Abstract
Tumor-infiltrating lymphocytes (TILs) represent the host immune response to cancer. CD8+ cytotoxic T cells (CTLs) have a central role in the elimination of tumors, while regulatory T cells (Tregs) can suppress the immune reaction. The aim of this study was to investigate the prognostic value of TILs, especially Tregs and CTLs, in hepatocellular carcinoma (HCC) patients after resection. CD3+, CD4+, CD8+, and FoxP3+ TILs were assessed by immunohistochemistry in tumor tissue from 141 randomly selected HCC patients. Prognostic effects of low- or high-density TIL subsets were evaluated by Kaplan–Meier and Cox regression analysis using the median values as cutoff. The density of intratumoral Tregs (P = 0.040) and peritumoral CTLs (P = 0.004) were an independent factor for overall survival (OS), but not for disease-free survival (DFS). The density of CD3+ and CD4+ TILs, and the prevalence of Tregs and CTLs were associated with neither OS nor DFS. The presence of low intratumoral Tregs with high intratumoral CTLs was a negative independent prognostic factor for OS (P = 0.001), while that of low intratumoral Tregs and low peritumoral CTLs independently correlated with improved DFS (P = 0.008). Moreover, the combined analysis of Tregs and CTLs displayed better prognostic performances than any of them alone. Additionally, higher density of intratumoral Tregs correlated with both the presence of liver cirrhosis (P = 0.025) and increased tumor size (P = 0.050). Tregs within tumor environment are promising prognostic parameters for HCC patients, and their combination with CTLs can predict prognosis more effectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor-infiltrating lymphocytes (TILs) are considered as one of the manifestations of host immune reaction against cancers. Patients with a prominent lymphocyte infiltration have improved prognoses [1, 2]. Nowadays, it has been clear that TILs are heterogeneous and contain various immune cell subsets, which can suppress or promote the progression of tumors. Therefore, if distinctions were made with respect to lymphocyte types, location, and their combinations, a more profound impact on prognosis will be observed compared with only the overall degree of lymphoid infiltration [3].
Regulatory T cells (Tregs) have a crucial role in impeding immune surveillance against cancer and hampering the development of effective antitumor immunity [4]. It has been reported that Tregs are increased in both the peripheral blood and the tumor microenvironment in many human carcinoma types [4] and can suppress proliferation and production of granzyme and perforin of autologous CD8+ cytotoxic T cells (CTLs) through direct cell-to-cell contact or via the release of cytokines [5–7]. The most specific Treg cells marker identified to date is the nuclear transcription factor known as FoxP3, which can define regulatory T cells in human tumors [8]. Accumulating studies have documented a link between the infiltration of FoxP3+ Tregs in several human carcinoma types and prognosis. The most comprehensive of these studies were in colorectal cancer with similar conclusion by numerous groups [3, 9]. However, mixed or conflicting results remain among several other cancer types [1, 10]. Importantly, more and more attention has been given to the balance between Tregs and CTLs. The concurrent low-Treg and high-CTL densities in Hodgkin’s lymphoma [11] and hepatocellular carcinoma (HCC) [12] have been reported to be more valuable than the single TIL subtypes.
However, the prognostic role of TILs, especially the combination of Tregs with CTLs, in patients with HCCs remains less clear. In this study, we examine the prognostic value of the density and prevalence of Tregs and CTLs alone or combined in intratumoral and peritumoral TILs. We suggest that Tregs and CTLs are independent predictors for survival and recurrence of HCC patients, respectively; and, more importantly, the existence of synergetic effect between Tregs and CTLs as regard HCC prognosis.
Patients and methods
Patients and samples
Archival specimens were obtained from 141 patients at the First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China) after written informed consent. The inclusion criteria included the following: (1) diagnosis of HCC confirmed by pathology; (2) without anticancer treatment and distant metastases before surgery; (3) underwent curative resection for HCC between 2005 and 2010, defined as macroscopically complete removal of the tumor with negative safety margin [12, 13]; (4) with complete clinicopathologic and follow-up data. The clinical classification of tumors was determined according to the TNM classification system of International Union Against Cancer (edition 6). The histologic grade of tumor differentiation was assigned by the Edmondson grading system [14]. Liver function was assessed by Child-Pugh score system [15]. Clinicopathologic characteristics are summarized in Table S1. The study protocol was approved by the Institutional Review Board of Key Lab of Combined Multi-organ Transplantation, Ministry of Public Health.
Follow-up
The last follow-up was completed on March 31, 2011. The mean follow-up time reached 22.7 months (range, 2.0–70.3). All patients were monitored prospectively by serum alpha-fetoprotein (AFP), abdominal ultrasonography, and chest X-ray every 1–6 months according to the postoperative time. For patients with test results suggestive of recurrence, computed tomography and/or magnetic resonance imaging were used to verify whether recurrence had occurred. A diagnosis of recurrence was based on typical imaging appearance in computed tomography and/or magnetic resonance imaging scan and an elevated AFP level. Overall survival (OS) was defined as the interval between surgery and death or between surgery and the last observation for surviving patients. The data were censored at the last follow-up for living patients.
Immunohistochemistry analysis
Immunohistochemistry was done on the formalin-fixed, paraffin-embedded tissue sections as previously described [5, 13]. The monoclonal antibodies used were CD3, CD4, CD8 (Novocastra, Newcastle, UK), and FoxP3 (Abcam, Cambridge, UK). Briefly, the sections were deparaffinized and rehydrated. Before blocking of endogenous peroxidase with methanol containing 3% H2O2, the sections were autoclaved at 121°C for 10 min in citrate buffer (pH 6.0) for antigen retrieval. After blocking with goat serum, the sections were reacted overnight with appropriately diluted primary antibodies. Slides were then incubated with labeled polymer horseradish peroxidase rabbit/mouse antibody for 15 min (Envision+ Detection System; Dako, Carpinteria, CA). Diaminobenzidine was used as the chromogen, and the nuclei were counterstained with hematoxylin.
Evaluation of immunohistochemical variables
The number of TILs was counted using a computerized image analysis system composed of a Leica DFC420 CCD camera, installed on a Leica DMIR2 light microscope (Leica, Wetzlar, Germany), and attached to a personal computer. Ten different high-power fields (×400), representing the densest lymphocytic infiltrates, were selected for each sample to ensure representativeness and homogeneity [12] and were counted by two independent investigators without knowledge of the clinicopathologic data. Variations in counts exceeding 5% were re-counted, and a consensus decision was made. “Peritumoral” was liver tissues adjacent to the tumor within a distance of 10 mm (supplementary Fig. 1a) [5, 13, 16]. The proportion of FOXP3+ lymphocytes among CD4+ lymphocytes and that of CD8+ lymphocytes among CD3+ T cells were calculated using the mean number of total fields, and the averages were compared.
Statistic analysis
Statistical analyses were performed with SPSS 15.0 software (SPSS, Chicago, IL). Cumulative survival time was calculated by the Kaplan–Meier method and analyzed by the log-rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. For all immunohistochemical markers, the cutoff for definition of subgroups was the median value. A secondary analysis was performed to assess the correlation of lymphocytic variables with clinicopathologic characteristics. For the comparison of individual variables, χ2 tests and Spearman’s coefficients tests were carried out as appropriate. Statistical significance was set at P < 0.05.
Results
Immunohistochemical characteristics
Higher densities of CD3+, CD4+, and CD8+ TILs were observed in peritumoral area than in intratumoral area (supplementary Fig. 1a–d). However, the density of FoxP3+ cells in intratumoral area was close to that in peritumoral area (supplementary Fig. 1e), thus resulting in significantly higher prevalence of FoxP3+ cells (FoxP3+/CD4+ TILs) in intratumoral area (P < 0.001, compared with peritumoral area; Table 1). Different from FoxP3+ cells, the prevalence of CD8+ cells (CD8+/CD3+ TILs) was similar in the two regions (Table 1).
Correlation between the studied TIL subsets is listed in Table S2. In intratumoral region, the densities of all TIL subsets correlated with each other (range of correlation coefficients, 0.23–0.66; P = 0.005 or <0.001). In peritumoral region, the densities of TIL subsets also correlated with each other except FoxP3+ cells. However, the association with TIL subsets from different regions seemed to be much weaker. In addition, a weak association was observed in the intratumoral prevalence between FoxP3+ and CD8+ cells (r = 0.22, P = 0.008; Table S3).
Prognostic factors
The disease-free survival (DFS) and OS rates were 59 and 65% at 3 years, 52 and 43% at 5 years, respectively, for the whole study population. On univariate analysis, age, AFP level, tumor number, vascular invasion, TNM stage, and tumor grading were predictors for DFS, while AFP level, tumor size, number, vascular invasion, and TNM stage for OS (Table 2).
The type of lymphocytic infiltration in the intratumoral or peritumoral area did not show any prognostic significance for DFS (Table 2; Figs. 1, 2a, c, e; supplementary Fig. 2a–d). For OS, only the density of intratumoral Tregs (FoxP3+) and peritumoral CTLs (CD8+), but not intratumoral CTLs, was found to be associated with prognosis (Fig. 2b, d, f; Table 2). Patients with low intratumoral Treg or peritumoral CTL number had longer mean survival time (MST) (57 or 53 months, respectively) than did those with high intratumoral Treg or peritumoral CTL number (34 or 35 months, respectively). In contrast, the prevalence of Tregs (FoxP3+/CD4+) or CTLs (CD8+/CD3+) in either intratumoral or peritumoral area had no prognostic value (Table 2; supplementary Fig. 2e–h).
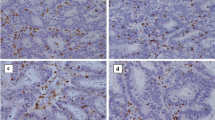
Tumor-infiltrating FoxP3+ and CD8+ cells. Consecutive sections were used for immunohistochemical study on (a, c, e, and g) FoxP3+ and (b, d, f, and h) CD8+ cells: (a, b) high FoxP3+ and low CD8+ cells; (c, d) low FoxP3+ and high CD8+ cells; (e, f) both low; (g, h) both high. Positive lymphocytes were stained brown (magnification, ×400)
Clinicopathologic features showing significance by univariate analysis were adopted as covariates for multivariate Cox proportional hazards analyses. The density of both intratumoral Tregs (FoxP3 +I ; P = 0.040) and peritumoral CTLs (CD8 +P ; 0.004) was independent prognostic factors for OS (Table 3). These two factors showed poor effects on patient outcome. In addition, AFP level was also associated with OS (P = 0.023). For DFS, only Age and TNM stage were revealed to be independent prognostic factors (P < 0.05, Table 3).
Combination of intratumoral Tregs with intratumoral or peritumoral CTLs
The combined influence of low versus high number of intratumoral Tregs and CTLs was also evaluated. First, the combination of CD8 +I with FoxP3 +I was evaluated. Using the median number as the cutoff, patients were classified into four groups: CD8 LoI FoxP3 HiI (n = 29), CD8 HiI FoxP3 LoI (n = 27), CD8 LoI FoxP3 LoI (n = 43), and CD8 HiI FoxP3 HiI (n = 42). Significant differences in both OS (P < 0.001) and DFS (P = 0.006) were found among the four groups with shortest MST in the CD8 LoI FoxP3 HiI group (21 and 13 months, respectively) and longest MST in the CD8 HiI FoxP3 LoI group (65 and 60 months, respectively) (supplementary Figs. 3a, b). Moreover, the MST of CD8 LoI FoxP3 HiI group is significantly shorter than those of the other three groups (Fig. 3a, b). Multivariate analysis revealed that CD8 LoI FoxP3 HiI was a negative independent prognostic factor for OS (HR = 0.03, P = 0.001; Table 3).
Kaplan–Meier analyses of disease-free survival and overall survival for the combination of intratumoral Tregs (FoxP3I) with intratumoral CTLs (CD8I) and with peritumoral CTLs (CD8P). High intratumoral Tregs concomitant with intratumoral Low CTLs (CD8 LoI FoxP3 HiI ) were associated with both shorter survival and higher recurrence (a, b), while the cocurrent presence of low intratumoral Tregs and low peritumoral CTLs (CD8 LoP FoxP3 HiI ) predicted prolonged survival and reduced recurrence (c, d)
Second, we analyzed the combination of CD8 +P with FoxP3 +I , as both of them were independent factors for OS. We grouped the patients in the same way: CD8 LoP FoxP3 HiI (n = 30), CD8 HiP FoxP3 LoI (n = 27), CD8 LoP FoxP3 LoI (n = 21), and CD8 HiP FoxP3 HiI (n = 26). Significant difference in OS was also found with the MST of CD8 LoP FoxP3 LoI much longer than those of the other three groups (P < 0.01, Fig. 3d; supplementary Fig. 3d). Interestingly, although they were not prognostic factors for DFS, the combined analysis of CD8 +P with FoxP3 +I showed the same result as for OS (P = 0.018, Fig. 3c). Moreover, multivariate analysis showed that CD8 LoP FoxP3 LoI was an independent prognostic factor for improved DFS (HR = 7.46, P = 0.008; Table 3). Finally, we revealed that the AUC of the combination of Tregs with CTLs was larger than that of Tregs or CTLs alone by receiver operating curve (ROC) analyses (Table 4).
Correlation of immunohistochemical variables with clinicopathologic features
Intratumoral Tregs were found to be associated with liver cirrhosis (P = 0.025) and tumor size (P = 0.050). The presence of liver cirrhosis or increased size of the primary tumor was prone to have higher density of intratumoral Tregs (Table 5). Moreover, the presence of liver cirrhosis also showed a positive association with the intratumoral prevalence of Tregs (FoxP3 +I /CD4 +I ) (P = 0.025, Table S4). Intratumoral CD8+ cells correlated positively with tumor number (P = 0.011). Neither peritumoral FoxP3+ nor peritumoral CD8+ TILs correlated with any clinicopathologic features (Table 5). In addition, significant association was observed between intratumoral CD3+ cells and Child-Pugh score (P = 0.042) and tumor number (P = 0.012) (Table S4).
Discussion
Tumor-infiltrating lymphocytes represent the host immune response to a tumor with CD8+ CTLs as critical positive responders and Tregs as main immunosuppressors. In this study, we investigated the relationship between host immune response and clinical outcome in HCC patients, especially focusing on infiltration of Tregs and CTLs in different tumor regions. First, our results indicated that the density of intratumoral Tregs and peritumoral CTLs had a negative impact on the overall survival of HCC patients, although none of them was associated with recurrence of HCC. Multivariate analysis revealed that both of them were also independent prognostic factors for OS. However, the prevalence of Tregs and CTLs showed no prognostic significance for HCC patients. Second, we showed that the combined analysis of Tregs and CTLs may improve the prediction of patient survival, regardless of their prognostic value in univariate analysis.
A few studies have investigated the prognostic value of Treg infiltration [5, 9, 12, 17–20], but conclusions were contradictory. Marked infiltration of Tregs in cancer stroma was reported to be an unfavorable prognostic factor in ovarian [17], pancreatic [18], and hepatocullular [5, 12] cancers, but indicated favorable outcome for patients with head and neck [19] and colorectal [9] cancers. No prognostic influence of Tregs was found in anal squamous cell carcinomas [20]. Our data showed that only the density of intratumoral Tregs was significantly correlated with survival, but not with recurrence. Also, we showed that the prevalence of Tregs in either region had no prognostic significance for survival and recurrence, inconsistent with a previous report, in which the prevalence of Tregs in HCC was significantly correlated with both survival and recurrence [21]. Perhaps, the different approach to calculate the prevalence of Tregs and CTLs led to the inconsistent results.
Tumor-infiltrating CD8+ CTLs are believed to be front fighters against cancers. They play a positive prognostic effect in some cancers [9, 22]. However, they have no prognostic role in other cancers [20, 23]. For HCCs, the role of CTL infiltration in prognostic value remains mixed. A positive prognostic effect was revealed in one report [2], while no prognostic significance in other reports [12, 21], as proved in this study. Two major points are suggested to be responsible for the compromised tumor-specific killing by CD8+ CTLs: the dysfunction of cytolytic machinery and increased inhibitory cells, including Tregs, in tumor environments [5, 6]. Interestingly, our data showed that peritumoral CTLs, unlike intratumoral CTLs, were inversely correlated with survival, different from a previous report [12]. It has been stated that the peritumoral liver tissues was the major target organ of HCC recurrence or metastasis [13]. Our results, which showed that the peritumoral CTLs rather than the intratumoral CTLs were an independent predictor for HCC prognosis, were in agreement with this conclusion. However, the observation of worse survival for patients with a high density of peritumoral CTLs is counter-intuitive due to antitumor response of CTLs. Functional studies of CTLs may help to explain the observed association with prognosis. In a report, the activated (granzyme B+) CTLs were found to be positively correlated with survival, but not the CD8+ CTLs [12].
Although two immune-related prognostic factors were found, Tregs or CTLs alone may be less effective in predicting prognosis of HCC patients, especially for recurrence. Therefore, we hypothesized whether the combined analysis of Tregs and CTLs could improve the prediction of patient survival. Three points are responsible for this hypothesis. First, in univariate analysis for DFS, neither CD8 +P alone nor FoxP3 +I alone was a prognostic factor. However, the combination of CD8 +P with FoxP3 +I showed a significantly poorer prognosis in the CD8 LoP FoxP3 LoI group than in the other three groups. Second, in combined analysis, the disparities of MST between high- and low-risk groups were much larger, and the differences were more significant. Third, according to ROC analysis, the predictive value of the combination of Tregs with CTLs was better than that of Tregs or CTLs alone (Table 5). Collectively, these results indicate that the combination of Tregs with CTLs could predict the outcome more effectively.
We also found that high-density Tregs correlated with the presence of liver cirrhosis [24]. As we know, most HCCs occur based on liver cirrhosis. Hirohashi et al. further found that Tregs increased in a stepwise manner from hepatitis, pre-cirrhotic stage, and liver cirrhosis to HCC [21]. This result, together with ours, indicated that Treg infiltration was closely involved in the progression of hepatocarcinogenesis.
In conclusion, our data suggest that the type, density, and location of immune cells in HCC had a prognostic value, and that the combination of Tregs with CTLs can predict clinical outcome effectively. These results may provide a novel independent predictor for prognosis and thus help identify the high-risk patients who would benefit most from adjuvant therapy.
References
Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med (Maywood). 2011;236(5):567–79. doi:10.1258/ebm.2011.011007.
Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27(2):407–14. doi:10.1002/hep.510270214.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi:10.1126/science.1129139.
Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307.
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–39.
Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, et al. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41(4):722–30.
Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, et al. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45(2):254–62.
Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69(9):3995–4000.
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186–92.
Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010;127(4):748–58. doi:10.1002/ijc.25464.
Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–73. doi:10.1158/1078-0432.CCR-04-1869.
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–93.
Ju MJ, Qiu SJ, Gao Q, Fan J, Cai MY, Li YW et al. Combination of peritumoral mast cells and T-regulatory cells predicts prognosis of hepatocellular carcinoma. Cancer Sci. 2009;100(7):1267–74. doi:10.1111/j.1349-7006.2009.01182.x.
Varotti G, Ramacciato G, Ercolani G, Grazi GL, Vetrone G, Cescon M et al. Comparison between the fifth and sixth editions of the AJCC/UICC TNM staging systems for hepatocellular carcinoma: multicentric study on 393 cirrhotic resected patients. Eur J Surg Oncol. 2005;31(7):760–7. doi:10.1016/j.ejso.2005.04.008.
Schepke M, Roth F, Fimmers R, Brensing KA, Sudhop T, Schild HH et al. Comparison of MELD, Child-Pugh, and Emory model for the prediction of survival in patients undergoing transjugular intrahepatic portosystemic shunting. Am J Gastroenterol. 2003;98(5):1167–74. doi:10.1111/j.1572-0241.2003.07515.x.
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26(16):2707–16. doi:10.1200/JCO.2007.15.6521.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9.
Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–34. doi:10.1158/1078-0432.CCR-06-0369.
Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12(2):465–72. doi:10.1158/1078-0432.CCR-05-1886.
Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res. 2006;12(11 Pt 1):3355–60. doi:10.1158/1078-0432.CCR-05-2434.
Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13(3):902–11.
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. doi:10.1056/NEJMoa020177
Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61(13):5132–6.
Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125(7):1640–8. doi:10.1002/ijc.24556.
Acknowledgments
This study was supported by the Major State Basic Research Development Program (NO.2009CB522407), the Major National S&T Program (NO. 2008ZX10002-026), and the Program for Innovative Research Team of Zhejiang Province of China (NO. 2009R50038).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling EiC: Peter Wiernik.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12032_2011_6_MOESM1_ESM.tif
Fig. 1 Peritumoral tumor-infiltrating lymphocytes (TILs) outnumbered intratumoral TILs. (a) A typical tissue disk. T, tumor tissue; P, peritumor tissue. (b) Peritumoral lymphocytes form an aggregate. In a representative case, (c) in the peritumoral noncancerous area, lymphocytic infiltration was more abundant than that of (d) the intratumoral area. In each case, positive lymphocytes were stained brown (a, x100; b, x200; c and d, x400). (e) The density of FoxP3+ cells in intratumoral area was close to that in peritumoral area. (magnification, x200) (TIFF 10588 kb)
12032_2011_6_MOESM2_ESM.tif
Fig. 2 Kaplan–Meier analyses of disease-free survival and overall survival for intratumoral CD3+ (CD3I; a and b) and CD4+ (CD4I; c and d) TILs, and the prevalence of intratumoral CTLs among intratumoral CD3+ TILs (CD8I/CD3I; e and f) and intratumoral Tregs among intratumoralCD4+ TILs (FoxP3I/CD4I; g and h). None of them was associated with survival and recurrence (TIFF 2311 kb)
12032_2011_6_MOESM3_ESM.tif
Fig. 3 Kaplan–Meier pairwise analyses of disease-free survival and overall survival for the combination of intratumoral Tregs (FoxP3I) with intratumoral CTLs (CD8I) and with peritumoral CTLs (CD8P) (TIFF 1924 kb)
Rights and permissions
About this article
Cite this article
Chen, Kj., Zhou, L., Xie, Hy. et al. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol 29, 1817–1826 (2012). https://doi.org/10.1007/s12032-011-0006-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0006-x