Abstract
Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, has shown clinical activity in metastatic colorectal cancer patients when used as either a first-line or second-line treatment. Here, we evaluated the efficacy and safety of bevacizumab plus FOLFIRI (irinotecan, 5-fluorouracil, and leucovorin) or FOLFOX (oxaliplatin, 5-fluorouracil, and leucovorin) in metastatic colorectal cancer cases after failure to FOLFIRI and FOLFOX. Between October 2004 and February 2007, the data on 42 patients with metastatic colorectal cancer after failure of FOLFIRI and FOLFOX were reviewed retrospectively. All patients were treated with bevacizumab plus FOLFIRI or FOLFOX. The median patient age was 57.0 years. The ECOG performance status was 0 or 1 in 27 patients (64.3%). The number of previous chemotherapy regimens was ≥3 in 35 patients (83.3%). Thirty-nine patients were evaluable for response. Four patients had partial responses (PRs) and no patient had a complete response (CR), giving an overall response rate of 9.5%. Twenty-two patients (52.4%) had stable disease and 13 patients (31.0%) showed progressive disease. With a median follow-up time of 12.9 months (range 1.0–30.0 months), the median progression-free survival time and the median overall survival time were 5.3 and 9.5 months, respectively. Grade 3 or 4 neutropenia developed in 18 patients (42.9%), including febrile neutropenia in 4 patients (9.5%). Common non-hematologic toxicities were fatigue (21.4%), neuropathy (21.4%), and mucositis (21.4%). Grade 2 or 3 hypertension occurred in 4 patients (9.6%), and grade 1 or 2 proteinuria was seen in 16 patients (38.1%). The frequencies of adverse events related BV, such as bleeding, thrombosis, and gastrointestinal perforation, were within the ranges of previous reports. However, there were no treatment-related deaths. The combination of bevacizumab plus FOLFIRI or FOLFOX showed modest activity and was relatively tolerable in patients with metastatic colorectal cancer refractory to both FOLFIRI and FOLFOX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the second leading cause of death from cancer in the United States [1]. In Korea, CRC is the fourth most common malignancy, with 10,000 new cases and 5,000 deaths each year, and the disease is increasing in incidence [2]. About 20% of CRC patients are diagnosed in metastatic stages and, even if curative surgery is performed, about 40% of patients will experience local or distant recurrences [3].
For decades, 5-fluorouracil (5-FU) was the sole active agent for metastatic CRC. The management of patients with metastatic CRC has changed dramatically over the last 5 years, especially, with the introduction of irinotecan and oxaliplatin. Irinotecan and oxaliplatin are widely used in combination with 5-FU and leucovorin as first-line and second-line treatments for metastatic CRC [4–6].
Bevacizumab (BV) is a recombinant human monoclonal antibody targeting vascular endothelial growth factor (VEGF), which is a key mediator of tumor angiogenesis [7, 8]. The addition of BV to IFL (irinotecan, bolus 5-FU, and leucovorin) was associated with a high response rate (RR) as well as a significant longer time to progression (TTP) and overall survival (OS) in CRC patients [9]. In a second-line setting, BV has also shown improved RR, progression-free survival (PFS), and OS, when combined with FOLFOX (oxaliplatin, 5-FU, and leucovorin). However, patients in the BV-only arm showed PFS rates lower than patients in the control (FOLFOX alone) arm (2.7 vs. 4.7 months) [10].
There are no standard treatments for patients with metastatic CRCs that have progressed after treatment with both irinotecan-based and oxaliplatin-based chemotherapy. In addition, there is little information on the use of BV combination chemotherapy in patients who were heavily pretreated with regimens including irinotecan and oxaliplatin. In this article, we report a retrospective survey of the efficacy and safety of BV plus FOLFIRI (irinotecan, 5-FU, and leucovorin) or FOLFOX in consecutive patients with metastatic CRCs that had refractory to both FOLFIRI and FOLFOX.
Methods
Patients
Between October 2004 and February 2007, a total of 65 consecutive patients with metastatic CRC were treated with BV plus chemotherapy as a third or later-line treatment. To be eligible, patients had previously received at least one course of irinotecan-based chemotherapy and at least one course of oxaliplatin-based chemotherapy, and had histologically confirmed Stage IV CRC. Patients were included if progression to both FOLFOX and FOLFIRI was documented during prior chemotherapy or within 3 months thereafter. Other criteria for eligibility were (1) performance scores (PS) of 0, 1, or 2 on the Eastern Cooperative Oncology Group (ECOG) scale and (2) adequate hepatic [bilirubin <2.0 mg/dl, transaminases levels <3 times the upper normal limit (5 times for patients with liver metastasis), and serum albumin of >2.5 mg/dl], renal (creatinine <1.5 mg/dl), and bone marrow functions [absolute neutrophil count (ANC) >1,500/μl, hemoglobin >9.0 g/dl, and platelets >75,000/μl].
Forty-two of the sixty-five patients who received BV containing chemotherapy during this period were deemed to fit our eligibility criteria and were included in this analysis. Of the 23 excluded patients, 8 had no measurable disease, 8 did not have refractoriness both FOLFIRI and FOLFOX, 4 received other combination regimens with BV in third-line settings, and 3 did not have bone marrow or organ function that complied with the present criteria.
Study design and treatment
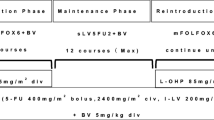
The Institutional Review Board of the Asan Medical Center approved this retrospective study. All patients had previously been intensively treated with combination chemotherapy. We therefore selected BV plus FOLFIRI to avoid cumulative oxaliplatin-induced neuropathy. In patients who had not tolerated irinotecan in previous treatments, we used BV plus FOLFOX.
Among the 42 patients, 18 patients received BV plus FOLFIRI and 24 patients received BV plus FOLFOX. BV (5 mg/kg) was administered intravenously (IV) on day 1 over 90 min, prior to administration of irinotecan and oxaliplatin. FOLFIRI consisted of irinotecan (150 mg/m2) IV over 2 h, and leucovorin (200 mg/m2) IV over 2 h, followed by a 5-FU (400 mg/m2) IV bolus on day 1, and 5-FU (1,200 mg/m2) by continuous IV over 22 h. FOLFOX consisted of oxaliplatin (85 mg/m2) IV over 2 h, and leucovorin (200 mg/m2) IV over 2 h, followed by a 5-FU (400 mg/m2) IV bolus on day 1, and 5-FU (1,200 mg/m2) by continuous IV over 22 h. Patients received BV plus FOLFIRI or BV plus FOLFOX once every 2 weeks. The treatment including BV plus FOLFIRI or FOLFOX was continued until progression.
Evaluation and statistical analysis
Our primary objective was to assess response rate, and secondary objectives were to evaluated OS, PFS, and toxicity. Descriptive statistics were reported as proportion and medians. Tumor responses were assessed by RECIST criteria every 6–8 weeks [11]. Radiologic evaluation consisted of a chest X-ray and an abdominopelvic CT scan. PFS was defined as the time from the commencement of treatment to disease progression or death. OS was defined as the time from the commencement of treatment to death from any cause. Survival curves were estimated using the Kaplan–Meier method. Safety was assessed in terms of toxicity and evaluated based on the NCI Common Toxicity Criteria for Adverse Events (CTCAE), version 3.0. All analyses were performed using SPSS 12.0 for Windows.
Results
Patient characteristics
The median patient age was 57.0 years. The ECOG performance status was 0 or 1 in 64.3% of patients. The number of previous chemotherapy regimens was ≥3 in 35 patients (83.3%). Prior to this study, all patients had progressed during or shortly after FOLFIRI and FOLFOX. No patients were pretreated BV as a first- or second-line treatment (Table 1).
Response to treatment
The median number of cycles of BV treatment was 5.5 (range 1–26). Four patients had partial responses (PRs) and no patient had a complete response (CR), giving an overall response rate of 9.5%. Twenty-two patients had stable disease (SD) (52.4%), and thus the disease was controlled in 26 patients (61.9%) (Table 2).
Survival outcome
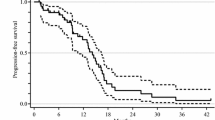
Of the 42 patients, 15 (35.7%) remained alive at a median follow-up time of 12.9 months (range 1–30 months). The median PFS was 5.3 months (range 1.0–13.6 months), the median OS was 9.5 months (range 1.0–25.7 months), and the 1-year OS rate was 31.5% (Fig. 1). Treatment failure was caused by disease progression (86.1%), inability to tolerate treatment (11.1%), or other reason (2.8%).
Safety and toxicity
The 42 patients received 297 cycles of chemotherapy. Grade 3 or 4 neutropenia developed in 18 patients (42.9%), including febrile neutropenia in 4 patients (9.5%). Common non-hematologic toxicities were fatigue (21.4%), neuropathy (21.4%), and mucositis (21.4%). Grade 2 or 3 hypertension occurred in 4 patients (9.6%) and grade 1 or 2 proteinuria was noted in 16 patients (38.1%). Deep vein thrombosis (DVT) and pulmonary embolism (PE) were observed in one patient, respectively. The patient who had DVT was treated without anticoagulation or any intervention (grade 2). While the patient with PE (grade 4) was treated with low molecular weight heparin and bevacizumab was discontinued. Bleeding events (grade 2) developed in two patients (4.8%) and all of them had minor bleeding at suspicious site of locoregional recurrence. There were two reports (4.8%) of gastrointestinal perforation. The presentation of these events varied in type and severity, from the incidental finding of air to perforation with peritonitis and abscess. One case was asymptomatic and the other one that underwent the primary repair of small intestine was recovered without complication. However, there were no treatment-related deaths (Table 3).
Discussion
This study demonstrated that the combination of BV with either FOLFIRI or FOLFOX was associated with an overall response rate of 9.5% in patients with metastatic CRC refractory to both FOLFIRI and FOLFOX. The median PFS and OS values were 5.3 and 9.5 months, respectively.
The combination of 5-FU/leucovorin with either irinotecan or oxaliplatin has been widely used as a first-line or second-line chemotherapy regimen in patients with metastatic CRC [4–6]. When combinations of these three standard drugs have failed, however, there are few accepted treatment options. Only a few studies on third-line or later treatment in patients with metastatic CRC pretreated with 5-FU/leucovorin, irinotecan, and oxaliplatin have been published (Table 4). Mitomycin-based chemotherapy or capecitabine single agent treatment showed minimal activities in these settings [12–15]. In contrast, cetuximab combined with irinotecan has shown promising activity after failure of both irinotecan-based and oxaliplatin-based chemotherapy. In these studies, the response rates were 20–25% and median TTP and OS values were 4.7–5.5 months and 9.8–10.4 months, respectively [16, 17]. Recently, two randomized phase III trials of monoclonal antibody against epidermal growth factor receptor were reported in patients with chemotherapy-refractory metastatic CRC: The results from cetuximab monotherapy in third-line patients has shown a statistically significant improvement in OS compared with best supportive care (BSC) [18]. In a study by Van Cutsem et al. [19], significant improvement of median PFS was shown for treatment with panitumumab compared with BSC alone in patients with chemo-refractory CRC.
Although the response rate was low in the present study, our PFS and OS data were comparable to those of previous cetuximab studies [16, 17, 20]. As the majority of our patients had received three or more previous courses of chemotherapy, and as 15 (35.7%) patients had poor performance status (ECOG ≥ 2), the results of the current study are encouraging. A small pilot study showed that the combination of BV plus FOLFIRI resulted in modest efficacy with an objective response rate of 28.5%, a median TTP of 7.0 months, and a median OS of 12.1 months, in patients with metastatic CRC that had progressed after irinotecan and oxaliplatin chemotherapy [21]. These findings are comparable to those of our study. The TRC-0301 study showed, however, that the combination of BV and 5-FU/leucovorin was associated with a low objective response (1%), a low PFS (3.5 months), and a low OS (9.0 months) [22]. These data suggest that a BV-containing regimen may bring about disease stabilization in only some patients. The between-study discrepancy may be related to differences in patient selection and the combinations of chemotherapy used.
It is interesting to note that four of our patients showed PRs. All four of these patients had earlier demonstrated resistance to chemotherapy with both FOLFIRI and FOLFOX. The PRs can be partly explained by the “normalization” of the vasculature by BV treatment, with resultant decreases in tumor interstitial pressures, which in turn enhanced the delivery of the drugs co-administered with BV [23]. In patients with breast cancer, eight patients who were refractory to cyclophosphamide and methotrexate treatment crossed over to a regimen of BV with cyclophosphamide. One patient showed PR and five patients revealed SD. Taken together, the data support the finding that BV may circumvent resistance to cytotoxic agents in patients with tumors resistant to such drugs [24]. No patient in the present study were pretreated with BV-containing regimens because BV was not reimbursed at the study period as a first- or second-line treatment in Korea.
The safety profile seen in this study was similar to those observed in previous studies featuring BV-containing regimens. The frequencies of BV-related adverse events, such as hypertension, thrombosis, or bowel perforation, were within the ranges of previous reports [9, 10, 22, 25].
In conclusion, these data demonstrate that the addition of BV to FOLFIRI or FOLFOX provides interesting PFS and OS for patients with metastatic CRCs refractory to both irinotecan- and oxaliplatin-based chemotherapy. A large-scale prospective study is needed to determine the value of combinations of BV with other chemotherapy, in third-line and later-line settings, and the present study serves to emphasize the importance of such work.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96.
Shin HR, Jung KW, Won YJ, Park JG. Annual report of the Korea central cancer registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103–14.
Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–77. doi:10.1007/s11605-006-0061-3.
Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004;22:1209–14. doi:10.1200/JCO.2004.11.037.
Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med 2005;352:476–87. doi:10.1056/NEJMra040958.
Ragnhammar P, Hafstrom L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40:282–308.
Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med 1999;77:527–43. doi:10.1007/s001099900019.
Gordon MS. Vascular endothelial growth factor as a target for antiangiogenic therapy. J Clin Oncol. 2000;18 Suppl 21:S45–6.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi:10.1056/NEJMoa032691.
Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44. doi:10.1200/JCO.2006.09.6305.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi:10.1093/jnci/92.3.205.
Lim DH, Park YS, Park BB, Ji SH, Lee J, Park KW, et al. Mitomycin-C and capecitabine as third-line chemotherapy in patients with advanced colorectal cancer: a phase II study. Cancer Chemother Pharmacol. 2005;56:10–4. doi:10.1007/s00280-004-0963-2.
Ardavanis AS, Ioannidis GN, Orphanos GS, Rigatos GA. Salvage treatment with single-agent capecitabine in patients with heavily pretreated advanced colorectal cancer. Anticancer Res. 2006;26:1669–72.
Gubanski M, Naucler G, Almerud A, Lidestahl A, Lind PA. Capecitabine as third line therapy in patients with advanced colorectal cancer. Acta Oncol 2005;44:236–9.
Rosati G, Rossi A, Germano D, Reggiardo G, Manzione L. Raltitrexed and mitomycin-C as third-line chemotherapy for colorectal cancer after combination regimens including 5-fluorouracil, irinotecan and oxaliplatin: a phase II study. Anticancer Res. 2003;23:2981–5.
Pfeiffer P, Nielsen D, Yilmaz M, Iversen A, Vejlo C, Jensen BV. Cetuximab and irinotecan as third line therapy in patients with advanced colorectal cancer after failure of irinotecan, oxaliplatin and 5-fluorouracil. Acta Oncol. 2007;46:697–701. doi:10.1080/02841860601009455.
Vincenzi B, Santini D, Rabitti C, Coppola R, Beomonte Zobel B, Trodella L, et al. Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: a single centre phase II trial. Br J Cancer. 2006;94:792–7. doi:10.1038/sj.bjc.6603018.
Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi:10.1056/NEJMoa071834.
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi:10.1200/JCO.2006.08.1620.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi:10.1056/NEJMoa033025.
Kwon HC, Oh SY, Lee S, Kim SH, Kim HJ. Bevacizumab plus infusional 5-fluorouracil, leucovorin and irinotecan for advanced colorectal cancer that progressed after oxaliplatin and irinotecan chemotherapy: A pilot study. World J Gastroenterol. 2007;13:6231–5. doi:10.3748/wjg.13.6231.
Chen HX, Mooney M, Boron M, Vena D, Mosby K, Grochow L, et al. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol. 2006;24:3354–60. doi:10.1200/JCO.2005.05.1573.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi:10.1126/science.1104819.
Burstein HJ, Spigel D, Kindsvogel K, Parker LM, Bunnell CA, Partridge AH, et al. Metronomic chemotherapy with and without bevacizumab for advanced breast cancer: a randomized phase II study. Brest Cancer Res Treat. 2005;94 Suppl 1:A4.
Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–705. doi:10.1200/JCO.2005.05.112.
Jeung HC, Rha SY, Cho BC, Yoo NC, Roh JK, Roh WJ, et al. A phase II trial of S-1 monotherapy in metastatic colorectal cancer after failure of irinotecan- and oxaliplatin-containing regimens. Br J Cancer. 2006;95:1637–41. doi:10.1038/sj.bjc.6603468.
Acknowledgment
This study was also supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A062254).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, B.W., Kim, T.W., Lee, JL. et al. Bevacizumab plus FOLFIRI or FOLFOX as third-line or later treatment in patients with metastatic colorectal cancer after failure of 5-fluorouracil, irinotecan, and oxaliplatin: a retrospective analysis. Med Oncol 26, 32–37 (2009). https://doi.org/10.1007/s12032-008-9077-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-008-9077-8