Abstract
The aim of the study was to investigate whether the presence of matrix metalloproteinase-2 (MMP-2) and its inducer, extracellular matrix metalloproteinase inducer (EMMPRIN), in primary cutaneous malignant melanoma (PCMM) might help to predict patient prognosis. Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded sections of PCMM from 150 patients. Association of clinical variables (gender, age, tumor location, thickness, Clark level and AJCC stage) with EMMPRIN and MMP-2 expression were analyzed by Fisher’s exact test. Survival rates were subsequently estimated using the Kaplan–Meier method and compared using the log-rank test. The expression of EMMPRIN and MMP-2 was detected in 117/150 (78.0%) and 115/150 (76.7%) of patients with PCMM, respectively. Higher positive rates of both EMMPRIN and MMP-2 expression were significantly correlated with increased tumor thickness (both P = 0.004), higher Clark level (P = 0.02 and 0.03) and higher AJCC stage (both P = 0.006). A significant correlation was found between the expression of EMMPRIN and MMP-2 in PCMM (r = 0.89, P = 0.01). Kaplan–Meier analysis demonstrated that patients who had EMMPRIN+/MMP-2+ expression had a significantly decreased 3-year disease-free survival (P = 0.005) and 5-year overall survival (P = 0.006). In multivariate analyses, tumor thickness and EMMPRIN+/MMP-2+ co-expression were the significant predictors of clinical outcome. EMMPRIN and MMP-2 may be independent biomarkers for disease recurrence and overall survival in patients with PCMM. A combined detection of EMMPRIN/MMP-2 co-expression may benefit us in prediction of a poor survival of PCMM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of primary cutaneous malignant melanoma (PCMM) is increasing more rapidly than any other cancers in China. Surgical excision of localized PCMM (American Joint Committee on Cancer [AJCC] stages I and II; approximately 75% of diagnoses) may lead to cure in many patients. The overall 5-year survival rate for these patients (AJCC stages I and II) is approximately 80%, suggesting that approximately 20% of stage I and II patients may have micrometastatic disease at the time of diagnosis [1, 2]. Several histopathological parameters, including tumor thickness, ulceration, satellites and lymph node status, have been reported to have prognostic significance in PCMM [3]. However, the assessment of molecular factors, more strictly related to tumor cell biology and influencing the development and progression of PCMM, could help to identify high-risk patients for disseminated disease and could improve overall clinical management of PCMM.
The metastatic potential of tumor cells is dependent upon their ability to disengage themselves from environmental cues that serve to regulate and constrain them. Typically, successful metastasis requires alterations in cytoskeletal architecture, expression of surface adhesion molecules and penetration of the basement membrane. Results of investigations into tumor biology can contribute to the development of novel promising therapies, such as protease inhibitors for myeloma treatment [4, 5]. Extracellular matrix metalloproteinase inducer (EMMPRIN), also known as CD147 or basigin, is highly expressed on the outer surface of carcinoma cells, but not on normal mucosal cells [6]. EMMPRIN was initially identified following the observation that interstitial collagenase (MMP-1) production was induced during the co-culturing of tumor cells and fibroblasts. Further studies revealed that EMMPRIN is capable of inducing the expression of several MMPs other than MMP-1, including MMP-2, MMP-3, MMP-9 and MMP-11 [7, 8]. Thus, tumor cells can interact with adjacent normal cells to produce MMPs via EMMPRIN on their surface and, in turn, invade lymphatic tissue and blood vessels and penetrate through the ECM to adjacent organs with the help of MMPs. The roles of EMMPRIN and MMPs in tumor invasiveness have been confirmed immunohistochemically in several types of cancer cells and surrounding tissue, including gliomas [9], hepatocellular carcinoma [10], prostate cancer [11] and thyroid carcinoma [12]. Moreover, the expression of MMPs is reported to correlate with the clinical prognosis of patients with breast carcinoma and other types of cancers. Yoshino et al. [13] demonstrated that the interaction and/or balance between MMP-2 and tissue inhibitor of metalloproteinase (TIMP)-2 in vivo may play important roles in the process of tumor growth, invasion and metastasis of malignant melanoma. However, the expression of EMMPRIN is unclear in clinical samples of PCMM. In the present study, we have investigated for the first time the immunohistochemical expression of EMMPRIN and MMP-2 in a large series of patients with PCMM, analyzed the correlation with clinicopathological parameters and elucidated the prognostic role.
Materials and methods
Patients and tissue samples
The study was approved by the Research Ethics Committee of Ministry of Public Health of China. Informed consent was obtained from all of the patients. From the files, we retrieved 150 patients with PCMM consecutively diagnosed and treated in our institution between January 2000 and December 2005. Treatment consisted of wide surgical excision of the primary lesion, and regional lymph node dissections were performed in all patients with suspected regional metastases. The clinical records of the patients were reviewed, and the relevant information was recorded in standardized forms. Follow-up data was obtained from these records and from the tumor registry records. Follow-up was terminated in May 22, 2009. Patients without documented follow-up were excluded from case selection. Lymph node status and the presence of metastasis were verified by both clinical and pathological examination. The demographic data and clinical parameters assessed are outlined in Table 1.
Each tumor, after surgical resection, was fixed in formalin and embedded in multiple paraffin blocks. Prognostic parameters were evaluated in sections taken from the block with the largest tumor thickness. Tumor was identified on hematoxylin and eosin-stained sections and on adjacent sections stained immunohistochemically for melanoma-associated antigens, including S100 protein, melan A and HMB-45. Independent histopathological reviews were performed by two pathologists on separate occasions.
Immunohistochemistry analysis
Immunostaining was performed using the alkaline phosphatase-anti-alkaline phosphatase method (Dako, Glostrup, Denmark). After dewaxing, the sections were digested with 0.1% trypsin in TRIS-buffered saline for 15 min at 37°C. They were then processed in a 600 W microwave oven, at maximum power, 5 times for 2 min each time in citrate buffer (pH = 6). After removal of excess normal serum, incubation of the primary antibody (mouse anti-human EMMPRIN monoclonal antibody (#sc-71038, 200 μg/ml, Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) diluted 1:100, mouse anti-human MMP-2 polyclonal antibody (#sc-53630, 200 μg/ml, Santa Cruz Biotechnology, Inc. Santa Cruz, CA. USA) diluted 1:150 against respective target proteins was performed overnight at 4°C. After washing, peroxidase-labeled polymer and substrate-chromogen were then employed to visualize the staining of the interested proteins.
The quality (number, intensity and pattern) of every staining procedure for EMMPRIN and MMP-2 has been comparatively evaluated using consecutive control sections, and the immunostaining was scored by two independent experienced pathologists, who were blinded to the clinicopathological data and clinical outcomes of the patients. The scores of the two pathologists were compared, and any discrepant scores were trained by re-examining the stainings by both pathologists to achieve a consensus score. The number of positive-staining cells in ten representative microscopic fields was counted, and the percentage of positive cells was calculated. Given the homogenicity of the staining of the target proteins, tumor specimens were scored in a semiquantitative manner based on the percentage of tumor cells that showed immunoreactivity. The staining results of MMP-2 were classified into negative (0, staining of ≤10% of cells) or positive (1, staining of >10% of cells); the staining results of EMMPRIN staining were classified into three patterns: negative (0, less than 10% of cells stained), moderate (1, 10–50% of cells stained) and strong (2, more than 50% of cells stained) [17].
Statistical analysis
The software of SPSS version 13.0 for Windows (SPSS Inc., IL, USA) and SAS 9.1 (SAS Institute, Cary, NC) was used for statistical analysis. Continuous variables were expressed as \( \overline{X} \pm s \). Association between EMMPRIN and MMP-2 expression and various clinicopathological characteristics were analyzed using the χ2 test. Correlation between EMMPRIN and MMP-2 expression was analyzed using Spearman’s rank correlation analysis. Two endpoints were examined for survival analyses: disease-free survival (DFS) and overall survival (OS). DFS and OS curves were plotted according to the Kaplan–Meier method, the log-rank test being used to determine the significance of differences between clinicopathological parameters. The Cox proportional hazards model was used for multivariate analysis. Differences were considered statistically significant when P was less than 0.05.
Results
Expression and location of EMMPRIN and MMP-2 in PCMM
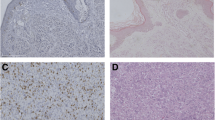
The expression of EMMPRIN and MMP-2 was detected in 117/150 (78.0%) and 115/150 (76.7%) of patients with PCMM, respectively. Their signals concentrated primarily within the cytoplasm and membrane (EMMPRIN) and cytoplasm (MMP-2) of the tumor cells. In contrast, non-cancerous adjacent tissues showed no EMMPRIN and MMP-2 immunoreactivity.
The correlation of EMMPRIN and MMP-2 expression in all patients was assessed. The percentage of positive MMP-2 expression in EMMPRIN-negative, moderate and strong tumor cells were 72.7, 86.6 and 92.0%, respectively. MMP-2 expression was significantly correlated with EMMPRIN expression by Spearman’s rank correlation analysis (r = 0.89, P = 0.01).
Correlation of EMMPRIN and MMP-2 expression with the clinicopathological features of PCMM
The correlation of EMMPRIN and MMP-2 expression with the clinicopathological features of patients with PCMM is summarized in Table 2. Higher positive rates of both EMMPRIN and MMP-2 expression were significantly correlated with increased tumor thickness (both P = 0.004), higher Clark level (P = 0.02 and 0.03) and higher AJCC stage (both P = 0.006). However, among clinical factors, EMMPRIN and MMP-2 expression did not correlate with location of the primary tumor, gender or age of the patients (P = 0.82, 0.56 and 0.60, respectively).
Prognostic implications of EMMPRIN and MMP-2 expression in PCMM
Kaplan–Meier analysis demonstrated that patients who had EMMPRIN+/MMP-2+ expression had a significantly decreased 3-year DFS (P = 0.005; Fig. 1a, Table 3) compared with those with EMMPRIN−/MMP-2−, EMMPRIN−/MMP-2+ and EMMPRIN+/MMP-2−. The predictive value of EMMPRIN/MMP-2 co-expression (P = 0.003, 0.01, 0.009) for 3-year recurrence-free survival maintained significance after the model had been adjusted for independent prognostic factors such as thickness, Clark level and AJCC stage, respectively. In addition, patients with EMMPRIN/MMP-2 co-expression had significantly decreased 5-year OS (P = 0.006; Fig. 1b, Table 3) compared with those with EMMPRIN−/MMP-2−, EMMPRIN−/MMP-2+ and EMMPRIN+/MMP-2–. Multivariate analysis was also conducted using a Cox proportional hazards model. Results are shown in Table 4. The tumor thickness (T1–T2 versus T3–T4) and EMMPRIN/MMP-2 co-expression (EMMPRIN+/MMP-2+ versus EMMPRIN−/MMP-2−) were significant for 3-year DFS (P = 0.02 and 0.01) and for 5-year OS (P = 0.02 and 0.008), respectively.
Kaplan–Meier survival curves for EMMPRIN and MMP-2 expression in PCMM. a 3-year DFS of 150 patients with PCMM, as a function of EMMPRIN+/MMP-2+ versus EMMPRIN−/MMP-2− expression (P = 0.005); b 5-year OS of 150 patients with PCMM, as a function of EMMPRIN+/MMP-2+ versus EMMPRIN−/MMP-2− expression (P = 0.006)
Discussion
The primary objective of this study was to evaluate EMMPRIN and MMP-2 staining in a large series of PCMM. Our findings indicate that the presence of EMMPRIN and MMP-2 in melanoma cell cytoplasm and plasma membrane, demonstrated by immunohistochemical staining, is significantly correlated with shorter survival, and a significant difference was observed in 5-year survival between patients with EMMPRIN+/MMP-2+ and EMMPRIN−/MMP-2− expression. Moreover, the recurrence rate was increased, especially in the first three postoperative years, in patients with EMMPRIN+/MMP-2+ co-expression compared with patients with EMMPRIN−/MMP-2−, EMMPRIN−/MMP-2+ and EMMPRIN+/MMP-2− staining. Cox’s proportional hazard model results indicate that EMMPRIN/MMP-2 co-expression is an independent predictor for the 3-year risk of disease recurrence. The results of this study suggest that EMMPRIN/MMP-2 co-expression is an important marker for recurrence and survival, independent of other factors. This finding adds further weight to the current evidence that EMMPRIN and MMP-2 expression is potentially relevant to tumor progression.
EMMPRIN is a transmembrane glycoprotein with two immunoglobulin-like domains and over-expressed on the surface of malignant tumor cells [18]. Depending on the cell system, EMMPRIN can stimulate production of MMP-1 (interstitial collagenase), MMP-2 (gelatinase A) and MMP-3 (stromelysin 1) but has no effect on their physiological inhibitors TIMP-1 or TIMP-2, hence modifying the collagenolytic balance toward MMP production and activation [19, 20]. Our results show that increased EMMPRIN expression in PCMM tissues has a trend toward the positive correlation with MMP-2 expression. Besides, there is a distinct positive correlation between EMMPRIN expression and clinicopathological features, which are related to tumor progression. These findings may reflect the multifunctional role of the EMMPRIN protein in PCMM, not only as a cell–cell adhesion molecule involved in cell–cell and cell–ECM interactions, but also as a mediator of tumor invasion via stimulating MMPs. These results are consistent with recent reports concerning the role of EMMPRIN, from an MMP stimulator to an angiogenic promoter [7, 21, 22].
MMPs, particularly MMP-2, are a group of zinc-dependent endopeptidases capable of degrading the extracellular matrix of the parenchymal and vascular basement membranes [23]. MMP-2 expression was found to be correlated with activation of extracellular signal-regulated kinase (ERK), with which EMMPRIN expression strongly correlates [24]. While fibroblasts normally constitutively express MMPs at low levels, dysregulated MMP expression and activation aids in cancer cell migration. It has been widely documented that tumorigenic cells expressing EMMPRIN induce MMP expression by neighboring fibroblasts [25, 26]. Of the MMPs induced by EMMPRIN, malignant progression has been most closely correlated with the expression of MMP-2 in several forms of cancer, and these increased levels are typically indicative of poor prognostic outcome [27]. Antibodies to EMMPRIN have potential anti-cancer therapeutic utility and have been shown to decrease MMP expression leading to an inhibition of tumor cell invasion [28]. In our series of patients with PCMM, statistic analysis shows that positive expression of EMMPRIN and MMP-2 in tumor cells has significant correlation with poor prognosis. These findings further confirm previous reports that EMMPRIN and MMP-2 immunoreactivities are most intense in the cells of the tumorous tissues and their positive expression shows the invasive potential and significant correlation with poor survival [29].
The present findings show that the co-expression of EMMPRIN and MMP-2, independent of other factors, is significantly associated with worse outcome in patients with PCMM. EMMPRIN/MMP-2 may be considered an important early prognostic marker, which may aid patient selection for adjuvant therapies and/or represent a valid target for treatment, as it appears to be related to the pathogenesis and progression of PCMM.
Abbreviations
- PCMM:
-
Primary cutaneous malignant melanoma
- EMMPRIN:
-
Extracellular matrix metalloproteinase inducer
- MMPs:
-
Matrix metalloproteinases
- ECM:
-
Extracellular matrix
References
Veit-Haibach P, Vogt FM, Jablonka R, et al. Diagnostic accuracy of contrast-enhanced FDG-PET/CT in primary staging of cutaneous malignant melanoma. Eur J Nucl Med Mol Imaging. 2009;36:910–8.
Fang X, Zhang X, Li J. Up-regulation of human leukocyte antigen G expression in primary cutaneous malignant melanoma associated with host-vs-tumor immune response. J Huazhong Univ Sci Technolog Med Sci. 2008;28:219–21.
High WA, Stewart D, Wilbers CR, et al. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53:89–100.
Biswas C, Zhang Y, DeCastro R, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–9.
Li RS, Huang L, Guo HM, et al. Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J Cell Physiol. 2001;186:371–9.
Zucker S, Hymowitz M, Rollo EE, et al. Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am J Pathol. 2001;158:1921–8.
Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99:520–8.
Muramatsu T, Miyauchi T. Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol. 2003;18:981–7.
Gu J, Zhang C, Chen R, et al. Clinical implications and prognostic value of EMMPRIN/CD147 and MMP2 expression in pediatric gliomas. Eur J Pediatr. 2009;168:705–10.
Jia L, Xu H, Zhao Y, et al. Expression of CD147 mediates tumor cells invasion and multidrug resistance in hepatocellular carcinoma. Cancer Invest. 2008;26:977–83.
Zhong WD, Han ZD, He HC, et al. CD147, MMP-1, MMP-2 and MMP-9 protein expression as significant prognostic factors in human prostate cancer. Oncology. 2008;75:230–6.
Tan H, Ye K, Wang Z, Tang H. CD147 expression as a significant prognostic factor in differentiated thyroid carcinoma. Transl Res. 2008;152:143–9.
Yuichiro Y, Kageshita T, et al. Clinical relevance of serum levels of matrix metallopeptidase-2, and tissue inhibitor of metalloproteinase-1 and -2 in patients with malignant melanoma. J Dermatol. 2008;35:206–14.
Breslow A. Thickness, cross-sectional area, and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902–8.
Clark WH, From L, Bernadino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705–26.
American Joint Committee on Cancer. Malignant melanoma of the skin. In: Fleming I, Cooper J, Henson D, editors. AJCC cancer staging manual. 5th ed. Philadelphia: Lippincott-Raven; 1997. p.163-70.
Ishibashi Y, Matsumoto T, Niwa M, et al. CD147 and matrix metalloproteinase-2 protein expression as significant prognostic factors in esophageal squamous cell carcinoma. Cancer. 2004;101:1994–2000.
Taylor PM, Woodfield RJ, Hodgkin MN, et al. Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene. 2002;21:5765–72.
Guo H, Zucker S, Gordon MK, et al. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem. 1997;272:24–7.
Sameshima T, Nabeshima K, Toole BP, et al. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett. 2002;157:177–84.
Toole BP. EMMPRIN (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol. 2003;54:371–89.
Tang Y, Nakada MT, Kesavan P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–9.
Nelson AR, Fingleton B, Rothenberg ML, et al. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–49.
Lim M, Martinez T, Jablons D, et al. Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett. 1998;441:88–92.
Biswas C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun. 1982;109:1026–34.
Biswas C. Collagenase stimulation in cocultures of human fibroblasts and human tumor cells. Cancer Lett. 1984;24:201–7.
Morgia G, Falsaperla M, Malaponte G, Madonia M, Indelicato M, Travali S, et al. Matrix metalloproteinases as diagnostic (MMP-13) and prognostic (MMP-2, MMP-9) markers of prostate cancer. Urol Res. 2005;33:44–50.
Redondo P, Lloret P, Idoate M, Inoges S. Expression and serum levels of MMP-2 and MMP-9 during human melanoma progression. Clin Exp Dermatol. 2005;30:541–5.
Sier CF, Zuidwijk K, Zijlmans HJ, et al. EMMPRIN-induced MMP-2 activation cascade in human cervical squamous cell carcinoma. Int J Cancer. 2006;118:2991–8.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, T., Zhu, J. Evaluation of EMMPRIN and MMP-2 in the prognosis of primary cutaneous malignant melanoma. Med Oncol 27, 1185–1191 (2010). https://doi.org/10.1007/s12032-009-9357-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-009-9357-y