Abstract
The objective of this study was to investigate the expressions of angiogenic factors and elucidate their angiogenic and prognostic roles in hepatocellular carcinoma (HCC) with background of hepatitis B virus (HBV). We evaluated microvessel density (MVD) of HCC, and investigated immunohistochemical expression of vascular endothelial growth factor (VEGF), angiopoietins (Ang-1 and Ang-2), and matrix metalloproteinases-9 (MMP-9) in 67 specimens of surgically resected HCC, which were all positive for hepatitis B surface antigen. We investigated the relationship between their expressions and clinicopathological factors or prognosis. The microvessel density (MVD) of tumor tissue and surrounding normal liver tissue was 93.1 ± 43.8/mm2 and 30.4 ± 14.8/mm2, respectively. The MVD of well-differentiated HCC was significantly less than that of poorly differentiated HCC. MVD was positively correlated with VEGF and Ang-2 expression (P = 0.0023 and 0.0265, respectively). There was less tumor recurrence in low Ang-2 and low MMP-9 group than high Ang-2 and/or high MMP-9 group (P = 0.002). In Cox regression model, portal vein thrombus and intrahepatic metastasis was the risk factors of tumor recurrence (P = 0.003 and 0.001, respectively). Our study showed that the expression of VEGF and Ang-2 were positively correlated with MVD. Ang-2 expression and/or MMP-9 expression might be a significant predictive factor for recurrence after resection in HCC patients with the background of HBV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) has the characteristics of vascular invasion and intrahepatic metastasis. There are accumulating evidences that tumor angiogenesis plays an important role in the progression and metastasis of HCC, and a wide variety of angiogenic factors involved in this process [1–6]. Among the identified angiogenic factors, vascular endothelial growth factor (VEGF) is a potent angiogenic factor that plays a critical role in mediating angiogenesis in HCC [1, 7]. The VEGF can function on various types of cells, such as endothelial cells [8], hepatic stellate cells [9], endothelial progenitor cells, and hemangiocytes [10], to induce vascular changes in HCC. Therefore, blockade of VEGF-mediated pathways, either by anti-VEGF neutralizing antibody or tyrosine kinase inhibitors that target VEGF receptors, suppresses carcinogenesis and angiogenesis in HCC [11]. In addition to VEGF, several other angiogenic factors in HCC have recently been identified. Angiogenetin (Ang-1 and Ang-2), the ligands for Tie2, play critical roles in angiogenesis in concert with VEGF [2]. Ang-1 binds to Tie2 and maintains and stabilizes mature vessels. Meanwhile, Ang-2 competitively binds to Tie2 and antagonizes the stabilizing action of Ang-1, which results in destabilization of vessels. In the presence of VEGF and ang-2, the destabilized vessels may undergo angiogenic changes [2, 12]. Matrix metalloproteinases-9 (MMP-9) specially degrades extracellular matrix proteins and is involved in tissue remodeling and angiogenesis [5, 6]. Recent studies have shown that the synergistically proangiogenic activity of Ang-2 and VEGF was at least partly mediated via induction of MMP-9 in HCC [3]. In recent study, antiangiogenic therapy has already entered clinical trials in HCC patients and promising antitumor activity has been observed [13].
Microvessel density (MVD) of tumor is effective to evaluate new vascularity of tumor. However, the relationship of MVD of HCC and clinicopathological features and postoperative survival is still controversial [7, 14, 15]. In this article, we will discuss the MVD of HCC and clinicopathological features in our group of patients, and investigate the relationship between MVD and angiogenic factors. In addition, the prognostic significance of MVD, VEGF, Ang-1, Ang-2 and MMP-9 will also be investigated.
Materials and methods
Patients
Fresh surgically resected specimens of HCC and noncancerous liver tissue were obtained from 67 patients with HCC who underwent partial hepatectomy in Renming Hospital, Wuhan University, from 2000 to 2005. The medium age was 63 years (range: 44–79 years). There were 67 patients who were positive for hepatitis B surface antigen. Macroscopically, 20 cases were well differentiated, 40 cases were moderately differentiated, and seven cases were poorly differentiated. About 53 patients underwent curative resection, and 14 cases underwent palliative resection. Resected HCC specimens were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylineosin for pathological examination. Histological grade was classified according to the criteria of Edmondson and Steiner.
Microvessel density (MVD) determination
MVD was assessed based on the method as Weidner recommended [16]. After immunostaining with anti-CD34 antibody (mouse monoclonal IgG2a), large and small microvessels and single brown immunostained endothelial cells separate from adjacent microvessels and stromal structures were included in the microvessel count. A KS-400 image analyzing system (Carl Zeiss Vision GmbH, Hallbermoss, Germany) was used for this study. The MVD count was calculated as the number of microvessels found within 1 mm2 of the microscopic field under a magnification of ×100. For each area, the average MVD of the five highest MVD counts was recorded as the MVD of that area. The MVD of the tumor was thus calculated as the average MVD of five highest MVD counts within the tumor.
Immunohistostaining
Formalin-fixed, paraffin-embedded specimens of tumors and associated periphery were selected for analysis. Sections measuring 4 μm in thickness were deparaffinized in xylene, rehydrated, and stained with hematoxylin-eosin solution for histopathological examination. After deparaffinization in xylene and rehydration in a graded series of ethanol, immunohistochemical procedures were performed using the Vectastain ABC peroxidase kit (Vector Laboratories, Burlingame, CA). Briefly, sections were treated with an antigen retrieval procedure in 0.01 M sodium citrate buffer (pH 6.0) for 40 min at 95°C and incubated in methanol containing 0.3% hydrogen peroxide at room temperature for 20 min to block endogenous peroxidases. The sections were then incubated with normal protein block serum solution at room temperature for 20 min to block nonspecific staining, followed by overnight incubation at 4°C with anti-VEGF (rabbit polyclonal IgG, Santa Cruz Biotechnology, Santa Cruz, CA), anti-angiopoietin-1 (goat polyclonal IgG, Santa Cruz Biotechnology), anti-angiopoietin-2 (goat polyclonal IgG, Santa Cruz Biotechnology), and anti-MMP-9 (rabbit polyclonal IgG, Santa Cruz Biotechnology). Sections were washed three times for 5 min in phosphate-buffered saline (PBS) before incubation at room temperature for 20 min with a biotin-conjugated secondary antibody (goat anti-rabbit for VEGF and MMP-9, rabbit anti-goat for Ang-1 and Ang-2), and finally with peroxidase-conjugated streptavidin at room temperature for 20 min. The peroxidase reaction was developed with 3,3′-diaminobenzidine tetrachloride. The sections were counterstained with Meyer’s hematoxylin. Other sections treated with Tris-buffered saline instead of the primary antibody served as the negative control.
The intensity of immunohistochemical staining for VEGF, Ang-1, Ang-2, and MMP-9 in these fields was scored on a scale ranging from level 0 to 3. Level 0–3 represented negative, faint, moderate, and strong staining, respectively. The vascular epithelium in the normal liver field of the same specimen expressed faint levels of Ang-1, Ang-2, and MMP-9 and the bile duct epithelium in the normal liver field of the same specimen expressed faint levels of VEGF. Accordingly, these levels of staining were used as an internal control within the sample, which was arbitrarily designed as intensity level 1.
Statistical analysis
Unless otherwise indicated, numerical data are presented as mean ± SD. Differences in the proportions of categorical data were tested using the Chi-square test. Unless otherwise indicated, differences in mean values of numerical data were tested using the two-tailed Student’s t-test. Survival was assessed using the Kaplan–Meier method, and comparisons were made using the log-rank test. A P value < 0.05 denoted the presence of a statistically significant difference. All analyses were performed using SPSS statistical software (version 11.0, Chicago, IL).
Results
The distribution pattern of angiogenesis in tumor
The tumor region and surrounding normal liver tissue were included in the assessment of all HCC cases evaluated in this study. Within HCC, condensed, short, and twisted blood vessels were observed. There were not more condensed CD34 staining in the peripheral region in comparison with central and intermediate regions of tumor (Fig. 1a). In the surrounding normal liver tissue, positive CD34 staining was also observed in the Glisson’s triangle and the central veins, which was not twisted and condensed as those within the tumor (Fig. 1b).
a The CD34-stained photomicrography of HCC tissue sample. The micro blood vessels are condensed, short and twisted with an even distribution pattern. Original magnification, ×100. b The CD34 stain photomicrography of surrounding normal liver tissue. The micro blood vessels of Glisson’s triangle are round and not twisted compared to blood vessels in tumor. Original magnification, ×100
MVD inside tumor and pathoclinical meanings
The MVD of tumor tissue (93.1 ± 43.8/mm2; range, 10–229/mm2) was significantly higher than that of the surrounding normal liver tissue (30.4 ± 14.8/mm2; range, 14–109/mm2). We did not observe the relationship of MVD with liver cirrhosis, tumor size, portal vein invasion, and intrahepatic metastasis. But we found that the MVD of well-differentiated HCC was significantly less than that of poorly differentiated HCC. The MVD was 40.2 ± 25.9/mm2 for well-differentiated HCC, 98.5 ± 40.5/mm2 for moderately differentiated HCC, and 105.9 ± 43.7/mm2 for poorly differentiated HCC (Table 1).
The expression of VEGF, Ang-1, Ang-2, and MMP-9
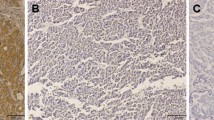
Immunohistochemical staining was assessed by two investigators independently without knowledge of the corresponding clinicopathological data. In case of disagreement, a pathologist joined evaluation and resolved disagreement. VEGF, Ang-1, Ang-2, and MMP-9 immunoreactivity was found in both the cytoplasm of cancer cells and the hepatocytes of adjacent liver tissue. Usually, the expression of VEGF, Ang-2, and MMP-9 was considerably less intense in hepatocytes than that in tumor (Fig. 2). VEGF expression was not detected or faintly stained in 13 cases, moderately stained in 41 cases, and strongly stained in 13 cases. Ang-1 expression was not detected or faintly stained in 21 cases, while 38 cases showed moderate staining and eight cases showed strong staining. Ang-2 expression was not detected or faintly stained in 21 cases, moderately stained in 33 cases and strongly stained in 13 cases. MMP-9 expression was not detected or faintly stained in 12 cases, while 27 cases showed moderate staining and 28 cases showed strong staining (Table 2).
a The anti-VEGF stained photomicrography of tissue sample of HCC. Positive staining can be found in the cytoplasm of tumor cells. Original magnification, ×400. b The anti-Ang-2 stained photomicrography of tissue sample of HCC. Positive staining can be found in the cytoplasm of tumor cells. Original magnification, ×400. c The anti-Ang-1s stained photomicrography of tissue sample of HCC. Positive staining can be found in the cytoplasm of tumor cells. Original magnification, ×400. d The anti-MMP-9 stained photomicrography of tissue sample of HCC. Positive staining can be found in the cytoplasm of tumor cells and stromal cells. Original magnification, ×400
Relationship of MVD and VEGF, Ang-1, Ang-2, and MMP-9 expression
According to the expression of these factors, the patients were divided into strong expression group (intensity 3), moderate expression group (intensity 2), and weak or negative group (intensity 0/1). For VEGF, the MVD is 133.2 ± 35.5/mm2 in strong expression group, 89.3 ± 39.4/mm2 in moderate expression group, and 58.2 ± 28.9/mm2 in weak or negative expression group, which are significantly different (P = 0.0023). For Ang-2, the MVD is 123.2 ± 36.3/mm2 in strong expression group, 99.5 ± 37.6/mm2 in moderate expression group, and 70.3 ± 42.2/mm2 in weak or negative expression group, which are significantly different (P = 0.0265). For Ang-1 and MMP-9, there is no significant difference among the MVD of strong expression group, moderate expression group, and weak or negative expression group (Table 3).
Relationship of postoperative recurrence and MVD, VEGF, Ang-1, Ang-2, and MMP-9 expression
We subdivided patients into two further groups of high MVD and low MVD based on the median value of MVD (76/mm2). For VEGF, Ang-1, Ang-2, and MMP-9, we subdivided patients into high expression group (intensity 2, 3) and low expression group (intensity 0/1). The postoperative disease-free survival was analyzed with Kaplan–Meier method. For MVD, VEGF, and Ang-1, there is no significant difference between high expression group and low expression group. There is significant difference between high Ang-2 group and low Ang-2 group (log-rank test, P = 0.037) (Fig. 3a). There is also significantly different between high MMP-9 group and low MMP-9 group (log-rank test, P = 0.001) (Fig. 3b). Furthermore, there is less tumor recurrence in low Ang-2 and low MMP-9 group than high Ang-2 and/or high MMP-9 group (log-rank test, P = 0.001) (Fig. 3c). We further analyzed risk factors of tumor recurrence with Cox regression method. Portal vein thrombus (risk ratio 2.8, P = 0.003) and intrahepatic metastasis (risk ratio 3.6, P = 0.001) were the risk factors of tumor recurrence (Table 4). Other factors such as age, sex, tumor size, histological grade, and angiogenic factor did not showe any relationship with tumor recurrence.
Relationship of overall survival and MVD, VEGF, Ang-1, Ang-2, and MMP-9
For MVD, VEGF, Ang-1, Ang-2, and MMP-9, we subdivided patients into high expression group and low expression group. The overall survival was analyzed with Kaplan–Meier method. No significant relationship was noted for any of the five angiogenic factors examined, as well as other clinicopathological factors such as age, sex, tumor size, histological grade, intrahepatic metastasis, and portal vein thrombus (data not shown).
Discussion
Our results showed robust angiogenesis within tumor, and the MVD of well-differentiated HCC was significantly less than that of poorly differentiated HCC. MVD was positively correlated with VEGF and Ang-2 expression. There was less tumor recurrence in low Ang-2 and low MMP-9 group than high Ang-2 and/or high MMP-9 group. In Cox regression model, portal vein thrombus and intrahepatic metastasis were the risk factors of tumor recurrence.
Our results showed that the MVD of HCC is related to the tumor differentiation. HCC with poor differentiation performs a more robust angiogenesis. Contrarily, MVD is not related to other clinicopathological factors such as cirrhosis, tumor size, capsular invasion, capsular formation, portal vein invasion, and intrahepatic metastasis. There is controversy about the relationship between MVD of HCC and biologic characteristics of tumor. One study showed that MVD assessed by immunohistochemical analysis with CD34 antibody was inversely related to tumor size [17]. A recent study showed MVD of HCC was significantly correlated with tumor capsule formation and tumor size [7]. The discrepancy could partly be due to particular tumor biological factors, accompanying liver disease, or small study population. For example, HCC is accompanied by HBV in all the cases in our study, which is significantly different in comparison with Japan and European studies, where HCC is mainly accompanied by HCV [14, 18].
Of the total 67 cases in our study, VEGF was highly expressed in 53 cases, Ang-1 was highly expressed in 46 cases, Ang-2 was highly expressed in 46 cases, and MMP-9 was highly expressed in 55 cases. Our result also showed that the expression of VEGF and Ang-2 of HCC was positively related to the angiogenesis of tumor. The MVD in high expression group of VEGF, as well as Ang-2, was significantly higher than low expression group. Our result demonstrated that tumor cells of HCC highly expressed many angiogenic factors, which play a critical role in the angiogenesis of HCC. Several studies reported similar results of angiogenic factors including Ang-2, MT3-MMP [19], MMP-2, MMP-9 [20], Trefoil peptides-3 (TFF-3) [21], Thrombospondin-1 [22], Basic fibroblast growth factor (bFGF) [23]. VEGF plays a critical role in mediating angiogenesis in HCC [1, 7]. Ang-2 antagonizes the stabilizing action of Ang-1 and results in destabilization of vessels. In the presence of VEGF and Ang-2, the destabilized vessels may undergo angiogenic changes [2, 12]. MMP-9 specially degrades extracellular matrix proteins and is involved in tissue remodeling and angiogenesis [5, 6]. Recent studies have shown that the synergistically proangiogenic activity of Ang-2 and VEGF was at least partly mediated via induction of MMP-9 in HCC [3]. However, further studies are necessary to understand the detailed mechanism of VEGF, Ang-2, and MMP-9 in the angiogenesis of HCC.
In our study, we investigated the relationship between tumor recurrence and MVD, VEGF, Ang-1, Ang-2, and MMP-9. The results showed that Ang-2 and MMP-9 were related to tumor recurrence. But none of these angiogenic factors showed a relationship with total survival time. This results did not mean that angiogenesis is not important for tumor invasion and development. Contrarily, angiogenesis plays a fundamental role in the growth and progression of HCC. MVD might be the result of synthetic actions of many angiogenetic factors and might not relate to biologic characteristics when beyond a threshold value. Other studies showed controversial results about relationship between angiogenic factors and survival times. One study showed that strong Ang-2 expression and/or high nuclear expression of HIF-1∝ was a significant predictive factor for recurrence after curative resection in HCC patients [14]. Another study showed that only the MVD of tumor was significantly correlated with intrahepatic recurrence and disease-free survival [7]. The inconsistency may be caused by particular tumor biological factors, such as the tumor location and accompanying liver disease, or by small study population, different case selection, or heterogeneous therapy patterns.
Today, blockade of VEGF-mediated pathways has already entered clinical trials in HCC patients and promising antitumor activity has been observed. The status of angiogenesis in HCC provides a potential therapeutic target. Our study showed that HCC highly expressed angiogenic factors such as VEGF, Ang-1, Ang-2, and MMP-9. Meanwhile, Ang-2 and MMP-9 were related to tumor recurrence. Further studies are necessary to understand the detailed mechanism of angiogenesis of HCC.
References
Mise M, Arii S, Higashituji H, et al. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology. 1996;23:455–64. doi:10.1002/hep.510230309.
Mitsuhashi N, Shimizu H, Ohtsuka M, et al. Angiopoietins and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology. 2003;37:1105–13. doi:10.1053/jhep.2003.50204.
Yoshiji H, Kuriyama S, Noguchi R, et al. Angiopoietin 2 displays a vascular endothelial growth factor dependent synergistic effect in hepatocellular carcinoma development in mice. Gut. 2005;54:1768–75. doi:10.1136/gut.2005.067900.
Yoshiji H, Kuriyama S, Yoshii J, et al. Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology. 2002;35:834–42. doi:10.1053/jhep.2002.32541.
Sun MH, Han XC, Jia MK, et al. Expressions of inducible nitric oxide synthase and matrix metalloproteinase-9 and their effects on angiogenesis and progression of hepatocellular carcinoma. World J Gastroenterol. 2005;11:5931–7.
Ishii Y, Nakasato Y, Kobayashi S, et al. A study on angiogenesis-related matrix metalloproteinase networks in primary hepatocellular carcinoma. J Exp Clin Cancer Res. 2003;22:461–70.
El-Assal ON, Yamanoi A, Soda Y, et al. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554–62. doi:10.1002/hep.510270613.
Yoshiji H, Kuriyama S, Yoshii J, et al. Vascular endothelial growth factor tightly regulates in vivo development of murine hepatocellular carcinoma cells. Hepatology. 1998;28:1489–96. doi:10.1002/hep.510280607.
Yoshiji H, Kuriyama S, Yoshii J, et al. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52:1347–54. doi:10.1136/gut.52.9.1347.
Young PP, Hofling AA, Sands MS. VEGF increases engraftment of bone marrow-derived endothelial progenitor cells (EPCs) into vasculature of newborn murine recipients. Proc Natl Acad Sci USA. 2002;99:11951–6. doi:10.1073/pnas.182215799.
Yau T, Chan P, Wong H, et al. Efficacy and tolerability of low-dose thalidomide as first-line systemic treatment of patients with advanced hepatocellular carcinoma. Oncology. 2007;72(Suppl 1):67–71. doi:10.1159/000111709.
Sugimachi K, Tanaka S, Taguchi K, et al. Angiopoietin switching regulates angiogenesis and progression of human hepatocellular carcinoma. J Clin Pathol. 2003;56:854–60. doi:10.1136/jcp.56.11.854.
Furuse J, Ishii H, Nakachi K, et al. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008;99:159–65.
Wada H, Nagano H, Yamamoto H, et al. Expression pattern of angiogenic factors and prognosis after hepatic resection in hepatocellular carcinoma: importance of angiopoietin-2 and hypoxia-induced factor-1 alpha. Liver Int. 2006;26:414–23. doi:10.1111/j.1478-3231.2006.01243.x.
Sun HC, Tang ZY, Li XM, et al. Microvessel density of hepatocellular carcinoma: its relationship with prognosis. J Cancer Res Clin Oncol. 1999;125:419–26. doi:10.1007/s004320050296.
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8.
Ng IO, Poon RT, Lee JM, et al. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838–45. doi:10.1309/FXNL-QTN1-94FH-AB3A.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Arai I, Nagano H, Kondo M, et al. Overexpression of MT3-MMP in hepatocellular carcinoma correlates with capsular invasion. Hepatogastroenterology. 2007;54:167–71.
Moon WS, Park HS, Yu KH, et al. Expression of betacellulin and epidermal growth factor receptor in hepatocellular carcinoma: implications for angiogenesis. Hum Pathol. 2006;37:1324–32. doi:10.1016/j.humpath.2006.04.022.
Khoury T, Chadha K, Javle M, et al. Expression of intestinal trefoil factor (TFF-3) in hepatocellular carcinoma. Int J Gastrointest Cancer. 2005;35:171–7. doi:10.1385/IJGC:35:3:171.
Poon RT, Chung KK, Cheung ST, et al. Clinical significance of thrombospondin 1 expression in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4150–7. doi:10.1158/1078-0432.CCR-03-0435.
Poon RT, Ng IO, Lau C, et al. Correlation of serum basic fibroblast growth factor levels with clinicopathologic features and postoperative recurrence in hepatocellular carcinoma. Am J Surg. 2001;182:298–304. doi:10.1016/S0002-9610(01)00708-5.
Acknowledgments
The authors appreciate the contributions of Dr. Dong-Ying Chen (Department of pathology, Sun-Yat-Sen Medical College, Sun-Yat-Sen University) for pathological support during the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, ZB., Shen, SQ., Ding, YM. et al. The angiogenic and prognostic implications of VEGF, Ang-1, Ang-2, and MMP-9 for hepatocellular carcinoma with background of hepatitis B virus. Med Oncol 26, 365–371 (2009). https://doi.org/10.1007/s12032-008-9130-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-008-9130-7