Abstract
The purpose of this study was to compare the outcome and resectability of patients with gastric cancer recurrence after curative surgery detected by follow-up endoscopy, according to the presence or absence of symptoms. All patients with gastric carcinoma, who underwent a curative gastrectomy, were retrospectively identified. We analyzed outcome and survival in patients compliant with routine follow-up who presented symptomatic and asymptomatic recurrence. Of the 119 resected patients, 63.0% had a recurrence, with an overall survival of 20.0 months. Fourteen patients were asymptomatic when recurrence was detected, whereas 61 patients were symptomatic. Median time to recurrence was 16.0 m for both groups. A local curative re-resection was possible in 2/14 (asymptomatic) and 1/61 (symptomatic). Asymptomatic patients had a longer median postrecurrence survival time of 9.0 months, compared with 2.0 months in the symptomatic patients (p=0.034). The median overall survival was greater in the asymptomatic vs symptomatic group (25.0 vs 20.0 months), although this did not reach statistical significance. The results from this study advocate that the presence or absence of symptoms is a good surrogate marker to assess biologic aggressiveness. The value of routine follow-up endoscopy to permit a higher rate of re-resection in asymptomatic patients remains to be established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the second leading cause of cancer-related deaths in Mexico and throughout the world [1, 2]. Surgical resection is the only curative treatment; however, the resectability rate is low, with curative resection rates reported between 30 and 40%. In Mexico, the resectability rate is even lower (12.4–28.6%), probably related to advanced stages at diagnosis [3, 4].
It is known that the majority of patients develop recurrence with locally invasive or metastatic disease, which will eventually lead to death. Only approximately 20% of all gastric cancers will have an isolated local recurrence during the first few years after surgery [5]. It is hoped that endoscopic examination will detect a significant proportion of such recurrences at an asymptomatic stage when curative resection may still be possible, although it remains questionable that routine endoscopic examination could accomplish this and improve survival.
Most clinicians perform postoperative surveillance for these patients, although there is no consensus on the regimen and frequency of follow-up after curative surgery. Thus, the practice of follow-up intervals and choice of tests varies considerably among clinicians. This absence of guidance is unsurprising, given the paucity of high-quality evidence and the complete lack of randomized controlled trials [6].
An effective postoperative surveillance strategy after potentially curative treatment should have at least three required elements: scheduled surveillance must identify tumor recurrence in asymptomatic patients, early therapeutic intervention must improve the outcomes of asymptomatic patients, and the costs and risks of surveillance must be acceptable [7]. Accordingly, assuming that routine follow-up would increase the detection of symptom-free recurrences and be beneficial in terms of survival, patients with asymptomatic “early” recurrence detected by routine follow-up should live longer than those with cancer-related symptoms at the time of detection.
The purpose of this study was to compare the outcome and potential resectability of patients with recurrence after curative surgery, followed by clinical examination, routine endoscopy, and imaging studies indicated at the discretion of the treating physician, according to the presence or absence of symptoms when recurrence was detected.
Materials and methods
All patients with gastric carcinoma, who underwent a curative gastrectomy from 1980 to 2006 at the Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran in Mexico City, were retrospectively identified. Patient hospital records and office charts were reviewed. Demographics, histology, stage, treatment, and recurrence were analyzed. The majority of patients were followed after curative resection for gastric cancer based on National Comprehensive Cancer Network guidelines [8]. No additional standard protocol for follow-up was practiced.
Recurrence was classified as either symptomatic or asymptomatic. Asymptomatic recurrence was defined as recurrence discovered by a routine laboratory, imaging, or endoscopic test, usually found in context of a physician-scheduled visit. Symptomatic recurrence was defined as a patient-initiated finding or complaint that resulted in a work-up documenting recurrence, detected at a patient-initiated visit or a physician-scheduled consult. Clinical symptoms that were thought to be related to recurrence were dysphagia, weight loss, palpable tumors, pain or other symptoms that were due to the recurrence found in the further diagnostic work-up. All symptoms had to be of new onset, e.g., all patients were free of symptoms for at least 2 months postoperatively. All patients with recurrence, symptomatic, and asymptomatic had complete radiographic imaging of the chest, abdomen, and pelvis.
Both symptomatic and asymptomatic recurrences were categorized as locoregional, peritoneal, or distant, as previously defined [9]. Locoregional recurrences were in the gastric bed, regional gastric lymph nodes, or the anastomosis. Peritoneal recurrences included positive cytology in ascitic fluid, carcinomatosis, or ovarian metastasis. Distant metastases were defined by the organ site or as distant lymph nodes outside of the regional basin. Patients, who presented with two or three different types of recurrences, were classified in the group that conferred the worst prognosis.
The recurrence-free survival (RFS) was calculated from curative gastrectomy to recurrence, the disease-specific survival (DSS) from recurrence to death, and the overall survival (OS) from curative gastrectomy to death. The RFS, DSS, and OS were estimated using the Kaplan–Meier method. For each of these time intervals, patients with symptomatic recurrence were compared with patients with asymptomatic recurrence. The log-rank test was used to evaluate differences in survival between groups. For the differences in baseline characteristics between symptomatic and asymptomatic patients, the Mann–Whitney test, Fisher’s exact test and, the χ 2 test were used. A p value of less than 0.05 was considered significant. The statistical analysis was performed using the SPSS software version 15.0 (SPSS Inc, Chicago, IL) and was revised by a medical statistician.
Results
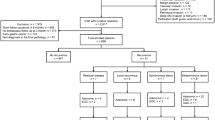
A total of 126 patients underwent a curative gastrectomy for gastric cancer between 1980 and 2006. Five patients were excluded who were subsequently lost to follow-up and other two because the gastric recurrence presented in the stump, as these cases should be analyzed differently from local recurrence, because the biological behavior is quite different between the stump cancer and the local recurrence. There were 64 males and 55 females, with a mean age of 56.3 ± 15.3 (range of 25–91 years old). The median OS of the entire group was 43.0 months.
Overall recurrence
Of the 119 resected patients, 75 (63.0%) had a documented recurrence. Metastatic recurrence (n = 48, 64%) and peritoneal carcinomatosis (n = 16, 22%) were the most common patterns of failure, whereas locoregional disease as a unique site of recurrence was infrequent (n = 11, 15%). The median OS from resection to death for the entire cohort of 75 patients was 20.0 months (95% CI, 13.9–26.1).
Fourteen patients (19%) were asymptomatic when recurrence was detected, whereas 61 patients (81%) either consulted a physician because of symptoms suggesting recurrence or had such symptoms at the time they came to the hospital for a regular follow-up. Patient demographics and pathological characteristics among symptomatic and asymptomatic patients are listed in Table 1. No differences in clinicopathologic variables were evident between patients who had cancer-related symptoms when recurrent disease was diagnosed and those without symptoms, except for the depth of invasion. Asymptomatic patients more commonly presented with T1 or T2 lesions compared with symptomatic patients in which T3 and T4 lesions were more frequently encountered.
The characteristics of recurrences in both groups are shown in Table 2. Symptomatic patients had 36 metastatic recurrences and 16 peritoneal, which together comprised the 85.2% compared with 85.7% of metastatic recurrences in the asymptomatic group. The proportion of locoregional recurrences was similar between both groups (14.8 vs. 14.3%, respectively). The time between surgery and recurrence was 16.0 months for both the asymptomatic and symptomatic patients through the entire follow-up period. Figure 1 shows the RFS for the two groups.
Recurrence-free survival (RFS) from curative gastrectomy to recurrence is demonstrated for both symptomatic and asymptomatic patients. The time to recurrence was the same between these two groups throughout the entire follow-up period, with a median time to recurrence of 16.0 months for both group patients (P = NS)
Follow-up endoscopy
The number of endoscopies until recurrence did not vary between asymptomatic and symptomatic patients, with a mean of 2.4 endoscopies for both groups (Table 2). The time between endoscopies divided by the total number of endoscopies (P = 0.347) and the interval between the resection and first endoscopy (P = 0.555) was similar between both groups.
Treatment
A local curative re-resection was possible in 3 patients, 2/14 (14.3%) in the asymptomatic group and 1/61 (1.3%) in the symptomatic patients (P = 0.088) (Table 3). One is currently alive after 46 months (asymptomatic recurrence), another died 1 month after surgery because of infectious postoperative complications, while the last one died 21 months after recurrence due to disease progression.
Twenty patients with a recurrence had palliative surgery for locoregional recurrences, 18 (29.5%) in the symptomatic group and 2 (14.3%) in the asymptomatic group, with no statistically significant difference. Symptomatic patients received systemic palliative treatment more frequently compared to the asymptomatic group, 70.5 vs. 42.9%, respectively, although this did not reach statistical significance.
Survival
Figure 2 shows the disease-specific survival (DSS) from recurrence to death. Asymptomatic patients had a longer median postrecurrence survival time of 9.0 months, whereas symptomatic patients had a poor median postrecurrence survival of only 2.0 months after documented recurrence (P = 0.034). As demonstrated in Fig. 3, the median OS was greater in the asymptomatic vs. symptomatic group (25.0 vs. 20.0 months), although this did not reach statistical significance (P = 0.568).
Overall survival (OS) from curative gastrectomy to death is demonstrated for both symptomatic and asymptomatic patients. The median OS from resection to death was 25.0 months for the asymptomatic patients and 20.0 months for the symptomatic patients, although this difference did not reach statistical significance
Discussion
Most clinicians perform postoperative surveillance for resected patients with gastric cancer, although there is no consensus on the regimen and frequency of follow-up after curative surgery, even after considering that this disease is the second leading cause of cancer death worldwide. Local recurrence and distant metastases are frequently observed following potentially curative resection of gastric carcinoma, but re-resection is rarely possible. The prognosis of patients with recurrent gastric cancer is usually considered to be poor because of several factors, including a low resectability rate and a poor tolerance of treatment, with increased morbidity and mortality [9]. In patients carrying a low perioperative risk, an attempt of re-resection appears justified as no alternative therapy offers a chance for cure.
Given that most patients with systemic metastases are not candidates for resection and that the survival rate after liver, peritoneal or pulmonary resection is very low [10], almost exclusively local recurrences are amenable to surgical curative intent. For this reason, the follow-up with routine endoscopy is theoretically useful in detecting resectable tumor recurrence [11–16]. Some reports have documented a median survival time after re-resection that varies between 9 and 82 months [17–19]. Still, not in all studies these findings denote an increment in overall survival [11, 15, 16]. In spite of this, the detection of local recurrence by routine endoscopy allows a curative re-resection that varies between 0.6 and 2.8%, with only a single report that reached 10% [15]. Our results confirm a re-resection of 3 out of the total 75 (5.3%) resected tumors. One patient is currently alive after 46 months, another died 1 month after surgery because of infectious postoperative complications, and one died after 21 months of re-resection due to disease progression.
Because follow-up with routine endoscopy only allowed the detection of 1.7% (2/119) of asymptomatic recurrences amenable to re-resection in the current study, the utility of this follow-up strategy is questionable. Two previous studies have demonstrated no benefit in the overall survival between patients with intensive follow-up vs. regular follow-up [20] or those who did or did not comply with the surveillance program [15]. Thus, although data show that a small but significant proportion of patients with gastric cancer experience resectable tumor recurrences, it is questionable whether the favorable prognosis is caused by adherence to a surveillance program or its biological aggressiveness.
There are three previous studies that have compared patients whose recurrence was detected by routine follow-up prior to the development of clinical signs (asymptomatic group) and patients who developed clinical symptoms due to a recurrence that was detected subsequently (symptomatic group) [21–23]. As our results verified, the three studies showed an equal RFS (from curative gastrectomy to recurrence) between both groups, which indicates that the routine follow-up in the asymptomatic patients failed to identify the recurrences in an earlier time compared with patients who presented with symptoms secondary to recurrence. Conversely, the asymptomatic groups showed a statistical significant advantage in DSS (from recurrence to death) in the three studies, as well as in ours. However, only in the largest study by Bennet [23] this benefit translated in a statistical significant increment in OS (from curative gastrectomy to death) with a difference of 29.4 months for the asymptomatic patients and 21.6 months for the symptomatic patients (P < 0.05). The OS was 25.9 vs. 20.4 months for the asymptomatic and symptomatic groups, respectively in the Kodera study [22] and 25.0 vs. 20.0 months in the present study, although these differences did not reach statistical significance, probably because of smaller samples. Table 4 summarizes the results of these three studies, as well as ours.
As shown in Fig. 2, patients with asymptomatic recurrence achieve a longer survival when compared to patients with symptomatic recurrence commencing at the time of recurrence. These data suggest that symptomatic recurrence is a surrogate that is associated with more aggressive tumor biology, as suggested previously [23]. These two types of recurrence patterns appear to be biologically different and are associated with different survival outcomes.
According to these assumptions, it would be relevant to develop strategies that could predict the risk of recurrence with a more accurate approach after potentially curative surgery for gastric cancer. A scoring system may predict recurrence and can guide an intensive follow-up program in patients with high risk of recurrence, in order to detect asymptomatic patients with local recurrences in an early stage that can be rescued with surgical treatment. Formerly, a scoring system was proposed based on clinicopathological characteristics with an overall accuracy of 82.2% [24]. However, clinicopathologic findings are sometimes inadequate for predicting recurrence in individuals, so it is important to construct a new diagnostic system that predicts recurrence in patients with advanced gastric cancer after curative resection based on molecular analysis. Regarding this matter, Motoori et al. [25] constructed a diagnostic system using 29 genes identified by systematic analysis of gene expression profiling, which classified each case into a good-signature or poor-signature group, with an impact in overall survival (P = 0.0125), especially in patients with smaller tumors, less developed lymph node involvement, or earlier stages. The identification of high-risk patients would lead to consideration of additional therapeutic intervention and closer follow-up.
In conclusion, the results from this study advocate that the presence of symptoms implies more tumor aggressiveness, which determines a shorter survival. The value of routine follow-up endoscopy to permit a higher rate of re-resection in asymptomatic patients remains to be established.
References
Garcia M, et al. Global cancer facts & figures 2007. Atlanta: American Cancer Society; 2007.
Estadísticas Demográficas 2006. México: Instituto Nacional de Estadística, Geografía e Informática, 2008.
Leon-Rodriguez E, Dominguez A. Adjuvant chemotherapy in gastric cancer. Experience at the National Institute of Nutrition. Rev Invest Clin. 1992;44:221–7.
Green D, Ponce de Leon S, Leon-Rodriguez E, Sosa-Sanchez R. Adenocarcinoma of the stomach: univariate and multivariate analysis of factors associated with survival. Am J Clin Oncol. 2002;25:84–9.
Papachristou DN, Fortner JG. Local recurrence of gastric adenocarcinomas after gastrectomy. J Surg Oncol. 1981;18:47–53.
Whiting J, et al. Follow-up of gastric cancer: a review. Gastric Cancer. 2006;9:74–81.
Jensen E, Tuttle T. Preoperative staging and postoperative surveillance for gastric cancer. Surg Oncol Clin N Am. 2007;16:329–42.
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, 2009. Available at: http://www.nccn.org. Accessed April 30, 2009.
D’Angelica M, et al. Patterns of inicial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–16.
Lehnert T, Rudek B, Buhl K, Golling M. Surgical therapy for loco-regional recurrence and distant metastasis of gastric cancer. EJSO. 2002;28:455–61.
Lee SY, et al. The role of follow-up endoscopy after total gastrectomy for gastric cancer. EJSO. 2005;31:265–9.
Onodera H, et al. Surgical outcome of 483 patients with early gastric cancer: prognosis, postoperative morbidity and mortality, and gastric cancer. Hepatogastroenterology. 2004;51:82–5.
Kikuchi S, et al. Efficacy of endoscopic surveillance of the upper gastrointestinal tract following distal gastrectomy for early gastric cancer. Hepatogastroenterology. 2003;50:1704–7.
Hosokawa O, et al. Endoscopic surveillance for gastric remnant cancer after early cancer gastric. Endoscopy. 2002;34:469–73.
Eckardt VF, Giebler W, Kanzler G, Bernhard G. Does endoscopic follow-up improve the outcome of patients with benign gastric ulcers and gastric cancer? Cancer. 1992;69:301–5.
Porro A, Alberto A. Correspondence of: does endoscopic follow-up improve the outcome of patients with benign gastric ulcers and gastric cancer? Cancer. 1992;70:2741–2.
Shchepotin I, et al. Radical treatment of locally recurrent gastric cancer. Am Surg. 1995;61:371–6.
Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–42.
Sunagawa M, et al. Reoperation of recurrent gastric cancer ± a comparative study of a resected and non-resected group. Gan No Rinsho. 1984;30:1899–903.
Tan IT, So BY. Value of intensive follow-up of patients after curative surgery for gastric carcinoma. J Surg Oncol. 2007;96:503–6.
Böhner H, Zimmer T, Hopfenmuller W, Berger G, Buhr HJ. Detection and prognosis of recurrent gastric cancer—is routine follow-up after gastrectomy worthwhile? Hepatogastroenterology. 2000;47:1489–94.
Kodera Y, et al. Follow-up surveillance for recurrence after curative gastric cancer surgery lacks survival benefit. Ann Surg Oncol. 2003;10:898–902.
Bennet J, et al. Is detection of asymptomatic recurrence after curative resection associated with improved survival in patients with gastric cancer? J Am Coll Surg. 2005;201:503–10.
Marrelli D, et al. Prediction of recurrence after radical surgery for gastric cancer. A scoring system obtained from a prospective multicenter study. Ann Surg. 2005;241:247–55.
Motoori M, et al. Prediction of recurrence in advanced gastric cancer patients after curative resection by gene expression profiling. Int J Cancer. 2005;114:963–8.
Acknowledgments
Study conception and design: Villarreal-Garza, León-Rodríguez. Collection of data: Villarreal-Garza, Rojas-Flores, Castro-Sánchez. Analysis and interpretation of data: Villarreal-Garza, Villa, León-Rodríguez. Systematic review of literature and drafting of manuscript: Villarreal-Garza, García-Aceituno. Final approval of manuscript: Villarreal-Garza, Rojas-Flores, Castro-Sánchez, Villa, García-Aceituno, León-Rodríguez.
Conflict of interest statement
The authors of the manuscript “Improved outcome in asymptomatic recurrence following curative surgery for gastric cancer” declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villarreal-Garza, C., Rojas-Flores, M., Castro-Sánchez, A. et al. Improved outcome in asymptomatic recurrence following curative surgery for gastric cancer. Med Oncol 28, 973–980 (2011). https://doi.org/10.1007/s12032-010-9576-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9576-2