Abstract
Alzheimer’s disease (AD) is considered a prevalent neurological disorder with a neurodegenerative nature in elderly people. Oxidative stress and neuroinflammation due to amyloid β (Aβ) peptides are strongly involved in AD pathogenesis. Klotho is an anti-aging protein with multiple protective effects that its deficiency is involved in development of age-related disorders. In this study, we investigated the beneficial effect of Klotho pretreatment at different concentrations of 0.5, 1, and 2 nM against Aβ1–42 toxicity at a concentration of 20 μM in human SH-SY5Y neuroblastoma cells. Our findings showed that Klotho could significantly and partially restore cell viability and decrease reactive oxygen species (known as ROS) and improve superoxide dismutase activity (SOD) in addition to reduction of caspase 3 activity and DNA fragmentation following Aβ1–42 challenge. In addition, exogenous Klotho also reduced inflammatory biomarkers consisting of nuclear factor-kB (NF-kB), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) in Aβ-exposed cells. Besides, Klotho caused downregulation of Wnt1 level, upregulation of phosphorylated cyclic AMP response element binding (pCREB), and mRNA levels of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase 1 (HO-1) with no significant alteration of epsilon isoform of protein kinase C (PKCε) after Aβ toxicity. In summary, Klotho could alleviate apoptosis, oxidative stress, and inflammation in human neuroblastoma cells after Aβ challenge and its beneficial effect is partially exerted through appropriate modulation of Wnt1/pCREB/Nrf2/HO-1 signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is considered a prevalent neurological disorder with a neurodegenerative nature in elderly people, typified by progressive neuronal loss and neuroinflammation with ensuing deterioration of language and cognitive skills (Monsell et al. 2014). Although the exact mechanisms of AD pathology is not yet fully unraveled, recent evidences indicated that development of neuroinflammation and concurrent oxidative stress and decline of antioxidants are strongly involved in AD pathogenesis (Rojas-Gutierrez et al. 2017; Verdile et al. 2015; Zhou et al. 2016). Additionally, the neurotoxic amyloid β (Aβ) peptide fragments as a result of decomposition of amyloid precursor protein (APP) play a pivotal role in AD pathogenesis (Spires-Jones and Hyman 2014; Haass and Selkoe 2007; Karran et al. 2011).
Wnt signaling has a pivotal regulatory role in many biological processes in the central nervous system such as neurogenesis, neuronal differentiation, and even neuroprotection (Inestrosa and Arenas 2010; Salinas 2012). This signaling has been recognized as a neuroprotective factor against Aβ cytotoxicity and tau phosphorylation, and dysfunction of Wnt cascade could play a significant role in development of AD (Tapia-Rojas and Inestrosa 2018; (Inestrosa and Toledo 2008).
Protein kinase C (PKC) isoforms are involved in various cell functions such as differentiation, proliferation, and apoptosis (Mackay and Mochly-Rosen 2001), and overexpression of epsilon isoform of PKC (PKCε) might enhance Bcl-2 expression and exert neuroprotective effect (Weinreb et al. 2004). Downregulation of PKCε has been observed following 6-hydroxydopamine (6-OHDA) challenge of SH SY5Y cells (Tiong et al. 2010). PKCε attenuates amyloid beta-related pathology in transgenic mice (Choi et al. 2006), and its activation lowers Aβ neurotoxicity (Hongpaisan et al. 2011).
In addition, it has been shown that cAMP-response element binding protein (known as CREB) is a pivotal transcription factor that protects neurons against oxidative stress (Lee et al. 2009) and its expression is reduced in brains of people with AD and in Aβ-challenged neurons of rat hippocampus (Pugazhenthi et al. 2011). Amyloid beta exposure is also associated with increased inflammatory response and elevated expression of nuclear factor-κB (NF-κB) (Chong et al. 2005).
Klotho is an anti-aging agent that its deficiency is involved in rapid development of age-related disorders (Shiozaki et al. 2008) and in premature aging (Sopjani et al. 2015). The single-pass transmembrane protein Klotho is encoded by the KL gene and is a circulating agent that plays a pivotal role in cellular functions including metabolism that has been linked to pathogenesis of age-related disorders (Kuro-o et al. 1997). Klotho is expressed in the choroid plexus and to a lower degree in the hippocampal neurons (Li et al. 2004). Klotho upregulation ameliorates aging-related memory decline and oxidative stress burden in senescence-accelerated mice (Zhou et al. 2018) and could exert neuroprotective effect in a model of ischemic brain injury via inhibition of RIG-I/NF-kB signaling (Zhou et al. 2017b). In addition, Klotho could protect hippocampal neurons against Aβ and glutamate toxicity through mobilization of antioxidants (Zeldich et al. 2014). Klotho is involved in regulation of some intracellular signaling cascades such as protein kinase C (PKC), cAMP, and Wnt signaling (Sopjani et al. 2015; Wang and Sun 2009; Sedighi et al. 2019). In addition, part of Klotho beneficial effect in attenuation of nigrostriatal dopaminergic system damage in 6-OHDA model of Parkinson’s disease has been through inhibition of apoptosis, oxidative stress, and appropriate modulation of pCREB signaling (Baluchnejadmojarad et al. 2017). While the renal functions of Klotho are well-established, its roles in brain functions remain to be fully elucidated. Through its diverse roles in the brain, Klotho has been claimed as a new therapeutic target for neurodegenerative disorders like AD and multiple sclerosis (Zhou et al. 2017b). Hence, this study was designed and conducted to find out whether Klotho could ameliorate Aβ (1–42)-induced neurotoxicity in a cell model of AD and to unravel some involved mechanisms.
Material and Methods
Preparation of Amyloid Beta 1–42
Recombinant amyloid beta 1–42 (human Aβ1–42) (R&D Systems, Inc., USA) was prepared as mentioned before (Yeo et al. 2018). Shortly, Aβ1–42 peptide powder was solubilized in DMSO to have a 2 mM solution with additional dilution in PBS. It was a mixture of all forms of amyloid beta (monomers, oligomers, fibrils). This solution was incubated at 4 °C for 1 day.
Cell Culture
Human neuroblastoma cells (SH-SY5Y) (obtained from Pasteur Institute of Iran, Tehran, Iran) were cultured in DMEM/F12 cell culture medium in the presence of FBS (10%), penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37 °C and at an atmosphere of 5% CO2/95% air. The medium was regularly changed every 2 days. Once the cells were 80% confluent, they were used for further experiments. First, they were seeded at a density of 5000 cells/well in a 96-well microtiter plate for MTT assay. Second, cells at a density of 15,000/well in 48-well microtiter plates were pretreated with different concentrations of Klotho (recombinant human Klotho protein; R&D Systems, Inc., USA) (0.5, 1, and 2 nM) for 24 h and thereafter were exposed to Aβ1–42 (concentration of 20 μM) for further 24 h. Selection of this concentration range for Klotho was from an earlier study on its protective effect (at a concentration of 2 nM) against cisplatin ototoxicity in an auditory cell line (Park et al. 2012).
Cell Viability Assessment
Cellular viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (known as MTT) assay method. In this experiment, cells were cultured at a cell density of 5000 cells/well and incubated for 1 day for their attachment. After Klotho pretreatment at different concentrations (0.5, 1, and 2 nM) and Aβ exposure (concentration of 20 μM), MTT solution was added and resultant product was solubilized by cell culture medium. The plates were placed overnight at 37 °C and absorbance was obtained at 570 nm. Each experiment was done in triplicate.
ROS Estimation
Cellular production of ROS was estimated using 2′, 7′-dichlorofluorescin diacetate (known as DCFDA) (Sigma-Aldrich, USA). After its entrance into the cell, DCFDA is finally converted to fluorogenic 2′, 7′-dichlorofluorescein (DCF) that its quantity is proportionate with intracellular ROS. Shortly, Klotho and Aβ1–42-treated cells were lysed and lysate was centrifuged at 10,062×g for 10 min at 4 °C and the obtained supernatant was mixed with DCFDA at 37 °C for 30 min. Then, fluorescence was measured (excitation wavelength = 485 nm, emission wavelength = 528 nm). Obtained results were converted to as ng of DCF as an equivalent of ROS generated.
Measurement of SOD Activity
Activity of superoxide dismutase or SOD was determined with an assay kit (Cayman Chemical, USA). One unit of SOD activity is the quantity of the enzyme required to cause 50% dismutation. Our results were finally presented as relative SOD activity (% of control).
Assessment of Caspase 3 Activity
Measurement of caspase 3 activity was done as mentioned in an earlier report (Movsesyan et al. 2002). The test is based on the degradation of the p-nitroaniline (PNA) substrate by the enzyme. Shortly, cell lysates were incubated with assay buffer (pH 7.4) comprising dithiothreitol (DTT), CHAPS, HEPES, sucrose, EDTA, and pNA-specific substrate for 3 h at 37 °C. Finally, the amount of formed p-nitroaniline was measured at a wavelength of 405 nm.
Assessment of DNA Fragmentation
This test was done using Cell Death Detection ELISA PLUS kit (Sigma Aldrich, USA) according to its protocol.
Enzyme-Linked Immunosorbent Assays
Twenty-four hours following Aβ1–42 exposure, cells were harvested and washed once with cold PBS prior to incubation with hypotonic lysis buffer on ice. The cells were then centrifuged at 10,000×g in a centrifuge at 4 °C and the obtained lysate was used for ELISA assays. In this regard, the level of TNF-α (Sigma-Aldrich, USA), IL-1β (MyBioSource, Inc., USA), IL-6 (MyBioSource, Inc., USA), Wnt1 (MyBioSource, Inc., USA), pCREB (R&D Systems, Inc.), and PKCε (MyBioSource, Inc., USA) in the cell lysates was determined.
Quantitative Real-Time Polymerase Chain Reaction Experiments
For extraction of total RNA of samples, TRIzol reagent (Thermo Fisher Scientific, USA) was used. Synthesis of cDNA was conducted using the reverse transcription reagent (Takara Bio Inc., USA). SYBR Green qPCR Master mix (Thermo Fisher Scientific Inc., USA) was used to conduct real-time PCR. All reactions were done on Applied Biosystems StepOne Plus (USA), and relative expressions of target genes including β-catenin, Wnt1, and Nrf2 were normalized to GAPDH and calculated using the 2−∆∆Ct method. Used primers were as follows: Wnt1 forward, 5′ TGGTTTGCAAAGACCACCTCCA 3′, Wnt1 reverse, 5′ TGATTCCAGGAGGCAAACGCAT 3′, Nrf2 forward, 5′ CAGCTTTTGGCGCAGACATT 3′, Nrf2 reverse, 5′ GACTGGGCTCTCGATGTGAC 3′, HO-1 forward, 5′ GCCATGAACTTTGTCCGGTG 3′, HO-1 reverse, 5′ TTTCGTTGGGGAAGATGCCA 3′, GAPDH forward, 5′ AATCCCATCACCATCTTC 3′, and GAPDH reverse, 5′ AGGCTGTTGTCATACTTC 3′.
Statistical Analysis
Obtained results were brought as means ± S.E.M. One-way ANOVA was applied for data analysis with subsequent Tukey’s test for paired comparisons with significance level set at p < 0.05.
Results
The Effect of Klotho and Aβ1–42 on Cell Viability of Neuroblastoma Cells
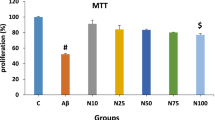
For assessment of cytotoxicity of Aβ1–42 and possible toxicity of Klotho in the neuroblastoma cells, we incubated cells with Klotho (0.5, 1, and 2 nM) for 24 h, then exposed them to Aβ1–42 for further 24 h, and finally, viability was determined using MTT method. Our results showed that cell viability in treated control groups (without Aβ1–42 challenge) does not significantly decrease in the presence of Klotho, even at its highest concentration (2 nM). In contrast, exposure of cells to Aβ1–42 for 24 h at a concentration of 20 μM significantly reduced cell viability (p < 0.001). In addition, pretreatment of these cells with various concentrations of Klotho (particularly at a concentration of 2 nM) significantly prevented viability reduction (p < 0.05 for Klotho at a concentration of 0.5 nM and p < 0.001 at concentrations of 1 and 2 nM) (Fig. 1).
The effect of amyloid beta 1–42 (Aβ) at a concentration of 20 μM and Klotho (KL) at different concentrations (0.5, 1, and 2 nM) on viability of SH-SY5Y cells. Cells were incubated 24 h with Klotho and then Aβ was added for additional 24 h. *p < 0.05; **p < 0.01; ***p < 0.001 (in comparison with control (Ctrl)); #p < 0.05, ###p < 0.001 (in comparison with Aβ)
The Effect of Klotho and Aβ1–42 on Oxidative Stress- and Apoptosis-Related Biomarkers in Human Neuroblastoma Cells
For assessment of oxidative stress and antioxidant system following Klotho and Aβ challenge, we measured lysate level of ROS (as an equivalent of DCF) (Fig. 2a) and SOD activity (Fig. 2b). In this regard, ROS level and SOD activity did not show a significant change in control + Klotho groups (those not exposed to Aβ). In contrast, exposure of SH-SY5Y neuroblastoma cells to Aβ for 24 h significantly increased ROS level (p < 0.001) and significantly reduced SOD activity (p < 0.001). Meanwhile, pretreatment of Aβ-exposed neuroblastoma cells with Klotho at a concentration of 2 nM significantly reduced ROS level (p < 0.001) and improved SOD activity (p < 0.05) when compared to Aβ group. In addition, the observed beneficial effect for Klotho was according to a concentration-dependent pattern. Regarding apoptotic biomarkers, caspase 3 activity (Fig. 2c) and DNA fragmentation (Fig. 2d) did not exhibit a significant alteration in control + Klotho groups (not challenged with Aβ) when compared to control group. In contrast, exposure of neuroblastoma cells to Aβ for 24 h significantly increased caspase 3 activity (p < 0.001) and DNA fragmentation (p < 0.001). Meanwhile, pretreatment of Aβ-exposed neuroblastoma cells with Klotho at a concentration of 2 nM significantly reduced caspase 3 activity (p < 0.01) and DNA fragmentation (p < 0.01) versus Aβ group. In addition, the observed beneficial effect for Klotho regarding apoptotic biomarkers followed a concentration-dependent algorithm.
The effect of amyloid beta 1–42 (Aβ) at a concentration of 20 μM and Klotho (KL) at different concentrations (0.5, 1, and 2 nM) on biomarkers of oxidative stress and apoptosis in SH-SY5Y cells. Cells were incubated 24 h with Klotho and then Aβ was added for additional 24 h. For oxidative stress, 2′,7′-dichlorofluorescein (DCF) as equivalent of reactive oxygen species (ROS) (a) and relative SOD activity (b) were measured. For apoptosis, optical density (OD) of caspase 3 activity (c) and DNA fragmentation (d) were measured. These measurements were done in duplicate. *p < 0.05; **p < 0.01; ***p < 0.001 (in comparison with control (Ctrl)); #p < 0.05; ## p < 0.01; ### p < 0.001 (in comparison with Aβ)
The Effect of Klotho and Aβ1–42 on Inflammatory Biomarkers in Human Neuroblastoma Cells
For assessment of inflammation following Klotho and Aβ exposure, we measured inflammatory indicators consisting of NF-kB, IL-1β, IL-6, and TNF-α. Determination of the lysate level of these parameters showed that the level of IL-1β (Fig. 3a), IL-6 (Fig. 3b), TNF-α (Fig. 3c), and NF-kB (Fig. 3d) did not show a significant change in Klotho-preincubated control groups (not exposed to Aβ). In contrast, exposure of neuroblastoma cells to Aβ for 24 h significantly raised NF-kB (p < 0.01), IL-1β (p < 0.01), IL-6 (p < 0.01), and TNF-α (p < 0.01). Furthermore, pretreatment of neuroblastoma cells with Klotho at a concentration of 2 nM significantly reduced NF-kB (p < 0.05), IL-1β (p < 0.01), IL-6 (p < 0.05), and TNF-α (p < 0.01) relative to Aβ group. In addition, the beneficial effect for Klotho followed a concentration-dependent pattern.
The effect of amyloid beta 1–42 (Aβ) at a concentration of 20 μM and Klotho (KL) at different concentrations (0.5, 1, and 2 nM) on biomarkers of inflammation consisting of interleukin 1β (IL-1β (a), interleukin 6 (IL-6) (b), tumor necrosis factor-α (TNF-α) (c), and the transcription factor NF-kB (d) in SH-SY5Y cells. Cells were incubated 24 h with Klotho and then Aβ was added for additional 24 h. These measurements were done in duplicate. *p < 0.05; **p < 0.01 (in comparison with control (Ctrl)); #p < 0.05; ##p < 0.01 (in comparison with Aβ)
The Effect of Klotho and Aβ 1–42 on Wnt1, pCREB, PKCε, Nrf2, and HO-1 in Human Neuroblastoma Cells
As depicted in Fig. 4, the supernatant level of Wnt1 (Fig. 4a) and its mRNA (Fig. 4b), PKCε level (Fig. 4c), and pCREB level (Fig. 4d), and mRNA levels of Nrf2 (Fig. 4e) and HO-1 (Fig. 4f) did not show a significant change in neuroblastoma cells pretreated with Klotho at concentrations of 05–2 nM when compared to the control group. In contrast, the level of Wnt1 and its mRNA non-significantly increased, PKCε level significantly reduced (p < 0.01), level of pCREB significantly increased (p < 0.05), mRNA level of Nrf2 did not have a significant change, and mRNA level of HO-1 did show a significant reduction (p < 0.01) in Aβ-exposed neuroblastoma cells in comparison with the control group. In addition, pre-incubation of neuroblastoma cells with Klotho at a concentration of 2 nM before Aβ exposure significantly reduced Wnt1 level (p < 0.05), significantly increased pCREB (p < 0.05) with no significant change of PKCε, and significantly increased Nrf2 mRNA (p < 0.05) and HO-1 mRNA (p < 0.01) relative to Aβ-exposed group.
The effect of amyloid beta 1–42 (Aβ) at a concentration of 20 μM and Klotho (KL) at different concentrations (0.5, 1, and 2 nM) on Wnt1 level (a) and its mRNA level (b), levels of epsilon isoform of protein kinase C (PKCε) (c), phosphorylated cyclic AMP response element binding (pCREB) (d), and mRNA levels of Nrf2 (e) and HO-1 (f) in SH-SY5Y cells. Cells were incubated 24 h with Klotho and then Aβ was added for additional 24 h. These measurements were done in duplicate. *p < 0.05; **p < 0.01 (in comparison with control (Ctrl)); #p < 0.05, ##p < 0.01 (in comparison with Aβ)
Discussion
The main goal of this research was assessment of neuroprotective potential of exogenous Klotho in the Aβ1–42-induced toxicity in SH-SY5Y cells. Our findings showed that challenge of neuroblastoma cells with Aβ1–42 leads to cellular toxicity, as shown by lower cell viability, increased formation of ROS, weakened antioxidant SOD activity, and higher rates of DNA fragmentation, and activity of caspase-3. Furthermore, cytotoxic effect of the peptide Aβ1–42 was also associated with increased levels of inflammatory biomarkers such as NF-kB, IL-1β, IL-6, and TNF-α and lower expression of PKCε and HO-1 and higher expression of pCREB with no significant alteration of Wnt1 and Nrf2. In contrast, Klotho pretreatment of Aβ1–42-exposed neuroblastoma cells partially and significantly restored back most of the mentioned parameters and its beneficial effect was in accordance with a concentration-dependent algorithm.
Aβ fragments including Aβ1–42 play pivotal role in pathogenesis of AD (Sun et al. 2015) that exert their harmful effects via disturbance of mitochondrial electron transport chain (Walsh et al. 2002), enhanced oxidative stress (Boyd-Kimball et al. 2005), and neuroinflammation (Sharma et al. 2016) and activation of apoptotic pathway (Yu et al. 2006). In our study, exposure of SH-SY5Y to Aβ1–42 for 24 h increased ROS level that is a biomarker of oxidative stress and this finding was in agreement with previous findings (Oguchi et al. 2017; Zhou et al. 2017a). In addition, antioxidant defensive system was weakened due to Aβ1–42 as shown by lower activity of SOD. The latter has also been reported before in the literature (Gill et al. 2017; Wang et al. 2012). Klotho treatment of Aβ-exposed SH-SY5Y cells mitigated oxidative stress burden, as demonstrated by lower level of ROS and improvement of SOD activity. In support of this finding, it has been shown that anti-aging protein Klotho could reduce injury of nigrostriatal dopaminergic pathway following 6-hydroxydopamine in a rodent model of Parkinson’s disease through suppression of oxidative stress (Baluchnejadmojarad et al. 2017) and Klotho is capable to function as an antioxidant effector to protect dopaminergic neurons against oxidant-induced degeneration (Brobey et al. 2015).
For assessment of apoptosis, DNA fragmentation and caspase 3 activity were determined. According to earlier reports, Aβ1–42 incubation could activate apoptotic cascade as shown by a higher number of TUNEL-positive neurons (Wang et al. 2018) and a higher ratio of Bax/Bcl2 (Li et al. 2018). Similar to our study, enhanced activity of caspase 3 as a reliable biomarker of apoptosis has also been reported following Aβ(1–42) exposure of SH-SY5Y cell lines (Xu et al. 2015). An earlier study has shown that part of toxic effect of Aβ(1–42) is mediated via enhancement of DNA fragmentation that is another indicator of apoptosis (Shi et al. 2010), and these findings are consistent with our results.
Aβ(1–42) exposure is also associated with enhanced inflammation with subsequent elevation of pro-inflammatory cytokines (Yeo et al. 2018). In this study, we showed increased level of IL-1β, IL-6, TNF-α, and NF-kB as valid biomarkers of inflammation, clearly indicating the occurrence of inflammation in SH-SY5Y cells following Aβ treatment. Klotho in this study also diminished severity of inflammation in Aβ1–42-challenged cells, as demonstrated by lower levels of pro-inflammatory indices. Consistent with our findings, it has been shown that part of protective effect of Klotho against ischemic brain injury is mediated through inhibition of downstream inflammatory cascade (Zhou et al. 2017b) and downregulation of Klotho in airways due to cigarette smoking is associated with induction of inflammation and overexpression of Klotho leads to an attenuation of airway inflammation (Krick et al. 2018).
In this study, Wnt1 level and its mRNA non-significantly increased following Aβ exposure in neuroblastoma cells. Consistent with this finding, it has been shown that Wnt1 protein expression is rapidly increased following Aβ1–42 exposure; however, its expression returns to normal levels with time (Chong et al. 2005). In addition, Klotho pretreatment at a concentration of 2 nM caused significant reduction of Wnt1 following Aβ challenge. Although weakened Wnt signaling may accentuate the appearance of pathological features of AD (Tapia-Rojas and Inestrosa 2018), for explanation of this finding, it has been claimed that part of protective effect of Klotho against lipopolysaccharide-induced inflammation in HK-2 cell lines is mediated through suppression of Wnt and nuclear factor-kB signaling cascades (Zhou et al. 2017c), and inhibition of Wnt and NF-kB pathways could exert a protective effect in HK-2 cells (Liang et al. 2017). Even the antagonizing effect of Klotho on Wnt1 has been reported (Fakhar et al. 2018). Another signaling that we studied to explore modes of action of Klotho was pCREB cascade. In this regard, Aβ challenge of SH-SY5Y cells increased pCREB level. It has been shown that part of Aβ toxicity may be due to higher degrees of activation of pCREB cascade, and inhibition of this pathway could exert a protective effect (Brewer et al. 2010). Klotho pretreatment further increased pCREB level following Aβ exposure. Consistent with our finding, an earlier study showed that Klotho could protect against oxidative stress in retinal tissue through upregulation of pCREB (Kokkinaki et al. 2013). Regarding PKCε, although its level significantly decreased following Aβ challenge, Klotho pre-incubation did not significantly elevate it. Previous findings have shown that Aβ peptide is capable to directly suppress PKC activation (Lee et al. 2004), and Klotho activates PKC signaling in kidney and testis (Imai et al. 2004). Part of protective effect of Klotho against Aβ neurotoxicity in this study was mediated through upregulation of Nrf2 and HO-1. In support of this fact, a recent study has shown that Klotho protein could inhibit H2O2-induced oxidative damage in endothelial cells, partly through enhancing Nrf2/HO-1 cascade (Cui et al. 2019).
To conclude, Klotho could alleviate apoptosis, oxidative stress, and inflammation in human neuroblastoma cells after Aβ challenge and part of its beneficial effect is mediated through appropriate modulation of Wnt1/pCREB/Nrf2/HO-1 signaling.
References
Baluchnejadmojarad T, Eftekhari SM, Jamali-Raeufy N, Haghani S, Zeinali H, Roghani M (2017) The anti-aging protein klotho alleviates injury of nigrostriatal dopaminergic pathway in 6-hydroxydopamine rat model of Parkinson’s disease: involvement of PKA/CaMKII/CREB signaling. Exp Gerontol 100:70–76. https://doi.org/10.1016/j.exger.2017.10.023
Boyd-Kimball D, Castegna A, Sultana R, Poon HF, Petroze R, Lynn BC, Klein JB, Butterfield DA (2005) Proteomic identification of proteins oxidized by Aβ (1–42) in synaptosomes: implications for Alzheimer’s disease. Brain Res 1044(2):206–215
Brewer GJ, Torricelli JR, Lindsey AL, Kunz EZ, Neuman A, Fisher DR, Joseph JA (2010) Age-related toxicity of amyloid-beta associated with increased pERK and pCREB in primary hippocampal neurons: reversal by blueberry extract. J Nutr Biochem 21(10):991–998. https://doi.org/10.1016/j.jnutbio.2009.08.005
Brobey RK, German D, Sonsalla PK, Gurnani P, Pastor J, Hsieh CC, Papaconstantinou J, Foster PP, Kuro-o M, Rosenblatt KP (2015) Klotho protects dopaminergic neuron oxidant-induced degeneration by modulating ASK1 and p38 MAPK signaling pathways. PLoS One 10(10):e0139914. https://doi.org/10.1371/journal.pone.0139914
Choi DS, Wang D, Yu GQ, Zhu G, Kharazia VN, Paredes JP, Chang WS, Deitchman JK, Mucke L, Messing RO (2006) PKCepsilon increases endothelin converting enzyme activity and reduces amyloid plaque pathology in transgenic mice. Proc Natl Acad Sci U S A 103(21):8215–8220. https://doi.org/10.1073/pnas.0509725103
Chong ZZ, Li F, Maiese K (2005) Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res 2(5):387–399
Cui W, Leng B, Wang G (2019) Klotho protein inhibits H2O2-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can J Physiol Pharmacol 97(5):370–376. https://doi.org/10.1139/cjpp-2018-0277
Fakhar M, Najumuddin, Gul M, Rashid S (2018) Antagonistic role of Klotho-derived peptides dynamics in the pancreatic cancer treatment through obstructing WNT-1 and frizzled binding. Biophys Chem 240:107–117. https://doi.org/10.1016/j.bpc.2018.07.002
Gill I, Kaur S, Kaur N, Dhiman M, Mantha AK (2017) Phytochemical ginkgolide B attenuates amyloid-beta1-42 induced oxidative damage and altered cellular responses in human neuroblastoma SH-SY5Y cells. J Alzheimers Dis 60(s1):S25–s40. https://doi.org/10.3233/jad-161086
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol 8(2):101–112
Hongpaisan J, Sun MK, Alkon DL (2011) PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J Neurosci 31(2):630–643. https://doi.org/10.1523/jneurosci.5209-10.2011
Imai M, Ishikawa K, Matsukawa N, Kida I, Ohta J, Ikushima M, Chihara Y, Rui X, Rakugi H, Ogihara T (2004) Klotho protein activates the PKC pathway in the kidney and testis and suppresses 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression. Endocrine 25(3):229–234. https://doi.org/10.1385/endo:25:3:229
Inestrosa NC, Arenas E (2010) Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 11(2):77–86. https://doi.org/10.1038/nrn2755
Inestrosa NC, Toledo EM (2008) The role of Wnt signaling in neuronal dysfunction in Alzheimer’s disease. Mol Neurodegener 3(1):9. https://doi.org/10.1186/1750-1326-3-9
Karran E, Mercken M, De Strooper B (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10(9):698–712
Kokkinaki M, Abu-Asab M, Gunawardena N, Ahern G, Javidnia M, Young J, Golestaneh N (2013) Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J Neurosci 33(41):16346–16359. https://doi.org/10.1523/jneurosci.0402-13.2013
Krick S, Grabner A, Baumlin N, Yanucil C, Helton S, Grosche A, Sailland J, Geraghty P, Viera L, Russell DW, Wells JM, Xu X, Gaggar A, Barnes J, King GD, Campos M, Faul C, Salathe M (2018) Fibroblast growth factor 23 and Klotho contribute to airway inflammation. Eur Respir J 52(1):1800236. https://doi.org/10.1183/13993003.00236-2018
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390(6655):45–51. https://doi.org/10.1038/36285
Lee W, Boo JH, Jung MW, Park SD, Kim YH, Kim SU, Mook-Jung I (2004) Amyloid beta peptide directly inhibits PKC activation. Mol Cell Neurosci 26(2):222–231. https://doi.org/10.1016/j.mcn.2003.10.020
Lee B, Cao R, Choi YS, Cho HY, Rhee AD, Hah CK, Hoyt KR, Obrietan K (2009) The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death. J Neurochem 108(5):1251–1265. https://doi.org/10.1111/j.1471-4159.2008.05864.x
Li S-A, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K (2004) Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct 29(4):91–99. https://doi.org/10.1247/csf.29.9
Li Y, Liu Q, Sun J, Wang J, Liu X, Gao J (2018) Mitochondrial protective mechanism of simvastatin protects against amyloid beta peptide-induced injury in SH-SY5Y cells. Int J Mol Med 41(5):2997–3005. https://doi.org/10.3892/ijmm.2018.3456
Liang H, Yang K, Xin M, Liu X, Zhao L, Liu B, Wang J (2017) MiR-130a protects against lipopolysaccharide-induced glomerular cell injury by upregulation of Klotho. Pharmazie 72(8):468–474. https://doi.org/10.1691/ph.2017.7525
Mackay K, Mochly-Rosen D (2001) Localization, anchoring, and functions of protein kinase C isozymes in the heart. J Mol Cell Cardiol 33(7):1301–1307. https://doi.org/10.1006/jmcc.2001.1400
Monsell SE, Mock C, Hassenstab J, Roe CM, Cairns NJ, Morris JC, Kukull W (2014) Neuropsychological changes in asymptomatic persons with Alzheimer disease neuropathology. Neurology 83(5):434–440. https://doi.org/10.1212/WNL.0000000000000650
Movsesyan VA, Yakovlev AG, Dabaghyan EA, Stoica BA, Faden AI (2002) Ceramide induces neuronal apoptosis through the caspase-9/caspase-3 pathway. Biochem Biophys Res Commun 299(2):201–207
Oguchi T, Ono R, Tsuji M, Shozawa H, Somei M, Inagaki M, Mori Y, Yasumoto T, Ono K, Kiuchi Y (2017) Cilostazol suppresses Abeta-induced neurotoxicity in SH-SY5Y cells through inhibition of oxidative stress and MAPK signaling pathway. Front Aging Neurosci 9:337. https://doi.org/10.3389/fnagi.2017.00337
Park SJ, Park SH, Chang JW, Choi J, Jung HH, Im GJ (2012) Protective effect of klotho protein against cisplatin ototoxicity in an auditory cell line. J Laryngol Otol 126(10):1003–1009. https://doi.org/10.1017/s0022215112001715
Pugazhenthi S, Wang M, Pham S, Sze CI, Eckman CB (2011) Downregulation of CREB expression in Alzheimer’s brain and in Abeta-treated rat hippocampal neurons. Mol Neurodegener 6:60. https://doi.org/10.1186/1750-1326-6-60
Rojas-Gutierrez E, Munoz-Arenas G, Trevino S, Espinosa B, Chavez R, Rojas K et al (2017) Alzheimer’s disease and metabolic syndrome: a link from oxidative stress and inflammation to neurodegeneration. Synapse. 71:e21990. https://doi.org/10.1002/syn.21990
Salinas PC (2012) Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol 4(2). https://doi.org/10.1101/cshperspect.a008003
Sedighi M, Baluchnejadmojarad T, Fallah S, Moradi N, Afshin-Majdd S, Roghani M (2019) Klotho ameliorates cellular inflammation via suppression of cytokine release and upregulation of miR-29a in the PBMCs of diagnosed Alzheimer’s disease patients. J Mol Neurosci 69(1):157–165. https://doi.org/10.1007/s12031-019-01345-5
Sharma S, Verma S, Kapoor M, Saini A, Nehru B (2016) Alzheimer’s disease like pathology induced six weeks after aggregated amyloid-beta injection in rats: increased oxidative stress and impaired long-term memory with anxiety-like behavior. Neurol Res 38(9):838–850. https://doi.org/10.1080/01616412.2016.1209337
Shi C, Wu F, Yew DT, Xu J, Zhu Y (2010) Bilobalide prevents apoptosis through activation of the PI3K/Akt pathway in SH-SY5Y cells. Apoptosis 15(6):715–727. https://doi.org/10.1007/s10495-010-0492-x
Shiozaki M, Yoshimura K, Shibata M, Koike M, Matsuura N, Uchiyama Y, Gotow T (2008) Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience 152(4):924–941
Sopjani M, Rinnerthaler M, Kruja J, Dermaku-Sopjani M (2015) Intracellular signaling of the aging suppressor protein Klotho. Curr Mol Med 15(1):27–37
Spires-Jones TL, Hyman BT (2014) The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 82(4):756–771. https://doi.org/10.1016/j.neuron.2014.05.004
Sun X, Chen WD, Wang YD (2015) β-Amyloid: the key peptide in the pathogenesis of Alzheimer’s disease. Front Pharmacol 6:221. https://doi.org/10.3389/fphar.2015.00221
Tapia-Rojas C, Inestrosa NC (2018) Wnt signaling loss accelerates the appearance of neuropathological hallmarks of Alzheimer’s disease in J20-APP transgenic and wild-type mice. J Neurochem 144(4):443–465. https://doi.org/10.1111/jnc.14278
Tiong CX, Lu M, Bian JS (2010) Protective effect of hydrogen sulphide against 6-OHDA-induced cell injury in SH-SY5Y cells involves PKC/PI3K/Akt pathway. Br J Pharmacol 161(2):467–480. https://doi.org/10.1111/j.1476-5381.2010.00887.x
Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Newsholme P (2015) Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediat Inflamm 2015:105828–105817. https://doi.org/10.1155/2015/105828
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416(6880):535–539
Wang Y, Sun Z (2009) Current understanding of klotho. Ageing Res Rev 8(1):43–51. https://doi.org/10.1016/j.arr.2008.10.002
Wang SW, Wang YJ, Su YJ, Zhou WW, Yang SG, Zhang R, Zhao M, Li YN, Zhang ZP, Zhan DW, Liu RT (2012) Rutin inhibits beta-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology 33(3):482–490. https://doi.org/10.1016/j.neuro.2012.03.003
Wang W, Wang W, Yao G, Ren Q, Wang D, Wang Z, Liu P, Gao P, Zhang Y, Wang S, Song S (2018) Novel sarsasapogenin-triazolyl hybrids as potential anti-Alzheimer’s agents: design, synthesis and biological evaluation. Eur J Med Chem 151:351–362. https://doi.org/10.1016/j.ejmech.2018.03.082
Weinreb O, Bar-Am O, Amit T, Chillag-Talmor O, Youdim MB (2004) Neuroprotection via pro-survival protein kinase C isoforms associated with Bcl-2 family members. FASEB J 18(12):1471–1473. https://doi.org/10.1096/fj.04-1916fje
Xu N, Xiao Z, Zou T, Huang Z (2015) Induction of GADD34 regulates the neurotoxicity of amyloid beta. Am J Alzheimers Dis Other Dement 30(3):313–319. https://doi.org/10.1177/1533317514545616
Yeo ETY, Wong KWL, See ML, Wong KY, Gan SY, Chan EWL (2018) Piper sarmentosum Roxb. confers neuroprotection on beta-amyloid (Abeta)-induced microglia-mediated neuroinflammation and attenuates tau hyperphosphorylation in SH-SY5Y cells. J Ethnopharmacol 217:187–194. https://doi.org/10.1016/j.jep.2018.02.025
Yu M-S, Suen K-C, Kwok N-S, So K-F, Hugon J, Chang RC-C (2006) Beta-amyloid peptides induces neuronal apoptosis via a mechanism independent of unfolded protein responses. Apoptosis 11(5):687–700
Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, Harris DA, Abraham CR (2014) The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem 289(35):24700–24715. https://doi.org/10.1074/jbc.M114.567321
Zhou X, Li Y, Shi X, Ma C (2016) An overview on therapeutics attenuating amyloid beta level in Alzheimer’s disease: targeting neurotransmission, inflammation, oxidative stress and enhanced cholesterol levels. Am J Transl Res 8(2):246–269
Zhou C, Zhao L, Zheng J, Wang K, Deng H, Liu P, Chen L, Mu H (2017a) MicroRNA-144 modulates oxidative stress tolerance in SH-SY5Y cells by regulating nuclear factor erythroid 2-related factor 2-glutathione axis. Neurosci Lett 655:21–27. https://doi.org/10.1016/j.neulet.2017.06.045
Zhou HJ, Li H, Shi MQ, Mao XN, Liu DL, Chang YR et al (2017b) Protective effect of Klotho against ischemic brain injury is associated with inhibition of RIG-I/NF-kappaB signaling. Front Pharmacol 8:950. https://doi.org/10.3389/fphar.2017.00950
Zhou Y, Kuang Y, Zhou J (2017c) Klotho protects against LPS-induced inflammation injury by inhibiting Wnt and NF-kappaB pathways in HK-2 cells. Pharmazie 72(4):227–231. https://doi.org/10.1691/ph.2017.6867
Zhou HJ, Zeng CY, Yang TT, Long FY, Kuang X, Du JR (2018) Lentivirus-mediated klotho up-regulation improves aging-related memory deficits and oxidative stress in senescence-accelerated mouse prone-8 mice. Life Sci 200:56–62. https://doi.org/10.1016/j.lfs.2018.03.027
Acknowledgments
This study has been adapted from a PhD thesis at Iran University of Medical Sciences (Tehran, Iran).
Funding
This work was financially supported (grant no. 95-01-87-27994) by Iran University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Mohsen Sedighi, Mona Amiri, and Malihe Aminzade performed the experiments. Tourandokht Baluchnejadmojarad, Siamak Afshin-Majd, and Mehrdad Roghani conceived and designed the study. Mehrdad Roghani coordinated and supervised the study. All the authors contributed equally to critical evaluation and interpretation of the results and to the preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors hereby declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sedighi, M., Baluchnejadmojarad, T., Afshin-Majd, S. et al. Anti-aging Klotho Protects SH-SY5Y Cells Against Amyloid β1–42 Neurotoxicity: Involvement of Wnt1/pCREB/Nrf2/HO-1 Signaling. J Mol Neurosci 71, 19–27 (2021). https://doi.org/10.1007/s12031-020-01621-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01621-9