Abstract
Ovarian cancer remains a highly lethal disease. The aim of the present study was to evaluate the usefulness of measuring serum matrix metalloproteinase-7 (MMP-7), CC chemokine ligand 18 (CCL18) and CC chemokine ligand 11 (CCL11) in comparison with serum cancer antigen 125 (CA 125) for diagnosis of epithelial ovarian cancer (EOC). This study included 51 patients with EOC, 27 patients with benign ovarian lesions and 29 healthy volunteers. Serum CA 125 was determined by microparticle enzyme immunoassay, while serum MMP-7, CCL18 and CCL11 were measured using enzyme-linked immunosorbent assay. The sensitivity and specificity were 86.3% and 92.9% for CA 125, 80.4% and 87.5% for MMP-7, 84.3% and 91.1% for CCL18 and, 68.6% and 62.5% for CCL11. Combination of CA 125, MMP-7, CCL18 and CCL11 gave a promising sensitivity of 100%, but specificity was decreased to 60.7%. The combined use of serum CA 125, MMP-7, CCL18 and CCL11 effectively detected early stages EOC with high sensitivity of 94.4%. Our data indicate that serum MMP-7, CCL18 and CCL11, in combination with CA 125 could be useful in diagnosis of EOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the leading cause of death among gynecological malignancies [1]. Despite advances in surgical management and chemotherapeutic options over the last three decades, the overall survival for ovarian cancer is extremely poor. This high death rate is related to the difficulty of detecting ovarian cancer at an early stage as well as the lack of effective therapies for advanced disease [2]. The epithelial tumors of the ovary account for 90% of ovarian malignancies [3].
Cancer antigen 125 (CA 125), a high-molecular weight glycoprotein, is currently the best clinical marker for epithelial ovarian cancer (EOC). However, it is a consistently poor diagnostic tumor marker in early stage ovarian carcinoma [4, 5]. In addition, falsely elevated levels are common in many benign conditions such as pregnancy, endometriosis and intra-abdominal infections [6]. Therefore, it is important to develop new markers for detection of EOC, particularly those that can be readily measured in the serum. Recent studies have evaluated the utility of multiple serum markers in combination with CA 125 for EOC diagnosis [7, 8].
Matrix metalloproteinases (MMPs) are a family of extracellular zinc-dependent neutral endopeptidases collectively capable of degrading essentially all extracellular matrix (ECM) components and they play important roles at different steps of tumor growth, invasion, and metastasis [9]. The role of several tissue MMP family members, including MMP-2, MMP-8, MMP-9 and membrane type-1 (MT1)-MMP in EOC has been well documented [10–12]. MMP-7, the smallest known member of MMPs, is consisting of two domains, a pro-peptide domain and a catalytic domain. Cleavage of the N-terminal pro-peptide domain yields the active form of the enzyme [13]. MMP-7 has broad proteolytic activity against a variety of ECM substrates such as elastin, fibronectin, proteoglycans and type IV collagen [14]. Overexpression of tissue MMP-7 was seen in many malignancies such as prostate, stomach, colorectal, lung, esophageal, liver, pancreas, skin, breast, head and neck [reviewed in reference 14]. Previous studies showed that MMP-7 was overexpressed in EOC surgical specimens and ovarian cancer cell lines [15–17].
Chemokines are a large group of low-molecular weight cytokines, which are implicated in many biological processes, such as migration of leukocytes, angiogenesis, and tumor growth and metastasis. Chemokines activate cells through binding to G-protein-coupled receptors. According to a new classification, chemokine ligands/receptors are named L/R. There are four subfamilies of chemokines ligands: CXCL, CCL, CX3CL and CL (X represents any amino acid). Six CXCR, ten CCR, and one receptor each for CX3C and C have been identified. The CCL chemokines (or β-chemokines) represent the largest family of chemokines and have adjacent cysteine residues. They generally attract monocytes, macrophages, T cells, B cells, basophils, eosinophils, dendritic cells (DCs), mast cells and natural killer cells [18]. Overexpression of chemokines and chemokine receptors appears to be a hallmark of cancer [18]; however, the functional significance of some of these chemokines is yet to be elucidated. CC chemokine ligand 18 (CCL18) is produced by monocytes/macrophages and DCs; so far no agonistic receptor has been identified for this chemokine [19]. CCL18 was more abundantly present in ovarian carcinoma ascites than CCL2, CCL3, CCL7, CCL20 and CXCL8 [20]. CC chemokine ligand 11 (CCL11) is an eosinophils-selective chemoattractant. CCL11 receptors (CCR2, CCR3 and CCR5) were reported to be overexpressed in ovarian cancer tissue relative to normal ovary [21]. While some authors did not observe a significant difference in serum CCL11 levels between patients ovarian cancer and healthy controls [22], others reported that levels of CCL11 in sera of patients with ovarian cancer was significantly decreased compared to healthy women [21].
Accumulating evidence indicates that numerous MMPs and chemokines have critical role in accelerated cancer growth [reviewed in references 9, 18]. The aim of the current study was to determine the diagnostic value of measuring serum MMP-7, CCL18 and CCL11 in patients with EOC. The results obtained were compared to serum CA 125. Moreover, we investigated whether there was a relationship between investigated serum markers and clinicopathological features.
Materials and methods
Subjects
This study was conducted on 78 Egyptian female patients admitted to the Obstetrics and Gynecology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt. The malignant group included 51 female patients with epithelial ovarian carcinoma. Tumors were classified histologically according to the World Health Organization (WHO) criteria [23] as serous (n = 27), endometrioid (n = 14) and mucinous (n = 10). Tumors were staged according to the criteria of the International Federation of Gynecology and Obstetrics (FIGO) [24], while tumor grading was determined as previously described [25]. The number of malignant patients with stage I, II, III and IV was 8, 10, 26 and 7, respectively; while the number of malignant patients with grade 1, 2 and 3 was 19, 15 and 17, respectively. The mean age ± SD of malignant group was 50.18 ± 11.42 (range 26–65 years). The final diagnosis of ovarian cancer was based on histopathological examination. The benign ovarian lesions group consisted of 27 female patients (9 with mature cystic teratoma, 7 with serous cystadenoma, 5 with ovarian fibroma, 4 with mucinous cystadenoma and 2 with simple serous cyst). The mean age ± SD of benign group was 47.28 ± 10.51 (range 24–62). Blood samples were collected from malignant and benign patients before surgery. A group of 29 female healthy volunteers matched for age (mean age ± SD: 48.39 ± 12.18; range 25–64), body mass index and menopausal status was also included in this study. The study was performed in accordance with declaration of Helsinki and was approved by the Research Ethics Committee of Ain Shams University, Cairo, Egypt. An informed consent was obtained from all subjects.
Biochemical measurements
Serum samples were prepared, aliquoted and stored at −80°C until analyses. Determination of CA 125 was performed by means of AxSYM microparticle enzyme immunoassay (MEIA) kit manufactured by Abbott Laboratories, Abbott Park, IL. USA. MMP-7 and CCL11 were measured by enzyme-linked immunosorbent assay (ELISA) using quantitative kits manufactured by R&D systems, Inc., Minneapolis, USA. CCL18 was measured using ELISA quantitative kit purchased from RayBiotech, Inc., Norcross, Georgia, USA. Measurements of MMP-7, CCL18 and CCL11 were carried out using ELISA plate reader, Lab systems Multiskan® plus, Helsinki, Finland. The precision and analytical recovery of CA 125, MMP-7, CCL18 and CCL11 serum assays were tested.
Statistical analysis
Intra-assay and inter-assay precision of CA 125, MMP-7, CCL18 and CCL11 serum assays were represented by the coefficient of variation (CV). The CV equals the standard deviation divided by the mean. The Kruskal–Wallis and Mann–Whitney U nonparametric tests were used for the statistical comparison of the variables between the various groups. The threshold value for optimal sensitivity and specificity was determined by receiver operating characteristic (ROC) curve, which was constructed by calculating the true-positive fraction (sensitivity %) and false-positive fraction (100-specificity %) of serum CA 125, MMP-7, CCL18 and CCL11 at several cutoff points [26]. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated according to standard statistical methods. Values of P lower than 0.05 were considered statistically significant. These analyses were performed using the Statistical Package for the Social Sciences (SPSS software, version 12.0, Chicago, Illinois) on a personal computer.
Results
Validation of MEIA/ELISA for quantification of serum markers
Intra-assay CV ranged from 3.2% to 4.8% for CA 125, 3.5% to 5.2% for MMP-7, 3.1% to 4.4% for CCL18 and 3.6% to 5.1% for CCL11. While inter-assay CV ranged from 2.9% to 5.7% for CA 125, 4.0% to 4.9% for MMP-7, 5.1% to 6.3% for CCL18 and 5.5% to 6.7% for CCL11. The calculated recovery ranged from 96.6% to 99.1% for CA 125, 95.6% to 99.8% for MMP-7, 94.1% to 99.3% for CCL18 and 96.1% to 99.7% for CCL11.
Levels of serum markers in investigated groups
Serum levels of CA 125, MMP-7 and CCL18 were significantly increased in ovarian cancer group compared to control group (P < 0.001); and in ovarian cancer group compared to benign ovarian lesions group (P < 0.001). Furthermore, serum CA 125, MMP-7 and CCL18 levels were significantly elevated in benign ovarian lesions group compared to control group (P < 0.001, P < 0.001 and P = 0.002, respectively). Serum CCL11 level was significantly decreased in patients with EOC compared to healthy volunteers (P = 0.010) and benign ovarian lesions group (P = 0.037) (Table 1).
Relation between investigated serum markers and clinicopathological factors of patients with EOC
Serum level of CA 125 was significantly increased in EOC patients with late stages (III, IV) compared to those with early stages (I, II) (P < 0.001); and in EOC patients with poorly differentiated tumors (grade 3) compared to those with well-differentiated and moderately differentiated tumors (grades 1, 2) (P = 0.001). The level of serum CA 125 was significantly elevated in patients with serous tumors compared to those with nonserous tumors (endometrioid and mucinous) (P = 0.001) (Table 2). There was no significant difference in serum level of MMP-7 with respect to stage, grade or pathological type of the EOC (Table 3). Serum CCL18 level was significantly elevated in EOC patients with early stages (I, II) compared to those with late stages (III, IV) (P = 0.010) (Table 4). Serum CCL11 level was significantly decreased in EOC patients with early stages (I, II) compared to those with late stages (III, IV) (P = 0.022); and in EOC patients with well-differentiated and moderately differentiated tumors (grades 1, 2) compared to those with poorly differentiated tumors (grade 3) (P = 0.018) (Table 5).
Sensitivity, specificity, PPV, NPV and accuracy of investigated serum markers
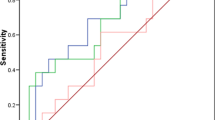
We constructed ROC curves for investigated parameters to calculate the best cutoff values to discriminate between malignant and nonmalignant (patients with benign ovarian lesions and healthy volunteers) groups. The calculated cutoff values were >35 U/mL for CA 125, >9.01 ng/mL for MMP-7 and >81.80 ng/mL for CCL18. Area under the curve (SE), 95% confidence limits range and P value were 0.973 (0.012), 0.950–0.997, P < 0.001, respectively, for CA 125; 0.907 (0.028), 0.853–0.961, P < 0.001, respectively, for MMP-7 and 0.930 (0.025), 0.881–0.978, P < 0.001, respectively, for CCL11 (Fig. 1). Moreover, the best cutoff value was <139.11 pg/mL for CCL11. Area under the curve (SE), 95% confidence limits range and P value were 0.638 (0.053), 0.534–0.743, P = 0.014, respectively, for CCL11 (Fig. 2). The highest overall values of sensitivity (86.3%) and specificity (92.9%) were observed for CA 125 followed by CCL18 (sensitivity: 84.3% and specificity: 91.1%), MMP-7 (sensitivity: 80.4% and specificity: 87.5%), and then CCL11 (sensitivity: 68.6% and specificity: 62.5%). CA 125 had higher PPV, NPV and accuracy than CCL18, MMP-7 and CCL11. The combined sensitivity of CA 125 with CCL18 (96.1%) was higher than combined sensitivity of CA 125 with either MMP-7 (94.1%) or CCL11 (92.2%). The combined sensitivity of MMP-7 with CCL18 (90.2%) was higher than combined sensitivity of CCL11 with either CCL18 (88.2%) or MMP-7 (86.3%). The combined CA 125, MMP-7, CCL18 and CCL11 had a sensitivity of 100%, but the specificity was only 60.7%, as shown in Table 6.
Discussion
The high ratio of death to incidence for patients with ovarian carcinoma is largely due to late-stage diagnosis, at a time when the disease has typically spread into the peritoneum beyond the pelvic region. Late-stage diagnosis of EOC can be attributed to the fact that the disease is relatively “asymptomatic” in its early stages, and that the symptoms of late-stage disease, such as abdominal discomfort, weight loss, diarrhea or constipation, vaginal bleeding, and shortness of breath, are nonspecific complaints [27, 28]. The relatively nonspecific nature of these symptoms underscores the need for disease diagnostics. Thus, in this study, we measured the concentration of CA 125, MMP-7, CCL18 and CCL11 in a series of 51 serum samples from EOC patients with different stages, grades and pathological types. The levels obtained were compared with those of 27 patients with benign ovarian lesions and 29 healthy controls. Moreover, we tested the possible relationship between the levels of investigated parameters and the clinicopathological factors of EOC patients. To our knowledge, no published studies have simultaneously assessed the diagnostic value of serum CA 125, MMP-7, CCL18 and CCL11 in patients with EOC.
In the current study, we revealed marked increase of serum CA 125 and MMP-7 levels in malignant group compared to either controls or benign group; and in benign group compared to control group. The diagnostic significance of CA 125 is well recognized in patients with EOC [4, 5, 7, 8]. Serum MMP-7 was previously reported to be increased in patients with EOC compared to healthy controls; however, it was noticed that such study was limited to serous ovarian carcinoma [29]. MMP-7 can promote cancer invasion by proteolytic cleavage of the ECM substrates [14]. MMP-7 also activates other MMPs, such as proMMP-2 and proMMP-9 to facilitate invasion of ovarian cancer [15]. Vascular endothelial growth factor was reported to induce secretion of pro-MMP-7 and pro-MMP-9 and activation of pro-MMP-2 in EOC DOV13 conditioned medium in a concentration-dependent manner [16]. In vitro studies show that MMP-7 expression correlated with EOC invasiveness and that lysophosphatidic acid induced EOC invasion through the secretion/activation of MMP-7 [17]. Modification of non-ECM proteins, such as insulin-like growth factor binding proteins, heparin-binding epidermal growth factor precursor and E-cadherin, by MMP-7 is one of the mechanisms by which MMP-7 plays a role in tumorigenesis [reviewed in reference 14]. A possible association was suggested between the MMP-7 A/G polymorphism and susceptibility to EOC [30].
Chemokines are produced by a variety of cell types including leukocytes and cancer cells [18]. In this work, serum CCL18 level was significantly higher in patients with EOC compared to either healthy volunteers or patients with benign ovarian lesions. We also observed a significant increase in serum CCL18 level in patients with benign ovarian lesions compared to healthy volunteers. CCL18 was reported to be significantly higher in ascitic fluids from patients with ovarian carcinoma compared to patients with nonovarian carcinoma; moreover, the ascitic fluid CCL18 was found to be lymphocytes chemoattractant indicating an important role for CCL18 in the recruitment of lymphocytes to the ovarian carcinoma environment [20]. Tumor-associated macrophages (TAMs) are demonstrated as the cellular source of the CCL18 present in the ovarian carcinoma ascitic fluid [20]. CCL18 is specifically induced in macrophages by alternative mediators such as IL-4, IL-13 and IL-10 [31]. These alternatively activated macrophages are presumed to be immunosuppressive, since they down-modulate Th1-mediated immunity and exert Th2-associated effector functions [32]. Moreover, DCs in ascites from patients with peritoneal carcinomatosis including those with EOC are reported to be rather immature and inadequate in antigen presentation [33]. Decreases in the expression of MHC class II antigens and costimulatory or accessory molecules on DCs associated with peritoneal carcinoma could provide a possible mechanism for the tumor to evade recognition by the host immune system [33]. Immature DCs were shown to express CCL18 mRNA [34, 35]. Overall, CCL18 could be involved in the immunosuppression of a host antitumor response by attracting tumor-infiltrating lymphocytes toward immature DCs and suppressive TAMs. The effects of these TAMs are of considerable importance in the context of malignant tumors, since a concomitant reduction in immune function is associated with tumor growth and progression. Inhibition of the immunosuppressive effects of these TAMs seems to be reasonable recommended therapeutic approaches to immunogenic cancers.
In the present work, serum CCL11 level was significantly lower in patients with EOC compared to healthy volunteers and patients with benign ovarian lesions. Recently, it was shown that cultured ovarian carcinoma cells absorbed soluble CCL11 [21] indicating that absorption by tumor cells could be responsible for the observed decrease of serum CCL11 in patients with EOC compared to healthy women and benign patients. CCL11 potently stimulated growth of ovarian carcinoma cell lines; this effect was associated with activation of MEK-1, ERK1/2, and STAT3 and upregulation of VEGF, IL-8, PDGF-BB, ICAM-1, bFGF and some chemokines [21]. Interestingly, eosinophils were rarely seen in ovarian tumor microenvironment [36]. This could be due to that most of produced CCL11 was rapidly bound to cognate receptors overexpressed by ovarian tumor [21]. It was shown that production of CCL11 was regulated by Th2 and Th1 related cytokines. The Th2-derived cytokine IL-4 upregulated while the Th1-derived cytokine IFN-γ inhibited CCL11 generation by fibroblasts [37].
Our data showed that elevated serum CA 125 level was significantly associated with late stages, poorly differentiated tumors and serous EOC patients. However, we could not find an association between serum MMP-7 level and any of the investigated clinicopathological factors (stage, grade and pathological type). Interestingly, serum CCL18 was significantly increased in EOC patients with early stages (I, II) compared to those with late stages (III, IV). In accordance with previously reported data [21], we found that EOC patients with early stages of disease had significantly decreased concentrations of serum CCL11 compared to patients with late stages. Our study also revealed that lower serum CCL11 level was significantly associated with well-differentiated and moderately differentiated tumors. Taken together, results obtained in this study emphasized the utility of CCL18 and CCL11 as markers for early detection of ovarian cancer. The data related to stage, grade and pathological type should be interpreted with caution due to small number of investigated patients.
Our study revealed that CA 125 had superior sensitivity, specificity, PPV, NPV and accuracy compared to CCL18, MMP-7 and CCL11. Previous studies have evaluated different combination of serum markers for ovarian cancer detection. Havrilesky et al. [7] reported that combination of CA 125, MMP-7, human epididymis protein 4 (HE4), glycodelin and plasminogen activator, urokinase receptor (Plau-R) gave a sensitivity and specificity of 78.9% and 93.2%, respectively for detection of early stage ovarian cancer. While for the detection of late stage disease, this biomarker panel showed an increase in sensitivity to 86.2% with an accompanying increase in specificity to 94.4%. Moreover, Palmer et al. [8] showed that combination of CA 125, MMP-7 and HE4 yielded a sensitivity of 72% at 98% specificity for detecting ovarian cancer. In the current study, combination of serum CA 125, MMP-7, CCL18 and CCL11 gave a promising sensitivity of 100%, but specificity was decreased to 60.7%. Sensitivity of serum CA 125 alone was 61.1% for detection of early stages (I, II) EOC. However, we found that MMP-7, CCL18 and CCL11 in combination with CA 125 provide high sensitivity (94.4%) for detection of early stages EOC.
In conclusion, the concurrent measurement of serum CA 125, MMP-7, CCL18 and CCL11 can improve the diagnosis of EOC. The current study emphasizes the potential diagnostic efficiency of serum CCL18 and CCL11 in early detection of EOC, which in turn can noticeably improve survival rates. However, larger prospective studies are warranted to validate the diagnostic value of investigated serum markers in detection of EOC.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96.
Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29.
D’Andrilli G, Giordano A, Bovicelli A. Epithelial ovarian cancer: the role of cell cycle genes in the different histotypes. Open Clin Cancer J. 2008;2:7–12.
Bast RC, Xu FJ, Yu YH, Barnhill S, Zhang Z, Mills GB. CA 125: the past and the future. Int J Biol Markers. 1998;13:179–87.
Seiden MV. Beyond CA125: the coming of age of ovarian cancer biomarkers. Are we there yet? Biomark Med. 2009;3:275–88.
Cohen LS, Escobar PF, Scharm C, Glimco B, Fishman DA. Three-dimensional power Doppler ultrasound improves the diagnostic accuracy for ovarian cancer prediction. Gynecol Oncol. 2001;82:40–8.
Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q, et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008;110:374–82.
Palmer C, Duan X, Hawley S, Scholler N, Thorpe JD, Sahota RA, et al. Systematic evaluation of candidate blood markers for detecting ovarian cancer. PLoS One. 2008;3:e2633.
Ala-aho R, Kahari V-M. Collagenases in cancer. Biochimie. 2005;87:273–86.
Davidson B, Goldberg I, Gotlib WH, Kopolovic J, Ben-Baruch G, Nesland JM, et al. The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Mol Cell Endocrinol. 2002;187:39–45.
Stadlmann S, Pollheimer J, Moser PL, Raggi A, Amberger A, Margreiter R, et al. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur J Cancer. 2003;39:2499–505.
Kamat AA, Fletcher M, Gruman LM, Mueller P, Lopez A, Landen CN, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12:1707–14.
Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11:143–52.
Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med. 2006;231:20–7.
Wang FQ, So J, Reierstad S, Fishman DA. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int J Cancer. 2005;114:19–31.
Wang FQ, So J, Reierstad S, Fishman DA. Vascular endothelial growth factor-regulated ovarian cancer invasion and migration involves expression and activation of matrix metalloproteinases. Int J Cancer. 2006;118:879–88.
Wang FQ, Smicun Y, Calluzzo N, Fishman DA. Inhibition of matrilysin expression by antisense or RNA interference decreases lysophosphatidic acid-induced epithelial ovarian cancer invasion. Mol Cancer Res. 2006;4:831–41.
Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26:453–67.
Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL 18) in normal and pathological processes. J Leukoc Biol. 2005;78:14–26.
Schutyser E, Struyf S, Proost P, Opdenakker G, Laureys G, Verhasselt B, et al. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J Biol Chem. 2002;277:24584–93.
Levina V, Nolen BM, Marrangoni AM, Cheng P, Marks JR, Szczepanski MJ, et al. Role of CCL11/eotaxin-1 signaling in ovarian cancer. Clin Cancer Res. 2009;15:2647–56.
Lambeck AJA, Crijns APG, Leffers N, Sluiter WJ, ten Hoor KA, Braid M, et al. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin Cancer Res. 2007;13:2385–91.
Scully RE, Sobin LH. Histological typing of ovarian tumours. International histological classification of tumours. World Health Organization. Heidelberg: Springer; 1999.
Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209–62.
Shimizu Y, Kamoi S, Amada S, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998;82:893–901.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36.
Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10.
Memarzadeh S, Berek JS. Advances in the management of epithelial ovarian cancer. J Reprod Med 2001;46:621–9; discussion 629–30.
Meinhold-Heerlein I, Bauerschlag D, Zhou Y, Sapinoso LM, Ching K, Frierson H, et al. An integrated clinical-genomics approach identifies a candidate multi-analyte blood test for serous ovarian carcinoma. Clin Cancer Res. 2007;13:458–66.
Li Y, Jin X, Kang S, Wang Y, Du H, Zhang J, et al. Polymorphisms in the promoter regions of the matrix metalloproteinases-1, -3, -7, and -9 and the risk of epithelial ovarian cancer in China. Gynecol Oncol. 2006;101:92–6.
Kodelja V, Muller C, Politz O, Hakij N, Orfanos CE, Goerdt S. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1α with a Th2-associated expression pattern. J Immunol. 1998;160:1411–8.
Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–42.
Melichar B, Savary C, Kudelka AP, Verschraegen C, Kavanagh JJ, Edwards CL, et al. Lineage-negative human leukocyte antigen-DR+ cells with the phenotype of undifferentiated dendritic cells in patients with carcinoma of the abdomen and pelvis. Clin Cancer Res. 1998;4:799–809.
Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713–7.
Vissers JL, Hartgers FC, Lindhout E, Teunissen MB, Figdor CG, Adema GJ. Quantitative analysis of chemokine expression by dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2001;69:785–93.
Negus RPM, Stamp GWH, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150:1723–34.
Miyamasu M, Misaki Y, Yamaguchi M, Yamamoto K, Morita Y, Matsushima K, et al. Regulation of human eotaxin generation by Th1-/Th2-derived cytokines. Int Arch Allergy Immunol. 2000;122:54–8.
Acknowledgments
The authors thank the Pathology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt, for providing the histopathological data of the patients included in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zohny, S.F., Fayed, S.T. Clinical utility of circulating matrix metalloproteinase-7 (MMP-7), CC chemokine ligand 18 (CCL18) and CC chemokine ligand 11 (CCL11) as markers for diagnosis of epithelial ovarian cancer. Med Oncol 27, 1246–1253 (2010). https://doi.org/10.1007/s12032-009-9366-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-009-9366-x