Abstract
Vasiformation is essential for the growth and metastasis of tumor. Vascular endothelial growth factor (VEGF) and connexin43 (Cx43) are important regulatory factors of vasiformation. This study aimed to find out the expression features of VEGF and Cx43 and their significance in pancreatic cancer. The expression levels of VEGF and Cx43 protein in the samples, which came from 100 patients of human pancreatic cancer tissues and adjacent normal pancreatic tissues, were examined by using immunohistochemical streptavidin–peroxidase (S–P) method, Western-blotting, and RT–PCR analyses. Compared with adjacent normal pancreatic tissues, RT–PCR showed that the expression of VEGF mRNA was significantly higher in pancreatic cancer tissues (0.788 ± 0.290, P < 0.01) and Cx43 mRNA was significantly lower in pancreatic cancer tissues (0.403 ± 0.204, P < 0.01). The expression of VEGF protein was higher in pancreatic cancer tissue (0.745 ± 0.254, P < 0.01) by Western-blot, and Cx43 protein was obviously lower in pancreatic cancer tissues (0.373 ± 0.164, P < 0.01). The immunohistochemical S–P method showed as follows: the positive expression rate of VEGF and Cx43 protein was 77 and 48% in pancreatic cancer tissues and 15 and 100% in adjacent normal pancreatic tissues; expressions of VEGF were related to tumor size, TNM stage, and lymph node metastasis (P < 0.05); there was a close relation between the expression of Cx43 and histological grades, TNM stage, and lymph node metastasis (P < 0.05). This present study suggests that VEGF is overexpressed and the expression of Cx43 is lower in pancreatic cancer. The expression of VEGF and Cx43 is significantly correlated with TNM stage and lymph node metastasis. Furthermore, VEGF and Cx43 may play an important role in the occurrence, development, and metastasis of pancreatic cancer. To examine VEGF and Cx43 may be of value in judging the malignancy degree and the prognosis of pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, it is believed that vascularization and the functional disorder of gap junctional intercellular communication (GJIC) which is mediated by connexin (Cx) on cytomembrane correlate with the occurrence, development and metastasis of human tumor [1–4]. Vascular endothelial growth factor (VEGF) plays an important role in the new vascularization of tumor [5–7]. Many species of malignant cells show up when their gap junction frameworks and functions are descent or absence, expression of connexin43 (Cx43) is also descent [8, 9]. Studies of VEGF and Cx43 expression in pancreatic cancer tumor tissue are seldom undertaken in recent years. We use immunohistochemical streptavidin–peroxidase (S–P) method, Western blot, and RT–PCR to detect the expression of VEGF and Cx43 in 100 patients with pancreatic cancer tissues and adjacent normal pancreatic tissues.
Methods
Material
From January 2004 to December 2008, one hundred patients with pancreatic cancer tissues who had unabridged case file were collected from the Department of Surgery in Affiliated Hospital of Guangdong Medical College. All of them were pathologic diagnosis and classified according to “The Diagnosis and Treatment Criterion for Common Cancer in China”, and none of the patients received chemotherapy or radiotherapy before surgical therapy. All specimens were stored at −196°C liquid nitrogen after being collected. The control group was normal tissues that parted from pancreatic cancer tissues about 5 cm. These patients included 27 with poorly differentiated adenocarcinoma, 41 with moderately differentiated adenocarcinoma, and 32 with well-differentiated adenocarcinoma. Among these cases, 19 were stage Ι, 27 were stage II, 34 were stage Ш, and 20 were stage IV, 61 were male, and 39 were female. The range of age was 21–76 years with a median age of 53. Mouse anti-human VEGF monoclonal antibody and S–P kit were purchased from Fuzhou Maixin Bio-tech Ltd, Fuzhou, China; Mouse anti-human Cx43 antibody was purchased from Wuhan Boster Bio-tech Ltd, Wuhan, China. Trizol kit, one-step RT–PCR kit and Western-blot relational kit were purchased from Invitrogen Bio-tech Ltd USA, and TaKaRa Bio-tech Ltd, Dalian, China, and Beyotime Bio-tech Ltd, Haimen, China.
RNA extraction and RT–PCR assay
The total RNA was extracted from pancreatic cancer tissue and adjacent normal pancreatic tissue by using Trizol kit, according to the manufacturer’s instruction. The concentration of total RNA was assayed by spectrophotometry (ODV = 1.826 and 1.910), and the integrity of RNA was determined by agarose gel electrophoresis. Two micrograms of total RNA was reversely transcribed using AMV reverse transcriptase. And the synthesis of cDNA was amplified by PCR. β-Actin served as the internal reference. The primers of VEGF, Cx43, and β-actin, which were synthesized by TaKaRa Bio-tech Ltd (Dalian, China), were shown in Table 1. The PCR conditions for VEGF and Cx43 were 30 cycles of 94°C (2 min), 94°C (45 min), 55°C (45 min), and 72°C (1 min). The final extension was at 72°C for 5 min. The internal reference was carried out in the same system, and the conditions were similar to VEGF and Cx43. The produces of RT–PCR were analyzed by agarose gel electrophoresis (1.5%), scanned by ultraviolet photometry (UVP), and their gray value was determined. The relational expression level of VEGF and Cx43 mRNA was calculated according to the proportionality of VEGF/Cx43 and β-actin (gray value).
Immunohistochemistry detection and assay
All of the specimens were fixed using 10% formaldehyde. Specimen sections were embedded by common paraffinum and observed by hematoxylin and eosin staining (H&E). Tumor histology was classified according to “The International Classified Criterion for Pancreatic Cancer Histology”. Serial sections of 4–5 μm were prepared from the cut surface of paraffin-embedded blocks. These sections separated paraffinum from water, and immunohistochemical staining was carried out by the standard S–P technique, and experimental procedure was conducted according to the S–P kit specification. Using the known sections as the positive substitution control and PBS-replaced primary antibody as the negative control. To judge the expression of VEGF and Cx43, cells whose cytomembrane and cytoplasm were dyed brown granula were regarded as positive ones by light microscope, positive cells were less than 10% of the whole visual field as negative response under high power lens were considered as negative and more than 10% as positive response.
Western-blot assay
The total protein extracted from pancreatic cancer tissues and adjacent normal pancreatic tissues were prepared using a lysis buffer. The protein concentration was detected by Bradford procedure (Bio-Rad) and was subjected to SDS–PAGE gel electrophoresis. After transferring to nitrocellulose membranes for 1 h, the membranes were blocked with 5% skimmed milk for 1 h. The membranes were labeled with primary antibody (mouse anti-human Cx43 antibody, mouse anti-human VEGF antibody, and anti-β-actin antibody) in suitable dilutions for 1 h at room temperature or overnight at 4°C, washed and treated with the HRP-conjugated secondary antibody (1:1,000 dilution) for 1 h at 4°C and added Western eluant 3 times. The protein was detected by BeyoECL kit (Beyotime, China), according to the manufacturer’s instruction. The expression of protein band was visualized by using enhanced chemiluminescence. The interest protein and inner reference β-actin were determined by using Kodak software package for the analysis of 2-D gel, and optical density value (ODV) was detected. The relational expression level of VEGF and Cx43 protein was calculated according to the proportionality of VEGF/Cx43 and β-actin (ODV).
Statistical analysis
All statistical analyses were performed using the SPSS 13.0 software package for Macintosh. Variables associated with VEGF and Cx43 expression were analyzed by the chi-square test, t’-test, and t-test. P < 0.05 was considered significant, and P < 0.01 was considered remarkably significant.
Results
The expression of VEGF and Cx43 mRNA and protein
The study showed that the expression of VEGF mRNA and protein was significantly higher in pancreatic cancer tissues than in adjacent normal pancreatic tissues (P < 0.05), and the expression of Cx43 mRNA and protein was obviously lower (P < 0.05). The results were shown in Tables 2 and 3.
The expression of VEGF/Cx43 and the relationship between them and clinical feature in pancreatic cancer
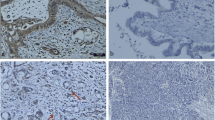
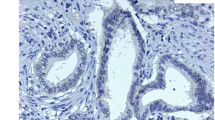
The expression of Cx43 protein was dyed brown granula on cytomembrane in normal pancreatic tissue. But it was expressed mainly in cytoplasm and was few dyed brown granula on cytomembrane in pancreatic cancer tissues (Fig. 1). The expression of VEGF protein was dyed brown granula in cytoplasm in pancreatic cancer tissues (Fig. 2).
The positive expression rate of VEGF protein was 77% (77/100)in pancreatic cancer tissues and 15% (15/100)in normal tissues (P < 0.01); the positive expression rate of Cx43 protein was 48% (48/100) in pancreatic cancer tissues and 100% (100/100) in normal tissues (P < 0.01).
The expressions of VEGF were related to tumor size, TNM stage, and lymph node metastasis (P < 0.05), and there was a close relation between the expression of Cx43 and histological grades, TNM stage, and lymph node metastasis (P < 0.05). The relationship between the expression of VEGF in pancreatic cancer tissues and clinicopathologic factors was given in Table 4, and Cx43 was listed in Table 5.
Discussion
As we know, vascular endothelial growth factor (VEGF) is the strongest, highest-specific angiogenesis factor and plays a vital role in tumor vascularization [10], and it is excreted more by tumor cell, labrocyte, and macrophage of tumor invasion in tumor tissues. It stimulates endothelial cells, promotes their cell proliferation, migration, and induces vasifaction by paracrine; it promotes constant tumor cell growth and enhances vasopermeability which causes fibrin deposition of surrounding tissue; it also helps in histo-leukocyte, fibroblast, and endothelial cell’s infiltration, which contributes to the formation of tumor’s groundplasm and the entry of tumors cell to new vessels. All these promote tumor metastasis [11–13]. Many researchers had found that VEGF overexpressed in a wide variety of tumor cells.
Pancreatic cancer is one of the solid tumors; new vasifaction ensures growth and metastasis. VEGF is one of the important expression products for inducing new vasifaction. In a great degree, the expression rate of VEGF can reflect new vasifaction in solid tumors.
In our study, we found out that the expression of VEGF on mRNA and protein levels in pancreatic cancer tissues was obviously higher than in adjacent normal pancreatic tissues, which suggested that the expression of VEGF plays an important role in the occurrence of pancreatic cancer. And the expression intensity of VEGF was related to tumor size, TNM stage, and lymph node metastasis. The result was similar to other researchers’ results. The result showed that VEGF was an important media for vasifaction and metastasis of tumor. We found the expression rate of VEGF ascended along with the progression of pancreatic cancer. It prompts us to believe that the progression of pancreatic cancer needs abundant new vasifactions for offering oxygen and nutrient substance. VEGF should be an important index to reflect the biological behavior of pancreatic cancer.
Gap junction (GJ) is an important framework where intercellular nutrient substance, metabolite, and signaling molecules are exchanged. It is composed of six connexins (Cx). GJ promotes cellular metabolism, stabilization of internal environment and controls cellular proliferation and differentiation by gap junctional intercellular communication (GJIC) which is mediated by Cx [14]. Many scholars [3, 15, 16] believe that the descent or absence of the expression of Cx causes the inhibition of GJIC function which induces the change in cellular internal environment and loss of control of cell’s proliferation, differentiation, and finally causes tumor development.
Tumor metastasis is a basic biological characteristic of malignant tumor. Compared with normal tissue’s stickiness, malignant tumor tissue’s stickiness is weaker. The lower stickiness is caused by the loss of GJ or the attenuation of GJIC in cells, and the attenuation of GJIC function causes loss of normal information control, contact inhibition, and density-dependent growth in tumor cells. It also causes the loss of organic integrity control action. As a result, tumor cells grow abnormally and induce the augmentation of local tension, which easily causes cellular defluvium and promote infestation of groundplasm gap [17, 18]. It is found that the abnormal expression of Cx and the defect in GJIC function are universal existence in tumor cells and transformants [19, 20]. Cx43 is an important factor of Cx, which not only has the function of Cx, but also is considered an anti-oncogene, whose decrease or loss of expression is closely related to tumor’s genesis, development, and metastasis. Many tumor cells such as glioma, lung cancer, breast cancer, prostatic carcinoma show decreased or loss of Cx43 expression and the GJIC function [21, 22]. Therefore, as a tumor suppressor, the expression of Cx43 can prompt malignant tumor’s invasive degree and assesses the prognosis.
In the study, we used three methods (RT–PCR, Western-blot, and immunohistochemical method) to detect the expression of Cx43. The result showed that the expression of Cx43 on mRNA and protein levels both lower in pancreatic cancer tissue. The result is consistent with the function of Cx43 in tumor cells. It verifies that Cx43 plays a negative interaction in pancreatic cancer. The result also prompts that pancreatic cancer tissues existed as a cause for the abnormal GJIC function. The expression of Cx43 was related to pathologic classification, TNM stage, and lymph node metastasis in pancreatic cancer tissues. With the development of tumor, we found that the expression of Cx43 gradually decreased. The difference in positive rate was statistically significant between stage Ι–II and stage Ш–IV. Compared with the expression of Cx43 in well-differentiated and moderately differentiated pancreatic cancer tissues, the expression of Cx43 was lower in poorly differentiated tissues. The result indicated that the decreased expression of Cx43 promoted the development of pancreatic cancer. The difference in the positive rate was remarkably significant between lymph node metastasis and no lymph node metastasis in our study. It should be showed that the decreased expression of Cx43 induced tumor cells to elude the control of normal growth and the immune surveillance of host which caused pancreatic cancer cells to diffuse and metastasize from distant places easily, and Cx43 should be one of the factors to affect lymph node metastasis of pancreatic cancer.
The study certified that VEGF and Cx43 played an important role in the genesis and development of pancreatic cancer. They should be a new biological marker to forecast the malignancy potency and prognosis of pancreatic cancer.
References
Simon KW, Roberts PC, Vespremi MJ, Manchen S, Schmelz EM. Regulation of beta-catenin and connexin-43 expression: targets for sphingolipids in colon cancer prevention. Mol Nutr Food Res. 2009;53(3):332–40. PMID: 17627324.
Upham BL, Blaha L, Babica P, Park JS, Sovadinova L, Pudrith C, et al. Tumor promoting properties of a cigarette smoke prevalent polycyclic aromatic hydrocarbon as indicated by the inhibition of gap junctional intercellular communication via phosphatidylcholine-specific phospholipase C. Cancer Sci. 2008;99:696–705. PMID: 18377422.
Sato H, Hagiwara H, Ohde Y, Senba H, Virgona N, Yano T. Regulation of renal cell carcinoma cell proliferation, invasion and metastasis by connexin 32 gene. J Membr Biol. 2007;216:17–21. PMID: 17565422.
Udaka N, Miyagi Y, Ito T. Connexin expression in mouse lung tumor. Cancer Lett. 2007;246:224–9. PMID: 16580773.
Duncan TJ, Al-Attar A, Rolland P, Scott IV, Deen S, Liu DT, et al. Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies? Clin Cancer Res. 2008;14:3030–5. PMID:18483368.
Wong ET, Brem S. Antiangiogenesis treatment for glioblastoma multiforme: challenges and opportunities. J Natl Compr Canc Netw. 2008;6:515–22. PMID: 18492463.
Tsirlis TD, Papastratis G, Masselou K, Tsigris C, Papachristodoulou A, Kostakis A, et al. Circulating lymphangiogenic growth factors in gastrointestinal solid tumors, could they be of any clinical significance? World J Gastroenterol. 2008;14:2691–701. PMID:18461654.
Wu J, Zhou HF, Wang CH, Zhang B, Liu D, Wang W, et al. Decreased expression of Cx32 and Cx43 and their function of gap junction intercellular communication in gastric cancer. Zhonghua Zhong Liu Za Zhi. 2007;29:742–7. (in Chinese) PMID: 18396685.
Avanzo JL, Mennecier G, Mesnil M, Hernandez-Blazquez FJ, Fukumasu H, da Silva TC, et al. Deletion of a single allele of Cx43 is associated with a reduction in the gap junctional intercellular communication and increased cell proliferation of mouse lung pneumocytes type II. Cell Prolif. 2007;40(3):411–21. PMID:17531084.
Breen E, Tang K, Olfert M, Knapp A, Wagner P. Skeletal muscle capillarity during hypoxia: VEGF and its activation. High Alt Med Biol. 2008;9:158–66. PMID: 18578647.
Oh SY, Kwon HC, Kim SH, Jang JS, Kim MC, Kim KH, et al. Clinicopathologic significance of HIF-1alpha, p53, VEGF expression, preoperative serum VEGF level in gastric cancer. BMC Cancer. 2008;8:123. PMID: 18452596.
Noma K, Smalley KS, Lioni M, Naomoto Y, Tanaka N, El-Deiry W, et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–93. PMID: 18439605.
Mimura K, Kono K, Takahashi A, Kawaguchi Y, Mizukami Y, Fujii H. Vascular endothelial growth factor partially inhibits the trastuzumab-mediated antibody-dependent cellular cytotoxicity of human monocytes. Oncology. 2007;72:172–80. PMID: 18097168.
De Maio A, Vega VL, Contreras JE. Gap junctions, homeostasis, and injury. Cell Physiol. 2002;191:269–82. PMID: 12012322.
Yano T, Fujimoto E, Hagiwara H, Sato H, Yamasaki H, Negishi E, et al. Connexin 32 as an anti-invasive and anti-metastatic gene in renal cell carcinoma. Biol Pharm Bull. 2006;29:1991–4. PMID: 17015938.
Zhang XF, Ren ZY, Fang FD, Zuo J, Su CB, Wang RZ, et al. Synergistic effect of all-trans retinoic acid and herpes simplex virus thymidine kinase gene on glioma. Ai Zheng. 2002;21:473–9. (in Chinese) PMID: 12452035.
Pollmann MA, Shao Q, Laird DW, Sandig M. Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Res. 2005;7:R522–34. PMID: 15987459.
Zhang ZQ, Hu Y, Wang BJ, Lin ZX, Naus CC, Nicholson BJ. Effective asymmetry in gap junctional intercellular communication between populations of human normal lung fibroblasts and lung carcinoma cells. Carcinogenesis. 2004;25:473–82. PMID: 14656943.
Peebles KA, Duncan MW, Ruch RJ, Malkinson AM. Proteomic analysis of a neoplastic mouse lung epithelial cell line whose tumorigenicity has been abrogated by transfection with the gap junction structural gene for connexin 43, Gja1. Carcinogenesis. 2003;24:651–7. PMID:12727792.
Willenberg HS, Schott M, Saeger W, Tries A, Scherbaum WA, Bornstein SR. Expression of connexins in chromaffin cells of normal human adrenals and in benign and malignant pheochromocytomas. Ann N Y Acad Sci. 2006;1073:578–83. PMID:17102126.
Kawasaki Y, Kubomoto A, Yamasaki H. Control of intracellular localization and function of Cx43 by SEMA3F. J Mebr Biol. 2007;217:53–61. PMID: 17665084.
Hernandez M, Shao Q, Yang XJ, Luh SP, Kandouz M, Batist G, et al. A histone deacetylation-dependent mechanism for transcriptional repression of the gap junction gene cx43 in prostate cancer cells. Prostate. 2006;66:1151–61. PMID: 16652385.
Acknowledgments
We appreciate the help from Dr. Han-Guo Jiang, Department of Pathology, Guangdong Medical College.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, QL., Wang, BR., Chen, GQ. et al. Clinical significance of vascular endothelial growth factor and connexin43 for predicting pancreatic cancer clinicopathologic parameters. Med Oncol 27, 1164–1170 (2010). https://doi.org/10.1007/s12032-009-9354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-009-9354-1