Abstract
We aimed to study the efficacy and safety of metronomic capecitabine in pretreated elderly patients with advanced gastric cancer. Eligible patients with advanced gastric cancer were treated with capecitabine at a fixed dose 1,000 mg daily (days 1–28 continuously, every 5 weeks) until disease progression or significant toxicity. Tumor response was assessed every 10 weeks by computed tomography scan using Response Evaluation Criteria in solid tumors. In total, 45 patients were enrolled, of whom 43 were evaluated for efficacy and 45 for safety. A median of 3 cycles (range 1–12) were administered. Metronomic chemotherapy had a disease control rate (DCR) at 8 weeks of 51.1% (95% CI 25.7–67.8), and the objective response rate was 20.9% (95% CI 13.1–38.5, 9 of 43 assessable patients). The median time-to-progression and median overall survival were 3.6 months (95% CI: 3.2–4.0 months) and 7.6 months (95% CI 7.0–8.2 months), respectively. Grade II neutropenia and thrombocytopenia were observed in 13.3 and 2.2% of patients, respectively. Grade II/III nonhematological toxicities included diarrhea (4.4%), stomatitis (13.4%), and hand–foot syndrome (15.5%). No grade IV toxicity, neutropenic fever or treatment-related deaths occurred. Metronomic capecitabine was effective and well tolerated as palliative treatment in elderly patients with advanced gastric cancer after fluoropyrimidine-based chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most common causes for cancer mortality and leads to approximately 160,000 deaths annually in China, the majority of cases occur in the elderly population [1]. Surgery remains the only established curative treatment for this disease in resectable stages [2]. Of all patients with gastric cancer, 80–90% are either diagnosed at an advanced stage when the tumor is inoperable or develop a recurrence within 5 years after surgery [3]. Chemotherapy for advanced gastric cancer (AGC) can improve either time-to-progression (TTP) or overall survival (OS) and is well tolerated [3]; although many Phases II and III chemotherapy trials for AGC were undertaken, only a minority of elderly patients (>65 years of age) were enrolled in those trials [4]. Many factors, such as patient compliance, performance status, toxicity of previous treatments, nutritional status, and treatment costs, play a role in choosing a drug for elderly patients or patients both after failure of previous lines of chemotherapy or in front-line when standard chemotherapy is contraindicated [5, 6]. Consequently, the type and the extent of systemic palliative chemotherapy that should be offered to elderly patients remain to be determined.
Despite the lack of evidence for benefit associated with administering salvage chemotherapy, it is a common practice to offer further chemotherapy for AGC patients after first-line failure, because patients and physicians have difficulty in accepting only supportive care without the possibility of systemic anticancer effects. For patients with recurrent, metastatic, or AGC, chemotherapy can improve survival and possibly provide significant palliation of symptoms [7, 8]. Given that no standard salvage treatment is available in those patients, limited investigation into palliative chemotherapy after first-line or second-line failure has been performed. Availability of an effective, less-toxic therapy might help extend potentially beneficial treatment to a great proportion of elderly patients.
Capecitabine is an oral fluoropyrimidine carbamate, which is enzymatically converted to 5-fluorouracil (5-FU) in several steps when absorbed from the gastrointestinal tract. The final step involves the enzyme thymidine phosphorylase, which is found at much higher levels in gastric cancers than in normal tissue, enabling the active drug 5-FU to be generated preferentially at the tumor site [9]. Capecitabine has been shown to be active as a single agent for the treatment of gastrointestinal tract tumors such as advanced gastric cancer [10], with response rates for AGC ranging from 19.4 to 34%; moreover, median survival duration in these studies was comparable with other double or triple combination chemotherapies [11]. In addition, in preclinical xenograft models, capecitabine was highly active against both 5-FU-sensitive and 5-FU-resistant tumors [12].
Metronomic chemotherapy is defined as daily low-dose administration of a cytotoxic agent at close, regular intervals, with no extent breaks, characterized by a good tolerability profile [13]. Metronomic capecitabine has been investigated as a single agent for treatment of several types of advanced tumors, showing to be active in advanced gastrointestinal tract cancers and breast cancer after prior treatment failure [14, 15]. On this basis, we investigated the safety and efficacy of metronomic capecitabine in elderly patients with AGC after fluoropyrimidine-based chemotherapy.
Patients and methods
Eligibility
Patients, who are older than 70 years, with histologically or cytologically confirmed AGC, after prior fluoropyrimidine-based chemotherapy, were eligible. All patients had at least one measurable lesion. Other eligibility criteria were Eastern Cooperative Oncology Group (ECOG) performance status ≤2, adequate organ function, and an expected survival of at least 3 months. Any prior antiangiogenic therapy must have been discontinued at least for 6 months before study entry. The exclusion criteria included unresolved bowel obstruction or malabsorption syndrome, brain metastasis, and an active infection with concurrent treatment that interfered with the study’s evaluation. All enrolled patients provided written informed consent.
Treatment schedule and dose modifications
Before treatment, each patient had a complete history and physical examination, a complete blood count, liver and renal function tests and electrolytes, ECG, chest radiograph, computed tomography (CT) scanning of the abdomen and pelvis, and if indicated, CT scans of the chest and a bone scan. Metronomic capecitabine was administered orally at a dose of 500 mg twice a day according to the intermittent schedule (28 days of treatment followed by a 7-day rest period, every 5 weeks) [16]. Treatment interruption or dose reduction was not indicated for the first occurrence of grade I or II toxicity. For hematological toxicity, treatment was interrupted in patients with grade III or IV event, and G-CSF was administered when the absolute neutrophil count decreased to 500 cells/μL. Metronomic capecitabine was permanently discontinued with grade IV nonhematological toxicity. The study was continued until disease progression, unacceptable toxicity, severe and unstable medical comorbidities, or if the patient chose to discontinue treatment.
Response evaluation and toxicity
Physical examination was carried out and chest X-rays were taken before each chemotherapy cycle, and complete blood counts were made and biochemical tests were performed before and on day 15 of each cycle. Response evaluation was made by CT scan every 2 cycles until the tumor progressed. Tumor response was classified on the basis of the response evaluation criteria defined by RECIST criteria, and responses were required to last longer than 4 weeks. The duration of response was defined as the interval from the onset of complete response [CR: the disappearance of all target lesions (those representative, by size and suitability of measurements, of all involved organs), confirmed at 4 weeks], partial response [PR: at least a 30% decrease in the sum of the longest diameters (LD) of the target lesions taking as reference the baseline LD sum, confirmed at 4 weeks], and stable disease (SD: neither sufficient shrinkage to qualify for PR, nor sufficient increase in qualify for progression, taking as reference the smallest LD sum since the treatment started, confirmed at 4 weeks) until disease progression [PD: at least a 30% increase in the sum of LD of the target lesions or new lesion(s)]. Objective response rate (ORR) was defined as CR + PR; disease control rate (DCR) was defined as CR + PR + SD. If death occurred before progression was documented, the date of death was assumed to be the date of progression. TTP was calculated from the date of entry into the study until the date of progression, and overall survival was the duration from the initiation of treatment to death or last known follow-up. Compliance with metronomic capecitabine treatment was monitored by questioning patients and counting their remaining pills at each outpatient visit. Safety was evaluated in all patients who received at least one cycle. Adverse events were graded according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0.
Statistical analysis
The expected number of patients for this study was calculated according to a Simon optimal two-stage design [17], assuming a response rate of 20%. With a power of 90%, this resulted in a sample size of 15 patients for first stage. The size of second stage was determined by the observed number of response and by the prespecified precision of 10%. There were three responders during the first stage of the study. Therefore, according to the study design, the sample size of the whole study was extended to at least 42 patients. TTP and OS were calculated using the Kaplan–Meier method. Log-rank test was used for multivariate and univariate analyses of response rate and to examine the effects of baseline factors on clinical outcome. Statistical analyses were performed using SPSS for Windows version 12.0 (SPSS Inc, Chicago, IL, USA).
Results
Patient characteristics
Forty-five patients were enrolled from March 2005 to August 2009; Table 1 lists the baseline characteristics. The median age was 74.5 years (range 71–81); 33 patients were men, and 12 were women. Thirty-six patients (80%) had a relatively good performance status of 0 or 1. Twenty-one patients had previously undergone surgery and recurrence (13 patients had received 2–6 cycles of adjuvant chemotherapy). All patients with AGC had received 5-FU-based first-line palliative chemotherapy. First-line chemotherapy was oxaliplatin plus 5-FU in 17 patients (37.8%), Epirubicin, cisplatin plus 5-FU in 15 patients (33.3%), capecitabine, epirubicin plus oxaliplatin in 6 patients (13.3%), docetexel, 5-FU plus oxaliplatin in 4 (8.9%), and 5-FU monotherapy in 3 patients (6.7%). Sixteen of 45 patients who had disease progression after first-line palliative chemotherapy turned to second-line chemotherapy. Second-line palliative chemotherapy included 5-FU plus cisplatin as intraperitoneal administration in 4 patients; standard capecitabine monotherapy in 3 patients; capecitabine plus recombinant human endostatin (endostar®) in 3 patients, oxaliplatin plus 5-FU in 3 patients; S-1 monotherapy in 2 patients; uracil-tegafur enterogranules (UFT-E) in 1 patient. Seventeen patients (37.8%) had liver metastasis, 13 (28.9%) had peritoneal metastasis, 11 (24.4%) had lymph node metastasis, and 4 (8.9%) had bone metastasis.
Efficacy
Two patients did not complete first cycle due to disease progression. Among the forty-three patients treated for at least 1 cycle, 9 patients achieved PR (response rate, 20.9%). Thirteen patients (30.2%) achieved SD, whereas 21 patients (48.8%) had progressive disease. The DCR (CR + PR + SD) was 51.1%. Response rate was not significantly influenced by age, gender, weight loss, PS, liver, peritoneal, lymph node or bone metastasis, gastrectomy, and response to prior first-line or second-line chemotherapy (Table 2).
TTP and survival
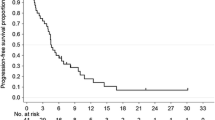
All 43 treated patients were assessable for TTP and survival, with median follow-up duration of 15 months (range 1–25 months). At the time of analysis, there were 37 deaths, 6 patients confirmed alive, and no patient lost to follow-up. Of the surviving patients, 3 remain progression free on therapy with metronomic capecitabine. The median TTP was 3.6 months (95% CI 3.2–4.0 months; Fig. 1). The median OS was 7.6 months (95% CI 7.0–8.2 months; Fig. 2). One-year survival rate was 28.5%. In a multivariate analysis of the 43 patients, OS was not affected by PS, metastatic sites, gastrectomy, or response to prior lines chemotherapy (Table 2).
Safety
The frequencies of treatment-related hematological and nonhematological adverse events were shown in Table 3. The most common treatment-related hematological adverse event was neutropenia, which occurred at grade III intensity in 4 patients (8.9%). No patient experienced grade IV neutropenia or febrile neutropenia. Grades II and III hand–foot syndrome and stomatitis were also relatively common, occurring in 7 patients (15.5%) and 6 patients (13.4), respectively. There were no treatment-related deaths. Treatment delays were observed in 9 patients (20%) as a result of hematological adverse events (4 patients; 8.9%), hand–foot syndrome (1 patient; 2.2%), anorexia (1 patient; 2.2%), and stomatitis (3 patients; 6.7%). There was no dose reduction with metronomic capecitabine.
Discussion
Palliative chemotherapy for advanced gastric cancer improves survival, when compared with the best supportive care [18]. The choice of the optimal chemotherapy regimen for the treatment of AGC needs to be based on a careful consideration of the value of the generally small benefit that we can give to patients. In particular, treatment decisions should take into account the high toxicity that is typical of most chemotherapy regimens commonly used in this setting. Elderly patients have a higher incidence of comorbidity, end-organ dysfunction, nutritional deficiencies, and gradual deterioration of PS. These factors prohibit elderly patients from receiving standard schedule treatments and clinical trails [19]. Metronomic chemotherapy as simplified administration may minimize the acute toxicity of cancer drugs and maintain the treatment for a long time in these patients suffering with advanced cancer who cannot benefit from the standard chemotherapy [20]. Several studies on this treatment approach with different drugs for other tumor types have been reported [21, 22]. Moreover, the administration in small doses is well tolerated and provides stable disease in cancer patients with such vulnerable and poor prognosis [23].
Capecitabine has demonstrated safety and efficacy as a single agent or combined with other active drugs against AGC [5]. Capecitabine at different daily doses has also been documented as an efficacious treatment in gastrointestinal tract cancers and advanced breast cancer [24, 25]. Oral capecitabine appears to be more convenient to administer than infused fluorouracil because it may obviate the need for central venous access and its associated risk of complications [26]. Besides the beneficial toxicity profile, metronomic capecitabine produced long median TTP and median survival for AGC and advanced colorectal cancer patients after failure of previous lines of chemotherapy or in frontline when standard chemotherapy is contraindicated, especially when the aims of medical treatment are to achieve disease control and to arrest tumor growth without affecting the patient’s quality of life [27]. Although standardized schedule of metronomic capecitabine time–dose does not yet exist, the reported daily doses of the agent ranging from 1,000 mg to 2,000 mg/day were well tolerated and allowed to achieve a prolonged stable disease [28, 29]. In a Phase II study that evaluated the safety and efficacy of the combination of continuous capecitabine 1,000 mg/day and celecoxib in the treatment of advanced cancer patients, nearly 30% of all patients, especially those with renal cell cancer and gastrointestinal tract tumors, had stable disease after 3 months of therapy [16]. Besides the good toxicity profile, metronomic capecitabine produced a response rate of 22.2%, median TTP 4.4 months and a median survival 9.5 months in the subgroup of metastatic colorectal cancer [14]. In this study, we chose to administer 1,000 mg daily because it has been shown that this very low dose, which is nearly 1/4 of the normal dose, does not compromise the antitumor effect of the drug.
Because elderly patients are generally excluded from cancer chemotherapy clinical trials, data to guide the treatment in this population AGC in an evidence-based fashion are lacking [30]. Comparing with single-agent chemotherapy, 5-FU-based combination chemotherapy demonstrated a statistically significant benefit in OS for elderly patients with AGD [31]. However, we should be cautious with 5-FU-based combination in the elderly patients due to the following limitations: (1) only a small, approximately one month, survival advantage was observed in the meta-analysis based on aggregate date [32]; (2) chemotherapy-related toxicities, such as neutropenia, anemia, stomatitis, diarrhea, and treatment-related deaths, occurred more frequently in the elderly [4]; (3) the early drop out rate was significantly higher, and 5-FU dose intensity was significantly lower in the elderly when treated with combination chemotherapy [33]; and (4) QOL, which could be impaired as the intensity of chemotherapy increases, has not been studied sufficiently. Ideally, elderly specific trials are needed to define the optimal treatment for these patients. Considering the ORR, OS, and safety results, our study provides the evidence that elderly patients with AGC could benefit from metronomic capecitabine monotherapy with minimal adverse events.
Unlike maximum tolerated dose (MTD) chemotherapy that presumably mainly targets (proliferating) tumor cells, frequent or continuous low-dose chemotherapy appears to inhibit preferentially the endothelial cell activity of the tumors’ growing vasculature [34]. In addition, sub-MTD capecitabine showed a trend toward the induction of TSP-1 expression in xenograft tumor tissues and plasma, known to be a mediator of the antiangiogenic effects of metronomic chemotherapy [35]. Moreover, latest evidence of metronomic chemotherapy in adult and pediatric cancer patients suggested that the efficacy of such treatment relied on antiangiogenic activity, restoration of anticancer immune response, and induction of tumor dormancy [36, 37]. In addition, metronomic chemotherapy in combination with antiangiogenic drugs provided interesting results in advanced colorectal cancer and ovarian cancer [38, 39]. Such combination can induce remarkable responses including sustained tumor regression, even of drug resistant tumors, as well as marked prolongation of survival with no serious toxicity in advanced hepatocellular cancinoma [40]. The treatment of metronomic capecitabine 500 mg twice daily in combination with cyclophosphamide and bevacizumab has been shown to be minimally toxic and effective in advanced breast cancer, achieving partial response of 46% and stable disease of 41% [41].
Metronomic capecitabine was well tolerated, and no treatment-related death was reported in this study. The treatment was minimally myelosuppressive, and the most frequent hematological toxicity was neutropenia. Although grade III neutropenia occurred in four patients treated with metronomic capecitabine, no patient experienced grade IV hematologic toxicities or febrile neutropenia. The most frequently observed grade II/III nonhematological toxicities were anorexia and hand–foot syndrome. Although toxicities led to treatment delay in 9 patients, no dose adjustment was required for capecitabine in these patients.
In conclusion, metronomic capecitabine demonstrated substantial efficacy with low toxicity in elderly patients with AGC after fluoropyrimidine-based chemotherapy.
References
Chen XM, Chen GY, Wang ZR, Zhu FS, Wang XL, Zhang X. Detection of micrometastasis of gastric carcinoma in peripheral blood circulation. World J Gastroenterol. 2004;10:804–8.
Hu JK, Li CM, Chen XZ, Chen ZX, Zhou ZG, Zhang B, Chen JP. The effectiveness of intravenous 5-fluorouracil-containing chemotherapy after curative resection for gastric carcinoma: a systematic review of published randomized controlled trials. J Chemother. 2007;19:359–75.
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev (abstract). 2010;17:3.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7.
Lee JL, Kang YK, Kang HJ, Lee KH, Zang DY, Ryoo BY, Kim JG, Park SR, Kang WK, Shin DB, Ryu MH, Chang HM, Kim TW, Baek JH, Min YJ. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer. 2008;19:584–90.
Seol YM, Song MK, Choi YJ, Kim GH, Shin HJ, Song GA, Chung JS, Cho GJ. Oral fluoropyrimidines (capecitabine or S-1) and cisplatin as first line treatment in elderly patients with advanced gastric cancer: a retrospective study. Jpn J Clin Oncol. 2009;39:43–8.
Park SH, Kang WK, Lee HR, Park J, Lee KE, Lee SH, Park JO, Kim K, Kim WS, Chung CW. Docetaxel plus cisplatin as second-line therapy in metastatic or recurrent advanced gastric cancer progressing on 5-fluorouracil-based regimen. Am J Clin Oncol. 2004;27:477–80.
Park SH, Kim YS, Hong J, Park J, Nam E, Cho EK, Shin DB, Lee JH, Lee WK, Chung M. Mitomycin C plus S-1 as second-line therapy in patients with advanced gastric cancer: a noncomparative phase II study. Anticancer Drugs. 2008;19:303–7.
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–81.
Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, Park JN, Han JY, Kang JH, Lee KS, Cho JY. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004;15:1344–7.
Koizumi W, Saigenji K, Ujiie S, Terashima M, Sakata Y, Taguchi T, Clinical Study Group of Capecitabine. A pilot phase II study of capecitabine in advanced or recurrent gastric cancer. Oncology. 2003;64:232–6.
Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer, M., Bugat R, Burger U, Garin A, Graeven U, McKendrick J, Maroun J, Marshall J, Osterwalder B, Pérez-Manga G, Rosso R, Rougier P, Schilsky RL. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. 2002;13:566–575.
Emmenegger U, Kerbel RS. Five years of clinical experience with metronomic chemotherapy: achievements and perspectives. Onkologie. 2007;30:606–8.
Petrioli R, Pascucci A, Francini E, Marsili S, Fiaschi AI, Civitelli S, Tanzini G, Battistelli S, Lorenzi M, Roviello F, Francini G. Multidisciplinary oncology group on gastrointestinal tumors. Continuous oral capecitabine at fixed dose in patients older than 75 years with metastatic colorectal and gastric cancer: a study of the Multidisciplinary Oncology Group on Gastrointestinal Tumors. Anticancer Drugs. 2008;19:91–6.
Taguchi T, Nakayama T, Masuda N, Yoshidome K, Akagi K, Nishida Y, Yoshikawa Y, Ogino N, Abe C, Sakamoto J, Noguchi S. Study of low-dose capecitabine monotherapy for metastatic breast cancer. Chemotherapy. 2010;56:166–70.
Steinbild S, Arends J, Medinger M, Häring B, Frost A, Drevs J, Unger C, Strecker R, Hennig J, Mross K. Metronomic antiangiogenic therapy with capecitabine and celecoxib in advanced tumor patients—results of a phase II study. Onkologie. 2007;30:629–35.
Simon R. How large should a phase II trial of a new drug be. Cancer Treat Rep. 1987;71:1079–85.
Wöhrer SS, Raderer M, Hejnal M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol. 2004;15:1585–95.
Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US foot and drug administration. J Clin Oncol. 2004;22:4626–31.
Franchi F, Grassi P, Ferro D, Pigliucci G, De Chicchis M, Castigliani G, Pastore C, Seminara P. Antiangiogenic metronomic chemotherapy and hyperthermia in the palliation of advanced cancer. Eur J Cancer Care (Engl). 2003;16:258–62.
Salem DA, Gado NM, Abdelaziz NN, Essa AE, Abdelhafeez ZM, Kamel TH. Phase II trial of metronomic chemotherapy as salvage therapy for patients with metastatic breast cancer. J Egypt Natl Canc Inst. 2008;20:134–40.
Kesari S, Schiff D, Doherty L, Gigas DC, Batchelor TT, Muzikansky A, O’Neill A, Drappatz J, Chen-Plotkin AS, Ramakrishna N, Weiss SE, Levy B, Bradshaw J, Kracher J, Laforme A, Black PM, Folkman J, Kieran M, Wen PY. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol. 2007;9354–9363.
Penel N, Clisant S, Dansin E, Desauw C, Dégardin M, Mortier L, Vanhuyse M, Bonodeau F, Fournier C, Cazin JL, Adenis A. Megestrol acetate versus metronomic cyclophosphamide in patients having exhausted all effective therapies under standard care. Br J Cancer. 2010;102:1207–12.
Nannini M, Nobili E, Di Cicilia R, Brandi G, Maleddu A, Pantaleo MA, Biasco G. To widen the setting of cancer patients who could benefit from metronomic capecitabine. Cancer Chemother Pharmacol. 2009;64:189–93.
Van Cutsem E, Hoff PM, Harper P, Bukowski RM, Cunningham D, Dufour P, Graeven U, Lokich J, Madajewicz S, Maroun JA, Marshall JL, Mitchell EP, Perez-Manga G, Rougier P, Schmiegel W, Schoelmerich J, Sobrero A, Schilsky RL. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer. 2004;90:1190–7.
Comella P, Franco L, Casaretti R, de Portu S, Menditto E. Emerging role of capecitabine in gastric cancer. Pharmacotherapy. 2009;29:318–30.
Calvani N, Orlando L, Nacci A, Sponziello F, Cinefra M, Cinieri S. Metronomic chemotherapy against cancer: from paradigm to clinical practice? Tumori. 2009;95:843–5.
Lokich J. Capecitabine: fixed daily dose and continuous (noncyclic) dosing schedule. Cancer Invest. 2004;22:713–7.
Sun JF, Wu RR, Norris C, Noone AM, Amankwa-Sakyi M, Slack R, Marshall JL. Safety of chronic low-dose capecitabine as maintenance therapy in gastrointestinal cancers. Gastrointest Cancer Res. 2009;3:134–40.
Lichtman SM, Wildiers H, Chatelut E, Steer C, Budman D, Morrison VA, Tranchand B, Shapira I, Aapro M. International society of geriatric oncology chemotherapy taskforce: evaluation of chemotherapy in older patients–an analysis of the medical literature. J Clin Oncol. 2007;25:1832–43.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin vs S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9.
Trumper M, Ross PJ, Cunningham D, Norman AR, Hawkins R, Seymour M, Harper P, Iveson T, Nicolson M, Hickish T. Efficacy and tolerability of chemotherapy in elderly patients with advanced oesophago-gastric cancer: a pooled analysis of three clinical trials. Eur J Cancer. 2006;42:827–34.
Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100:12917–22.
Ooyama A, Oka T, Zhao HY, Yamamoto M, Akiyama S, Fukushima M. Anti-angiogenic effect of 5-Fluorouracil-based drugs against human colon cancer xenografts. Cancer Lett. 2008;267:26–36.
Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–65.
Rozados VR, Mainetti LE, Rico MJ, Zacarías Fluck MF, Matar P, Scharovsky OG. The immune response and the therapeutic effect of metronomic chemotherapy with cyclophosphamide. Oncol Res. 2010;18:601–5.
Bocci G, Falcone A, Fioravanti A, Orlandi P, Di Paolo A, Fanelli G, Viacava P, Naccarato AG, Kerbel RS, Danesi R, Del Tacca M, Allegrini G. Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br J Cancer. 2008;98:1619–29.
Jurado JM, Sánchez A, Pajares B, Pérez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008;10:583–6.
Hsu CH, Shen YC, Lin ZZ, Chen PJ, Shao YY, Ding YH, Hsu C, Cheng AL. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010;53:126–31.
Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch A, Rocca A, Pietri E, Colleoni M. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–905.
Acknowledgments
This project was supported by the Shanghai Nature Science Fund, Shanghai, China (0552nm007).
Conflict of interest
The authors declared no conflicts of interest with respect to authorship and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, S., Shen, J., Hong, L. et al. Capecitabine “metronomic” chemotherapy for palliative treatment of elderly patients with advanced gastric cancer after fluoropyrimidine-based chemotherapy. Med Oncol 29, 100–106 (2012). https://doi.org/10.1007/s12032-010-9791-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9791-x