuick R, Thomas DG, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24:6646–56.
Cho G, Lim Y, Zand D, et al. Sizn1 is a novel protein that functions as a transcriptional coactivator of bone morphogenic protein signaling. Mol Cell Biol. 2008;28:1565–72.
Cho G, Bhat SS, Gao J, et al. Evidence that SIZN1 is a candidate X-linked mental retardation gene. Am J Med Genet A. 2008;146A:2644–50.
Cho G, Lim Y, Golden JA. SUMO interaction motifs in Sizn1 are required for promyelocytic leukemia protein nuclear body localization and for transcriptional activation. J Biol Chem. 2009;284:19592–600.
Li H, Liu Q, Hu X, et al. Human ZCCHC12 activates AP-1 and CREB signaling as a transcriptional co-activator. Acta Biochim Biophys Sin (Shanghai). 2009;41:535–44.
Acknowledgments
This work was supported by the Sun Yat-sen University 985 Program Initiation Fund Phase II, the Natural Science Fund of Guangdong Province (sequence number 07001523, 9451008901002400), and the Medical Research Fund of Guangdong Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Ql., Chen, Fj., Lai, R. et al. ZCCHC12, a potential molecular marker of papillary thyroid carcinoma: a preliminary study. Med Oncol 29, 1409–1417 (2012). https://doi.org/10.1007/s12032-011-0018-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0018-6