Abstract
HER-2/neu is overexpressed in 25–30% of breast tumors. Signaling through HER-2/neu leads to an increase in the production of vascular endothelial growth factor (VEGF) and enhances angiogenesis. We evaluated the effects of three specific anti-HER2/neu single chain-Fv (scFv) antibodies on the expression level of VEGF in HER2/neu-expressing breast cancer cell lines. A nonimmunized human scFv library was panned against three epitopes of HER2/neu. BT-474 human breast cancer cell line was treated with three specific anti-HER2/neu scFv antibodies and the amount of VEGF gene transcript was determined by quantitative real-time PCR. The expression of VEGF protein was analyzed by western blot. All three scFv antibodies along with their combination inhibited VEGF expression at both the gene and protein levels. Our results show that anti-HER2/neu recombinant antibodies can be considered as anti-angiogenic agents in HER2/neu-positive breast cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
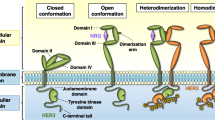
HER-2/neu (Human epidermal growth factor receptor 2, also known as ErbB2) is a 185-kDa transmembrane protein and a member of the human epidermal growth factor receptor (EGFR) family of tyrosine kinase receptors [1]. Overexpression of this protein has been observed in 25–30% of breast cancers due to its gene amplification [2]. The ErbB family plays vital roles in cell growth and differentiation processes in embryonic and adult tissues. These receptor proteins are normally coexpressed at different levels in diverse tissues, excluding the hematopoietic system. HER2/neu is expressed in numerous normal and malignant tissues of epithelial origin, including breast, ovary, lung, gastrointestinal, endometrium, and central nervous system [3]. HER2/neu has no known ligand and can form dimers when overexpressed. In contrast, other receptors of the ErbB family have several known ligands that promote dimer formation on binding. The ErbB receptors can form both homo- and heterodimers with HER2/neu acting as the preferred dimer partner. The activation of these receptors either by overexpression or by ligand binding leads to the activation of intracellular growth-promoting pathways and thus promotes tumor growth [4, 5]. Overexpression of HER2/neu dysregulates several critical pathways in the cell, including cell growth, proliferation, migration, and cell adhesion [6, 7]. Moreover, HER2/neu signaling leads to an increase in vascular endothelial growth factor (VEGF) and, therefore, is thought to enhance angiogenesis [8].
Preclinical data have identified a critical role for VEGF in endothelial cell proliferation, migration, and invasion [9]. In addition, VEGF has anti-apoptotic effects for endothelial cells, stimulating various inhibitors of programmed cell death [10–13]. Finally, VEGF is a potent regulator of vascular permeability. Research has shown that VEGF-overexpressing cancer cells are capable of inducing disruption of the endothelial cell basement membrane, possibly contributing to the ability of breast tumors to undergo successful metastasis [14].
Monoclonal antibodies are the most widely used form of cancer immunotherapy at this time. Herceptin, a humanized monoclonal antibody, is one of the antibodies that has been approved by the United States Food and Drug Administration (FDA) for the treatment of patients with HER2/neu-overexpressing breast cancers [15, 16]. However, only 30% of HER2/neu-overexpressing breast cancers respond to Herceptin as a single agent, and when it is used in combined chemotherapy, patients show secondary resistance [17, 18]. In addition to Herceptin, lapatinib is the only therapy approved by the FDA for use in patients with HER2/neu-positive metastatic breast cancer. Lapatinib, a small molecule, is a dual tyrosine-kinase inhibitor of HER1 and HER2/neu [19]. However, treatment with these drugs causes multiple life-threatening side effects that limit their use [20–22]. Thus, because of deficiencies in current therapies, the search for a new therapeutic agent is still ongoing.
Advances in recombinant DNA technology facilitated the production of smaller recombinant antibody fragments. Recombinant antibodies are available in a wide spectrum of formats. The most popular format appears to be the single-chain variable fragment (scFv). ScFv molecules are the smallest antibody fragments (26–27 kDa) [23]. Human scFv antibodies have improved pharmacokinetic properties because of their lower retention times in nontarget tissues, better target tissue penetration and clearance, and non-human anti-mouse antibody response [24, 25]. Because of these properties, human scFvs perform significantly better than antibody molecules in therapeutic applications.
In this study, we assessed the effects of three specific anti-HER2/neu scFv antibodies on the expression of VEGF in the HER2/neu-expressing breast cancer cell line.
Materials and methods
Selection of anti-HER2/neu scFv
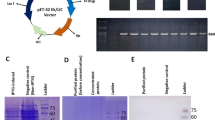
A phage antibody display library and scFv antibodies were produced essentially as described previously [26]. The specific anti-HER2/neu antibodies were selected by panning the phage library against three HER2/neu epitopes. Peptides [10 μg/ml in phosphate buffered saline (PBS)] were used to coat an immunotube (Nunc, Roskilde, Denmark) overnight at 4°C. The tubes were washed with PBS and blocked 2 h at 37°C with 10% fetal calf serum (FCS) and 2% skimmed milk in PBS. Then, the tubes were washed four times with PBS-Tween 20 (PBST) and four times with PBS. Phage supernatant (109 plaque-forming units (pfu) per ml) was added and incubated for 1 h at room temperature. The bound phagemids were eluted by adding log phase TG1 E. coli and incubating at 37°C for 1 h. The tubes were centrifuged and the pellets were plated onto LB agar plates. Helper phage M13KO7 was used to rescue the phage-transformed E. coli. Four rounds of panning were performed to select specific and high-affinity scFv antibodies against three HER2/neu epitopes (epitope I: TGRCEKCSKPCARVGYGL, epitope II: KIFGSLAFLPESFD, and epitope III: PPFCVARCPSG). MvaI DNA fingerprinting was performed on 20 colonies of each panned library to confirm enrichment of scFv clones. After PCR amplification of the scFv inserts, in order to select specific scFv against each peptide using the common fingerprinting pattern, each product was digested with MvaI (Roche Diagnostic GmbH, Mannheim, Germany) at 37°C for 2 h and run on 2% agarose gel.
Purification of scFv antibodies
The selected scFv antibody against each peptide was precipitated and purified by PEGylation. Phage rescue supernatant (30 ml) was mixed with 7.5 ml 20% PEG-8000/2.5 M NaCl and incubated on ice for 30 min. The mixture was centrifuged at 11,000g for 20 min then the supernatant was discarded and the pellet, containing scFv antibodies, was resuspended in 500 μl STE buffer and transferred to a fresh eppendorf tube. The tube was centrifuged at 14,000g for 10 min to remove the PEG and the supernatant was collected.
Measuring scFvs concentration
The scFv concentration in each phage rescue supernatant was measured by adding 10 μl of phage antibody supernatant to 1 ml TG1 E. coli in a logarithmic growth phase and incubating with shaking at 37°C for 1 h, The cultures were diluted serially and plated onto 2TY/ampicillin plates. The number of colonies per dilution was counted and scFv concentration titer per ml was determined.
Cell culture and antibody treatment
BT-474 HER2/neu-positive human breast cancer cell line (NCBI, Pasteur Institute, Tehran, Iran) was grown in RPMI-1640 medium (BioSera, Ringmer, UK) and supplemented with 15% fetal bovine serum (BioSera), 100 U/ml penicillin, and 100 mg/ml streptomycin. Cells were grown at 37°C in an incubator with 5% CO2. The cells were treated with anti-HER2/neu scFv antibodies (IC50 = 500 scFv/cell) and Herceptin (IC50 = 150 ng/ml) as the positive control [27]. After 48 h, real-time RT-PCR and western blot assays were performed.
Quantitative real-time RT-PCR
Total RNA was extracted from 106 cultured cells using TRIzol solution (Invitrogen, Paisley, UK) according to the manufacturer’s instructions. The quality and quantity of the extracted RNA were estimated by spectrometry. Total RNA (5 μg) was used for cDNA synthesis with the RevertAid First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania). Quantitative real-time RT-PCR was performed in triplicate (Chromo4 Real-time PCR Detector, Bio-Rad, CA, USA) using SYBR Green I. The amount of 18s RNA housekeeping gene transcripts was used as a reference for the level of VEGF gene expression. Amplification was carried out in a total volume of 20 μl containing 0.5 μg cDNA prepared as described above, 3.0 pmol each of VEGF (sense: 5′-CCCACTGAGGAGTCCAACAT-3′ and antisense: 5′-TTTCTTGCGCTTTCGTTTTT-3′) and 18s RNA (sense: 5′-CGAACGTCTGCCCTATCAACTT-3′, and antisense: 5′-ACCCGTGGTCACCATGGTA-3′) primers, 1× reaction mixture consisting of FastStart DNA polymerase, reaction buffer, dNTPs, DNA Master SYBR Green I (Applied Biosystems, CA, USA). Primers were designed by Primer3 open source software (SourceForge, USA).
Thermal cycling for both genes was initiated with a denaturation step at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 56°C for 30 s and elongation at 60°C for 40 s. Melting curve analysis of the amplification products was performed at the end of each PCR by cooling the samples to 60°C and then increasing the temperature to 95°C at 0.2°C/s.
The experiment was repeated thrice for statistical analysis.
Western blot analysis
A RIPA lysis buffer [containing 150 mM sodium chloride, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris, pH 8.0] was used to lyse 106 cells. Protease inhibitor cocktail (1×) (Sigma-Aldrich, St. Louis, MO, USA) was added to the RIPA buffer immediately before use. The lysates were centrifuged at 12,000 rpm for 20 min to pellet the cell debris, and the supernatants were collected. The protein concentration was determined by the Bradford assay. Protein (20 μg) was loaded onto a 12% gel for SDS-PAGE. After electrophoresis, the gels were blotted onto PVDF membranes. The blots were blocked with 5% skim milk in Tris-buffered saline Tween-20 buffer (10 mM Tris, pH 8.0, 150 mM NaCl, and 0.05% Tween-20) for 2 h at room temperature. The membranes were then incubated with the primary anti-VEGF antibody (ab1316, Abcam, Cambridge, MA, USA) and anti-β-actin antibody (ab6276, Abcam) in fresh 5% skim milk-PBST buffer at 4°C overnight. The membranes were washed and incubated with secondary HRP-conjugated goat anti-mouse IgG antibody (ab6728, Abcam). The bands were visualized in a Pierce ECL chemiluminescence system and quantified by densitometry with GelPro software.
Statistical analysis
The level of VEGF transcript expression was determined from the ∆Ct and 2(−∆Ct) formulas. Data were analyzed with the Mann–Whitney U test to compare gene expression level between antibody-treated and untreated cells. All data are presented as the mean ± standard error. A P value <0.05 was considered statistically significant.
Results
Anti-HER2/neu scFv antibodies
Figure 1 shows DNA fingerprinting of 20 panned clones against peptide I (A), peptide II (B), and peptide III (C). Common patterns were obtained for all colonies panned against each peptide, and one colony from each pattern was used for further investigation.
Inhibition of VEGF gene expression by anti-HER2/neu scFvs
As shown in Fig. 2, a significant down-regulation of VEGF gene expression was obtained after 48 h of treatment with 500 scFv/cell anti-HER2/neu scFv-I, scFv-II, scFv-III, and their combination (P < 0.05). The levels of VEGF gene expression were 2.2 × 10−4, 2 × 10−4, 2.6 × 10−4, and 2.1 × 10−4, respectively, and expression in untreated cells was 5.2 × 10−4.
Reduction of VEGF protein expression by anti-HER2/neu scFv
The expression of VEGF protein in the HER2/neu-positive breast cancer cell line, BT-474, was measured by western blot analysis. As shown in Fig. 3, after 48 h of treatment with 500 scFv/cell anti-HER2/neu scFv-I, scFv-II, and scFv-III separately and in combination, VEGF protein expression decreased.
Reduction of VEGF protein expression induced by anti-HER2/neu scFv antibodies in BT-474 human breast cancer cell line. After 48 h of treatment, cell lysates were immunoblotted with antibodies specific for VEGF and β-actin (internal control). Anti-HER2/neu scFv antibodies decreased the VEGF protein expression
Discussion
HER-2/neu overexpression is associated with markedly aggressive forms of cancer. Although the precise signaling pathways by which HER2/neu regulates more aggressive clinical phenotype are not fully understood, angiogenesis is well established as a vital step for tumorigenesis and metastasis [28, 29]. VEGF is one of the most important inducers of tumor angiogenesis [30, 31]. Clinical studies have shown that VEGF overexpression in the early-stage breast cancer is related to augmented metastatic potential [32, 33]. The majority of HER2/neu-positive breast cancers overexpress VEGF, and elevated serum VEGF has been reported in invasive breast cancers [34–38], suggesting that VEGF-induced angiogenesis may be vital for this type of breast tumor. Moreover, two important transcriptional factors, hypoxia-inducible factor 1 and Sp1, which both play a critical role in the PI3K-AKT pathway, are responsible for the up-regulation of VEGF by HER2/neu signaling [39]. Therefore, blocking HER2/neu signaling with anti-HER2/neu antibodies is a practical approach to antiangiogenic tumor therapy. Wen et al. [40] showed that activation of HER2/neu up-regulates the expression of two proangiogenic factors, VEGF and IL-8, and one antiangiogenic factor, TSP-1, in vitro and in vivo. Their results also indicated that inhibition of HER2/neu signaling by the anti-HER2/neu monoclonal antibody, trastuzumab (Herceptin), down-regulates VEGF production by inhibiting the PI3-AKT pathway. Although bevacizumab (Avastin), a humanized recombinant monoclonal antibody, is a VEGF-neutralizing antibody that specifically inhibits the binding of VEGF to its high-affinity receptors [41], it has been demonstrated that blocking of HER2/neu decreases the expression of VEGF in HER2/neu-overexpressing breast cancers [38, 42]. According to a hypothesis proposed by Pegram et al. [38], the up-regulation of VEGF in HER2/neu-overexpressing breast cancers contributes to the aggressive phenotype observed in HER2/neu-positive cases, and the angiogenic switch associated with HER2/neu can be attenuated by Herceptin. Therefore, a potentially useful approach is to target HER2/neu and VEGF simultaneously using anti-HER2/neu antibodies.
This study examined the effects of three specific scFv antibodies against HER2/neu on the expression of VEGF at both the gene and protein levels in comparison to the effects of Herceptin. All three scFv antibodies individually and in combination were able to significantly inhibit VEGF gene expression in the BT-474 cell line. Yen et al. [39] have shown that in the HER family, HER1/HER2 and HER2/HER3 heterodimer formations are the most potent inducers of VEGF gene expression. It has been also shown that monoclonal antibodies may suppress HER2/neu signaling in vitro by inhibiting dimerization [43]. Therefore, it seems that selected scFv antibodies block HER2/neu signaling through the inhibition of HER2/neu dimerization.
The results of real-time PCR showed that the gene expression levels of VEGF in cells treated with scFv-I, -II,-III, and their combination decreased by 41, 39, 50, and 40%, respectively, compared to untreated cells (P < 0.05). Levels of VEGF gene expression in scFv antibody-treated cells did not differ significantly from Herceptin-treated cells (positive control). This finding suggests that the inhibitory effect of scFv antibodies on VEGF gene expression is the same as the effect of Herceptin. The reduction in VEGF protein expression after treatment with scFv-I, scFv-II, scFv-III, and their combination was documented by western blot assay. These data were consistent with the real-time PCR results.
Our results document the inhibition of VEGF expression at both the mRNA and protein levels by anti-HER2/neu scFv antibodies. These results support a possible role for these recombinant antibodies as anti-angiogenic agents in HER2/neu-positive breast cancer immunotherapy.
References
Hung MC, Lau YK. Basic science of HER-2/neu: a review. Semin Oncol. (1999);26(4 Suppl 12):51–9.
Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96.
Natali PG, et al. Expression of the p185 encoded by HER2 oncogene in normal and transformed human tissues. Int J Cancer. 1990;45(3):457–61.
Brennan PJ, et al. HER2/Neu: mechanisms of dimerization/oligomerization. Oncogene. (2002);21(2):328.
Zaczek A, Brandt B, Bielawski KP. The diverse signaling network of EGFR, HER2, HER3 and HER4 tyrosine kinase receptors and the consequences for therapeutic approaches. Histol Histopathol. 2005;20(3):1005–15.
Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19(53):6102–14.
Zhou BP, Hung MC. Dysregulation of cellular signaling by HER2/neu in breast cancer. Semin Oncol. (2003);30(5 Suppl 16):38–48.
Petit AM, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151(6):1523–30.
Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280(6):C1358–66.
Gupta K, et al. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247(2):495–504.
Pidgeon GP, et al. Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer. 2001;85(2):273–8.
Tran J, et al. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res Commun. 1999;264(3):781–8.
Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280(6):C1375–86.
Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437(7058):497–504.
JP ShepardHM, Slamon DJ, Pirot Z, Maneval DC. Herceptin. Handb Exp Pharmacol. 2008;181:183–219.
FDA expands us of herceptin for early-stage breast cancer. Mayo Clin Womens Healthsource. 2007;11(6):3.
Burris H 3rd, et al. Phase II trial of trastuzumab followed by weekly paclitaxel/carboplatin as first-line treatment for patients with metastatic breast cancer. J Clin Oncol. 2004;22(9):1621–9.
Vogel CL, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26.
Nielsen DL, Andersson M, Kamby C. HER2-targeted therapy in breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35(2):121–36.
Metzger Filho O, Saini KS, Azim HA, Jr., Awada A (2010)Prevention and management of major side effects of targeted agents in breast cancer. Crit Rev Oncol Hematol.
Du XL, Xia R, Burau K, Liu CC (2010)Cardiac risk associated with the receipt of anthracycline and trastuzumab in a large nationwide cohort of older women with breast cancer, 1998–2005. Med Oncol.
Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009;48(7):964–70.
Nuttall SD, Irving RA, Hudson PJ. Immunoglobulin VH domains and beyond: design and selection of single-domain binding and targeting reagents. Curr Pharm Biotechnol. 2000;1(3):253–63.
Batra SK, et al. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr Opin Biotechnol. 2002;13(6):603–8.
Curigliano G, et al. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog Cardiovasc Dis. 2010;53(2):94–104.
Nejatollahi F, Hodgetts SJ, Vallely PJ, Burnie JP. Neutralising human recombinant antibodies to human cytomegalovirus glycoproteins gB and gH. FEMS Immunol Med Microbiol. 2002;34(3):237–44.
Nahta R, Esteva FJ. In vitro effects of trastuzumab and vinorelbine in trastuzumab-resistant breast cancer cells. Cancer Chemother Pharmacol. 2004;53:186–90.
Kern FG, Lippman ME. The role of angiogenic growth factors in breast cancer progression. Cancer Metastasis Rev. 1996;15(2):213–9.
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8.
Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87(7):1153–5.
Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem. 1996;271(2):603–6.
Foekens JA, et al. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61(14):5407–14.
Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist. (2000);5 Suppl 1: 37–44.
Konecny GE, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10(5):1706–16.
Schoppmann SF, et al. HER2/neu expression correlates with vascular endothelial growth factor-C and lymphangiogenesis in lymph node-positive breast cancer. Ann Oncol. 2010;21(5):955–60.
Linderholm B, et al. Overexpression of c-erbB-2 is related to a higher expression of vascular endothelial growth factor (VEGF) and constitutes an independent prognostic factor in primary node-positive breast cancer after adjuvant systemic treatment. Eur J Cancer. 2004;40(1):33–42.
Loureiro RM, et al. ErbB2 overexpression in mammary cells upregulates VEGF through the core promoter. Biochem Biophys Res Commun. 2005;326(2):455–65.
Pegram MD, Reese DM. Combined biological therapy of breast cancer using monoclonal antibodies directed against HER2/neu protein and vascular endothelial growth factor. Semin Oncol. (2002);29(3 Suppl 11):29–37.
Yen L, et al. Differential regulation of tumor angiogenesis by distinct ErbB homo—and heterodimers. Mol Biol Cell. 2002;13(11):4029–44.
Wen XF, et al. HER2 signaling modulates the equilibrium between pro—and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25(52):6986–96.
Scott LJ. Bevacizumab: in first-line treatment of metastatic breast cancer. Drugs. 2007;67(12):1793–9.
Yen L, et al. Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis. Oncogene. 2000;19(31):3460–9.
Montgomery RB, et al. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005;65(2):650–6.
Acknowledgment
The authors acknowledge Shiraz University of Medical Sciences for financial support and K. Shashok (Author AID in the Eastern Mediterranean) for improving the use of English in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nejatollahi, F., Asgharpour, M. & Jaberipour, M. Down-regulation of vascular endothelial growth factor expression by anti-her2/neu single chain antibodies. Med Oncol 29, 378–383 (2012). https://doi.org/10.1007/s12032-010-9796-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9796-5