Abstract
Myeloid differentiation factor 88 (MyD88) is an adaptor protein for the Toll-like receptor (TLR) and interleukin 1 receptor (IL-1R) families of innate immunity receptors that mediate inflammatory responses to cellular injury. TLR/IL1R/MyD88 signaling is known to contribute to retinal degeneration, although how MyD88 regulates neuronal survival, and the effect of MyD88 on the inflammatory environment in the retina, is mostly unknown. In this study, we tested the hypothesis that blocking MyD88-mediated signaling early in retinal degeneration promotes transition of microglia towards a neuroprotective anti-inflammatory phenotype, resulting in enhanced photoreceptor survival. We also tested whether systemic delivery of a pharmacologic MyD88 inhibitor has therapeutic potential. The rd10 mouse model of retinal degeneration was injected intraperitoneally with increasing doses of a MyD88 blocking peptide or control peptide early in degeneration, and inflammatory responses and photoreceptor survival were measured at specific time points using flow cytometry, cytokine profiling, and electroretinograms. Our results demonstrated that rd10 mice injected with a low dose of MyD88 inhibitor peptide showed increased rod photoreceptor function and reduced apoptosis compared with control peptide and uninjected mice. MyD88 inhibition also resulted in fewer microglia/macrophage cells in the photoreceptor layer whereas total peripheral and retinal macrophage were not changed. Furthermore, increased number of cells expressing the Arg1 marker of neuroprotective microglia in the photoreceptor layer and higher MCP-1 and anti-inflammatory cytokine IL-27 were associated with photoreceptor survival. Therefore, these data suggest that the MyD88 inhibitor modified the retina environment to become less inflammatory, leading to improved photoreceptor function and survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inherited retinal degenerations are common diseases of the retina in which photoreceptors dysfunction then die, resulting in gradual vision loss that eventually leads to complete blindness. The retina is often used to investigate general neuroinflammatory processes that occur in other CNS diseases because of its simpler anatomy, fewer cell types, and ease of characterizing neuronal function and survival. Several lines of evidence suggest that degenerating photoreceptors release molecules that stimulate inflammatory signaling, which leads to microglial recruitment and exacerbates photoreceptor death (Hua et al. 2007; Kaczorowski et al. 2008; Ko et al. 2011; Kohno et al. 2013). Additionally, early degenerative changes in numerous human retinal diseases and rodent models of retinal degenerations coincide with increased activated microglia and macrophages migrating into the retina and elevated pro-inflammatory cytokines (Akhtar-Schafer et al. 2018; Karlstetter et al. 2015; Roque et al. 1999). Therefore, a major focus of the retina field is characterizing regulators of neuroinflammation during photoreceptor death in an effort to delay or prevent retinal degeneration.
Neuroinflammation is a complex cascade of cellular events occurring in the CNS in response to injury and disease. In injured CNS, microglia and astrocytes act in concert with macrophages and other non-neural cells to sustain the neuroinflammatory response by balancing specific cellular signals that often induce opposing effects. The cumulative response from these inflammatory cells may limit the propagation of neuronal damage by enhancing phagocytosis and secreting neuroprotective and anti-inflammatory molecules, but has the potential to induce detrimental processes, such as generating ROS and releasing neurotoxic and pro-inflammatory cytokines, which can lead ultimately to neuronal loss. Additionally, microglia/macrophages exist in various activation states depending on the damage condition, and different cytokines polarize microglia to pro-inflammatory or protective phenotypes (Geloso et al. 2017; Voet et al. 2019; David and Kroner 2011). Microglia/macrophage phenotypes influence the recovery of neural tissue after injury or disease. For example, macrophages with a neurotoxic phenotype, previously referred to as classical “M1” macrophages, express TNF and iNOS, and promote signaling pathways that result in neuronal death. In contrast, macrophages with an anti-inflammatory or reparative phenotype, traditionally referred to as alternatively-activated “M2” macrophages, express IL-10 and Arginase 1 (Arg1), and are associated with tissue repair. Therefore, strategies that promote polarization of microglia/macrophages to an anti-inflammatory phenotype and reduce excessive inflammation at critical times after injury may be more beneficial to injured neurons than completely eliminating all inflammation.
MyD88 is a central adaptor molecule that mediates signaling from the innate immune receptors Toll-like receptors (TLRs) and interleukin-1 receptor (IL-1R), leading to cytokine release and migration of microglia and macrophages to injury sites. MyD88 also influences the polarization of microglia/macrophage in acute and chronic CNS disease conditions. For example, TLR4/MyD88 activation converted macrophages from a protective anti-inflammatory phenotype to a pro-inflammatory phenotype (Yang et al. 2013), whereas blocking MyD88 in various disease models increased microglia with the anti-inflammatory phenotype and reduced neuronal apoptosis (Liu et al. 2018). The importance of MyD88-mediated signaling to photoreceptor survival was indicated in our previous study using a germline MyD88 knockout in a mouse model of retinal degeneration that led to delayed photoreceptor death and reduced microglia (Syeda et al. 2015), although the mechanism of survival was not defined. Additionally, mice lacking TLR4 showed improved survival of retinal ganglion cells after optic nerve crush (Nakano et al. 2017) and axotomy (Kilic et al. 2008) and had smaller infarct sizes in CNS injury models (Tang et al. 2007; Caso et al. 2007), whereas stimulating TLR4 induces retinal cell death (Patel and Hackam 2014; Yi et al. 2012; Kleinman et al. 2012; Templeton et al. 2013). Furthermore, injured photoreceptors release molecules that activate TLR/IL-1R/MyD88 signaling in glia and retinal pigment epithelium (RPE), and leads to microglial activation and further photoreceptor death (Kohno et al. 2013). However, little is known about how MyD88 regulates retinal neuron survival, particularly whether MyD88 influences polarization of microglia towards classic pro-inflammatory or alternative anti-inflammatory phenotypes during photoreceptor degeneration, and the consequences on photoreceptor survival.
In this study, we tested the hypothesis that blocking MyD88-mediated signaling early in retinal degeneration promotes transition of microglia towards a neuroprotective anti-inflammatory phenotype, resulting in enhanced photoreceptor survival and function. We also tested whether systemic delivery of a pharmacologic MyD88 inhibitor has therapeutic potential. Using the rd10 mouse model of retinal degeneration, we determined that inhibiting MyD88 reduced photoreceptor death and improved rod- but not cone-dependent retinal function. Photoreceptor survival was associated with increased reparative Arg1-positive microglia/macrophage in the outer retina and elevated MCP-1 and IL-27 cytokines. These findings implicate MyD88 in regulating microglia effects on rod degeneration, and suggest that MyD88 inhibition may be an effective therapeutic strategy for retinal degeneration via modulation of microglial/macrophage activation states.
Materials and Methods
Animals
All procedures involving mice were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmology and Vision Research and were approved by the Institutional Animal Care and Use Committee at the University of Miami. The retinal degeneration 10 (rd10) (B6.CXB1-Pde6brd10/J) mouse model is a commonly used model of retinitis pigmentosa that is homozygous for a mutation in a rod-specific visual transduction gene (Chang et al. 2007). Mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and were housed with a controlled 12 h light/dark cycle with free access to food and water. The cages for all the experimental and control mice were housed at equivalent distances from the overhead light, and experimental and control groups were from the same litters. Male and female mice were used, and no significant differences were found between the sexes except for body weight.
The MyD88 inhibitor peptide (abbreviated as MI) has a decoy MyD88 homodimerization sequence (underlined, DRQIKIWFQNRRMKWKKRDVLPGT), combined with a protein transduction (PTD) sequence derived from antennapedia for cellular permeability (IMG2005, Novus Biologicals, Centennial, Colorado). The control peptide consists of the PTD sequence only, and both were solubilized in sterile PBS. The mice were divided randomly into groups containing males and females and were injected intraperitoneally (IP) with three different concentrations (1, 2, and 3 mg/kg body weight) of the MyD88 inhibitor peptide (MI) or control peptide (Ctrl). The dose range is based on published studies that were similar to doses used to block MyD88 signaling and reduce ventricular dilatation and hypertrophy in a mouse model of acute myocardial infarction (Van Tassell et al. 2010) and reduce disease severity in a mouse EAE model of multiple sclerosis with a similar peptide (Dishon et al. 2018). The first injection was at post-natal day (P) P14, prior to retinal degeneration, then again at P17 and P21. Visual function was tested by electroretinography (ERG) at P25 and then retinas were immediately collected for flow cytometry, quantitative PCR (QPCR), or immunohistochemistry (IHC). Investigators were masked to the identity of the injected compound for all analyses.
Electroretinograms
Mice were anesthetized with a ketamine/xylazine mixture and the eyes were dilated using 2.5% phenylephrine hydrochloride ophthalmic solution. The mice were placed on a heating pad to maintain a constant body temperature and eyes were kept moist with artificial tears. Scotopic and photopic electroretinograms (ERGs) were performed as described previously (Patel et al. 2016), using a wildtype mouse to calibrate the system at the beginning of each experiment. Briefly, corneal silver wire electrodes were positioned onto both eyes, a ground electrode was inserted at the base of the tail, and a reference electrode was inserted on the forehead pointing down between the eyes. Dark adapted mice were placed into a Ganzfield light-emitting chamber and exposed to flashes of white light at an increasing intensity from 0 to + 2 log cds/m2. Cone responses were recorded after a brief period of light adaptation. The response to 10 flashes of light with an inter-stimulus time of 5 s were recorded and averaged using the UTAS system controlled by EM for Windows software (LKC Technologies, Gaithersburg, MD).
Immunohistochemistry
Eyes were immediately enucleated from euthanized mice (CO2/cervical dislocation) at P25 and fixed for 1 h in 4% paraformaldehyde, incubated overnight in 5% sucrose, followed by 1 h each in 10% sucrose, 20% sucrose, then a 1:1 mixture of 20% sucrose with Optimal Cutting Temperature Compound (OCT) (Sakura, Tissue-Tek). The eyes were embedded in OCT then frozen, and 10 μm cryostat-cut sections from the entire eye were mounted onto slides. To detect macrophage/microglia within the outer nuclear layer (ONL), slides were chosen that corresponded to the central retina for each mouse. The slides were first blocked with a solution of 10% goat serum in 0.3% Triton X-100/PBS, followed by incubation with rabbit anti-Iba1 primary antibody (1:200 dilution, Wako, Richmond, VA or Novus, Centennial, CO) and goat anti-Arg1 antibody (1:200 dilution, Novus) diluted in 2% goat serum in 0.3% Triton X-100/PBS, overnight at 4 °C. Following several washes in PBS, the slides were incubated in secondary antibodies (1:600 dilution, Invitrogen) for 1 h at room temperature, washed in PBS, counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize nuclei, and imaged with Zeiss Axiovert 200 fluorescent microscope at × 20 magnification. The specificity of immunostaining was established by comparison to control sections omitting the primary antibody or including an irrelevant antibody, and exposure times were kept constant between control and experimental slides. The images were coded to mask the treatment group and double-labeled Arg1-positive and Iba1-positive cells (all morphologies) were counted from 4 animals per group, using at least six 10 μm sections per animal. Cell density was calculated by dividing the number of cells by the total length of the retinal cross-section.
Flow Cytometry
Flow cytometry was used to quantify inflammatory cells in the retina and spleen and for measuring cell death. Mice were anesthetized then perfused with PBS, and the eyes and spleens were removed. Retinas were isolated and pooled for each animal then dissociated using the Neural Tissue Dissociation Kit P (Miltenyi Biotec, Auburn, CA), adapting the manufacturer’s protocol to accommodate small tissue amounts. To isolate inflammatory cells in the spleen, the tissue was isolated and mechanically disrupted using HBSS buffer without magnesium and calcium, then filtered through a 70 μm cell strainer and resuspended in RBC lysis buffer (Invitrogen), according to the manufacturer’s instructions. For inflammatory cell quantification, retina and spleen samples were resuspended in flow cytometry staining buffer (eBioscience), blocked with TruStain fcX (BioLegend) and incubated with anti-CD11b (PE, eBioscience), anti-CD45 (FITC, eBioscience), and anti-MHC II (APC, eBioscience) antibodies for 30 min, then fixed in 1% PFA. Immediately prior to analysis, DAPI and eBeads (eBioscience) were added to the samples and flow cytometry was performed on a BD LSRII flow cytometer using BDFACS Diva 8.1 acquisition software.
For detection of apoptosis and necrosis, the Alexa Fluor 488 Annexin V/Dead Cell Apoptosis Kit (Molecular Probes, Eugene, OR) was used according to the manufacturer’s directions. Briefly, dissociated retinas were resuspended in annexin binding buffer then incubated with Alexa Fluor 488 annexin V and propidium iodide for 15 min. DAPI and eBeads were then added and the samples were immediately analyzed using a BD LSRII flow cytometer. Annexin V detects phosphatidylserine (PS) externalization, which is an early marker of apoptosis. In normal viable cells, PS is on the cytoplasmic surface of the plasma membrane, whereas in apoptotic cells, PS is translocated from the inner to the outer leaflet of the plasma membrane and it is then bound by the fluorescently-labeled annexin V. Additionally, the cell-impermeant dead cell stain propidium iodide, in combination with annexin V staining, was used to distinguish dead non-apoptotic cells.
RNA Isolation and Quantitative PCR
Total RNA was isolated from dissected retinas using TRIZOL reagent (Invitrogen) and 1 μg of RNA was reverse-transcribed into cDNA using ThermoScript Reverse Transcriptase or SuperScript IV (Invitrogen), as previously described (Hackam et al. 2004). QPCR was performed in triplicate for each sample using PowerUp SYBR Green Master Mix (Applied Biosystems) in a Realplex2 Mastercycler (Eppendorf) using specific primers to mouse Arg1 (Forward: 5′ CTCCAAGCCAAAGTCCTTAGAG 3′; Reverse: 5′ AGGAGCTGTCATTAGGGACATC 3′), Ccl3 (Forward: 5′ GCAACCAAGTCTTCTCAGCG 3′; Reverse: 5′ TGGAATCTTCCGGCTGTAGG 3′) and Ccl5 (Forward: 5′ AGCAGCAAGTGCTCCAATCT 3′; Reverse: 5′ ATTTCTTGGGTTTGCTGTGC 3′). The primer sequences spanned an intron to avoid amplifying genomic DNA. The delta-delta Ct method was used for relative gene quantification using the reference gene (housekeeping control gene) ARP as in (Hackam et al. 2004), following the calculations in https://toptipbio.com/delta-delta-ct-pcr. The Arg1 qPCR product was purified and sequenced at GENEWIZ (South Plainfield, NJ) to confirm that the correct product was amplified.
Cytokine Profiling
Cytokine levels were analyzed in retinas from MyD88-inhibited and control mice using the LEGENDplex mouse inflammation panel 13-plex kit (BioLegend, San Diego, CA). Mice were injected IP with 2 mg/kg MyD88 inhibitor or control peptide at P14 (prior to photoreceptor degeneration) or at P18 (early photoreceptor degeneration); then, retinas were isolated 3 days post-injection and immediately frozen and stored at − 80 °C until analysis. Retinas were then thawed on ice and lysed in PBS containing protease inhibitors and 0.1% NP40. The lysate was diluted 1:1 with assay buffer, and an aliquot was used in the assay according to the manufacturer’s directions, and quantified using a CytoFlex S apparatus (Beckman Coulter). Data analysis was performed using the LEGENDplex v8.0 software.
Statistical Analysis
Student’s t test or ANOVA with Tukey post hoc test using GraphPad Prizm were used for comparison of the treatment groups. p values less than 0.05 were considered significant. Results are presented as average ± SD.
Results
Inhibiting MyD88 Reduces Photoreceptor Apoptosis in the rd10 Mouse Model of Retinal Degeneration
The rd10 mouse model has a well-described predictable rate of retinal degeneration in which primary rod photoreceptor death occurs rapidly during the third week of life, followed by delayed secondary cone photoreceptor degeneration (Barhoum et al. 2008; Gargini et al. 2007). The rd10 strain has slower and delayed degeneration than the rd1 mouse strain, used previously in combination with a MyD88 knockout (Syeda et al. 2015), which allows comparisons of post-development rod and cone photoreceptor death. Three different doses of MyD88 inhibitor peptide (MI), which blocks MyD88-mediated TLR and IL-1R signaling, or control peptide (C), were delivered by IP injections beginning at P14 prior to rod death, as described in the “Materials and Methods” section. Comparisons to uninjected rd10 mice are also shown for each experiment. No significant differences were observed in weights among the mice in the different experimental groups at the beginning and end of the study (data not shown).
To determine the effect of MI injections, photoreceptor death was quantified in treated mice during retinal degeneration at P25, corresponding to the peak of rod photoreceptor death, by measuring the number of apoptotic and necrotic cells in the retina using flow cytometry. We chose flow cytometry with Annexin V/propidium iodide colabeling (see “Materials and Methods”) as a quantitative approach that has less potential technical bias than counting photoreceptor rows or measuring ONL thickness from retina sections. Annexin V detection of phosphatidylserine externalization was used as an early marker of apoptosis, along with the cell-impermeant dead cell stain propidium iodide (PI). Annexin V–positive apoptotic cells can be distinguished from being in early or late apoptosis depending on whether they are also PI+. As shown in Fig. 1, inhibiting MyD88 signaling with 2 mg/kg of MI peptide reduced the number of cells in the early apoptotic phase (which are the Annexin V+/PI− cells) by 3-fold compared with the same dose of control peptide (p = 0.031), and reduced the number of cells in late apoptosis (which are the Annexin V+/PI+ cells) by 4-fold (p = 0.045) (Fig. 1a, b). The 2 mg/kg dose of MI peptide also led to fewer cells in early (p = 0.023) and late (p = 0.024) apoptosis compared with the untreated rd10 mice. In contrast, the 1.0 and 3.0 mg/kg doses of MI did not reduce the number of cells in early, late, or total apoptosis compared to the equivalent dose of control peptide, indicating that the protective effect is not dose-dependent. Additionally, the MI peptide did not significantly change the number of necrotic cells (which are the Annexin V−/PI+ cells) at any dose compared with the equivalent dose of control peptide or untreated mice (Fig. 1d, no significant differences between MI vs Ctrl, and MI vs UT, and Ctrl vs UT). Because photoreceptors are the only cell types in the rd10 retina undergoing apoptosis or necrosis (Arango-Gonzalez et al. 2014), we conclude that photoreceptor apoptosis was significantly reduced by the MyD88 inhibitor.
Inhibiting MyD88 signaling reduced the number of retina cells in early and late apoptosis. Flow cytometric analysis of cell death in the retina of mice treated with MI and control (Ctrl) peptides. a Quantification of the total number of cells in early apoptosis (Annexin V+/PI−); 2.0 mg/kg MI peptide was lower than Ctrl peptide and untreated (UT) rd10 mice. b Quantification of the total number of cells in late apoptosis (Annexin V+/PI+); 2.0 mg/kg MI peptide was lower than Ctrl and UT rd10 mice, and 1.0 mg/kg MI and Ctrl had fewer late apoptotic cells compared with UT rd10, but the MI and Ctrl were equivalent, indicating no therapeutic effect. c Quantification of the total number of apoptotic cells (total Annexin V+); 2.0 mg/kg MI was lower than Ctrl and UT mice. d Quantification of the total number of cells in undergoing necrosis (Annexin V−/PI+); no differences were found between treatments at any dose. e Representative flow cytometry plots for 2.0 mg/kg MI and Ctrl are shown. *p < 0.05; **p < 0.01. (n = 7–15 per group). Mean ± SD are shown
MyD88 Inhibition Increases Retinal Activity
To determine whether inhibiting MyD88 signaling protected the retina from functional deficits during retinal degeneration, we quantified light-evoked retina responses using ERGs on MI and control peptide–injected animals at each dose. Scotopic b-wave amplitudes, representing inner retina responses stimulated by rod photoreceptors, were significantly higher in 2 mg/kg MI–injected mice than in control or untreated mice over several light intensities (Fig. 2a). In contrast, none of the other doses of MI significantly increased b-wave amplitudes compared with their respective controls. Comparisons across all doses indicated that 2 mg/kg MI resulted in the highest b-waves of any of the doses. Scotopic rod photoreceptor-generated a-wave amplitudes were also highest in the 2 mg/kg MI–injected mice at the highest light intensity (Fig. 2b), although not at lower intensities. There were no significant differences in photopic (cone photoreceptor) responses at any light intensity (Fig. 2c, d). Together with the data showing reduced apoptotic cell death, these results demonstrate that inhibiting MyD88 signaling reduced rod photoreceptor degeneration in the rd10 mice (Fig. 2).
Inhibiting MyD88 signaling increased retina activity. a Quantification of light-evoked retina responses in the MI, Ctrl, and untreated animals using ERG. Light-evoked scotopic b-wave amplitudes (trough to peak), representing rod photoreceptor-driven inner retina responses, were significantly elevated in 2.0 mg/kg MI–injected mice compared with Ctrl and UT rd10 mice at different light intensities. Three light intensities are shown: 0, 1.0 and 2.0 log cd s/m2. b Analysis of scotopic a-wave amplitudes, representing rod photoreceptor responses; a-waves were significantly elevated in 2.0 mg/kg MI. c Quantification of photopic (cone photoreceptor-driven) responses; no differences were found between groups at any dose. *p < 0.05; **p < 0.01. (n = 5–17 per group). Mean ± SEM are shown
Inhibition of MyD88 Does Not Alter Retinal and Peripheral Macrophages and Microglia
During inflammation, TLR/MyD88 signaling is one of many pathways that promotes microglia activation, cytokine production, and migration of inflammatory cells to injury sites. To test whether blocking MyD88 signaling had an effect on these processes, we quantified microglial cells and infiltrating macrophages in the retina using flow cytometry. The number of CD45highCD11b+ macrophages and CD45lowCD11b+ microglia was not significantly different between MI and Ctrl groups at any dose (Fig. 3a, b). A similar profile was observed in the total number of activated (MHCII+) macrophages and microglia, in which no significant differences were observed between treatment groups at any dose (Fig. 3c).
Quantification of inflammatory cells in the retina and spleen. Flow cytometry was used to quantify a CD45highCD11+ macrophages, b CD45lowCD11+ microglia, and c MHCII+ activated myeloid (macroglia/macrophages) cells. d Representative flow cytometry plots of microglia and macrophage populations in the retina of mice treated with 2 mg/kg MI or Ctrl peptide. There were no significant differences in retinas from MI-treated mice compared with Ctrl peptide at any dose (n = 9–15 per group). Mean ± SD are shown
Given that MyD88 signaling is important for peripheral immune cell function and activation, flow cytometric analysis was also performed on spleens to determine whether peripheral immune alterations were occurring as a consequence of the treatment. The total number of CD45+ leukocytes and CD45+CD11b+ myeloid cells was found to be equivalent among groups at all doses (data not shown), suggesting that MI and control peptide treatments did not alter the peripheral immune response.
Inhibiting MyD88 Reduced the Number of Microglia/Macrophage in the Photoreceptor Layer
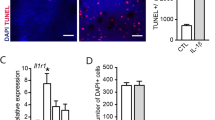
The position of microglia within the retina layers is important in relation to their phagocytic and pathogenic activities. While resting microglia mostly reside within the ganglion cell layer (GCL) and inner retina (Zeiss and Johnson 2004) (Zhao et al. 2015), during photoreceptor degeneration, microglia migrate to the outer nuclear layer (ONL) where they phagocytose dying photoreceptors. Therefore, immunohistochemistry for the marker protein Iba1 was used to determine whether the MyD88 inhibitor caused differential localization of microglia/macrophages within the retina. Following treatment with 2 mg/kg MI peptide, Iba1+ cells in the ONL adjacent to dying photoreceptors were reduced by half compared with control peptide treated mice (p = 0.0254) (Fig. 4a, b). Iba1-positive cells in the inner retina, which corresponds to the inner nuclear layer and ganglion cell layer combined, were approximately 2-fold higher in the control than the 2 mg/kg MyD88 inhibitor group but it did not reach significance (p = 0.06) (data not shown). The percent of cells in the inner and outer retina in each group was roughly equivalent due to the reduction in Iba1+ cells in both layers (percent of cells in photoreceptor layer: control 71.5%, MyD88 inhibitor 73.4%; percent of cells in inner retina: control 28.5%; MyD88 inhibitor 26.6%). No differences in distributions of Iba1+ cells were observed at any other dose between treatment groups (data not shown). Therefore, these data suggest that inhibiting MyD88 reduced migration of microglia/macrophage within the retina to the layer containing dying photoreceptors.
Reduced Iba1-positive cells in the ONL of mice injected with MyD88 inhibitor. a Representative images of Iba1-positive cells (pinkish red, indicated by asterisks) in retinas from mice injected with the MI or control peptide. DAPI-stained nuclei are blue. ONL outer nuclear layer, INL inner nuclear layer, GCL ganglion cell layer. Scale bar, 50 μm. b Quantification of Iba1-positive cells in the retina layers. *p = 0.025, (n = 3 per group). Mean ± SD is shown
Increased Arg1-Positive Microglia/Macrophage Is Associated with Photoreceptor Survival
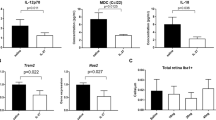
We next investigated whether the proportion of microglia expressing arginase 1 (Arg1), a marker associated with the neuroprotective microglia/macrophage phenotype, was altered by inhibiting MyD88 signaling. QPCR analysis on retinas from mice injected with 2 mg/kg inhibitor showed approximately two-fold higher Arg1 expression compared with control (Fig. 5a). In contrast, Arg1 expression was not altered in animals injected with 1 or 3 mg/kg. Additional genes typically associated with the reparative microglia/macrophage phenotype, including IL-10 and Ccl22, had undetectable expression at the time point analyzed. Markers of the neurotoxic microglia/macrophage phenotype, such as TNF, IL6, Ccl3 and Ccl5 genes, showed minimal expression and no significant differences between treatment groups at any dose (data not shown).
Increased Arg1-positive (M2-like) macrophages in mice injected with MyD88 inhibitor. a Quantification by qPCR of Arg1 gene expression in mice injected with MI or Ctrl peptide. **p < 0.01; ***p < 0.001, MI, n = 5; Ctrl, n = 6. b Arg1 immunostaining (asterisks, green) in the retina of mice injected with 2.0 mg/kg MI or Ctrl peptide. DAPI-stained nuclei are blue. ONL outer nuclear layer, INL inner nuclear layer, GCL ganglion cell layer. Scale bar, 50 μm. c Quantification of Arg1/Iba1-double positive cells. *p < 0.01 (n = 4 per group). Mean ± SD are shown
Next, retina sections from mice injected with 2 mg/kg MI and control peptides were immunostained with anti-Arg1 and anti-Iba1 antibodies at P25 (Fig. 5b). All Arg1-positive cells were also positive for Iba1. Quantification of the number of microglia of all morphologies residing in the ONL demonstrated that there was significantly more Arg1/Iba1-positive cells in mice injected with MI peptide than the control (p = 0.0058) (Fig. 5c). Therefore, a higher number of microglia/macrophage with a marker of the neuroprotective phenotype is associated with photoreceptor survival when MyD88 signaling is blocked, suggesting a possible cellular mechanism for reduced degeneration. No Iba1 + Arg1+ cells were observed in the ONL at P14, the time point immediately before treatment and prior to any detectable degenerative changes (n = 4 mice, data not shown).
Finally, we profiled levels of 13 inflammatory molecules and cytokines in the retinas (Table 1). Significantly higher levels of the chemoattractant cytokine MCP-1 were observed in retinas from MI-injected mice early in degeneration compared with control (p = 0.01). Also, the anti-inflammatory cytokine IL-27 had sustained levels in MI-injected mice early in degeneration whereas IL-27 levels dropped in the control peptide–injected mice (p = 0.03). No changes in cytokine levels were observed in mice injected with the inhibitor prior to degeneration. Increased IL-27 and MCP-1 levels are consistent with higher numbers of Arg1-positive microglia/macrophage, suggesting that blocking MyD88 signaling leads to an anti-inflammatory environment within the retina resulting in rod photoreceptor survival.
Discussion
MyD88 signaling plays a role in multiple inflammatory processes in the CNS, including cytokine release and activation of neurotoxic microglia phenotypes (Yang et al. 2013; Liu et al. 2018). The purpose of this study was to investigate whether systemic delivery of a pharmacologic MyD88 inhibitor rescued photoreceptors in a mouse model of inherited retinal degeneration and determine whether MyD88 inhibition induced changes in the inflammatory environment in the retina. Our findings demonstrate that inhibiting MyD88-mediated inflammation early in degeneration increased rod photoreceptor survival and improved retinal function, which was associated with increased microglia with the reparative neuroprotective phenotype in the photoreceptor layer and alteration of MCP-1 and IL-27. These data provide new information about how MyD88 inhibition leads to an anti-inflammatory/reparative microenvironment in the retina that favors photoreceptor rescue.
Microglia are dynamic cells that can switch phenotypes depending on the microenvironment and tissue injury. These cells have been classified into pro-inflammatory phenotypes that secrete neurotoxic molecules, and anti-inflammatory phenotypes that secrete anti-inflammatory and regenerative molecules (Geloso et al. 2017; Voet et al. 2019; David and Kroner 2011). Polarization towards the pro-inflammatory phenotype is directed through TLR4-MyD88-NF-κB signaling. We observed more Arg1-positive cells within the ONL, the region adjacent to the injured photoreceptors, in the MyD88 inhibited mice. Because Arg1-positive microglia/macrophage are associated with tissue repair and reduced inflammation, an increase of these cells is a possible mechanism by which reduced MyD88 leads to increased rod photoreceptor survival. Fewer Iba1-positive microglia/macrophage in the ONL were also observed in mice injected with the MyD88 inhibitor, although the total number of microglia/macrophage in the retina was not reduced when measured by flow cytometry. This suggests that MyD88 may influence migration of inflammatory cells within the retina layers towards dying photoreceptors as well as polarization to an Arg1+ phenotype, but is not primarily responsible for proliferation or migration of these cells into the retina itself.
Our findings are supported by previous studies in other animal models of CNS disease showing that blockade of MyD88 signaling promotes a neuroprotective macrophage/microglia phenotype. Liu et al. demonstrated more Arg1+ microglia in a rodent induced seizure model after inhibiting MyD88 and in MyD88 deficient mice (Liu et al. 2018). Increased Arg1+ cells and reduced pro-inflammatory cytokines were also reported in a traumatic brain injury (TBI) model in mice lacking TLR4/MyD88 signaling (Yao et al. 2017). Several groups demonstrated that only Arg1+ reparative microglia/macrophages were upregulated without corresponding changes in pro-inflammatory cells, such as in a spinal cord injury model (Hayakawa et al. 2014) and at late stages of disease in an infection model (Hanke et al. 2012). Similarly, increased anti-inflammatory markers and more Arg1+ microglia without a change in total Iba1+ cells were reported in a Sca6 mouse model lacking MyD88 (Aikawa et al. 2015). We also did not observe a corresponding change in pro-inflammatory genes or markers of pro-inflammatory microglia; it is possible that pro-inflammatory microglia were reduced at early stages of degeneration and not at the time points we tested, as observed elsewhere in the CNS in the induced seizure model (Liu et al. 2018). Future studies in rd10 mice using additional markers of pro-inflammatory cells and testing earlier time points may reveal changes in the pro-inflammatory macrophage population. It would also be interesting to determine at what time point the Arg1+ cells begin to migrate to the photoreceptor layer in response to MyD88 inhibition.
The inability to detect reduced expression of pro-inflammatory cytokines may also reflect different roles of MyD88 in different cell types (immune cells, glia, neurons). Indeed, TLR4/MyD88 is expressed on other cell types within the retina, including photoreceptors and Muller glia. Muller glia are astrocyte-like cells that span the retina, express MyD88, and respond to TLR4/MyD88 inhibition (Yi et al. 2012; Kumar and Shamsuddin 2012). Muller glia cross-talk with microglia during photoreceptor injury via inflammatory molecules and growth factors, and their activation often occurs acutely after neuronal damage and prior to activation and recruitment of microglia (Wang et al. 2011). How Muller glia and microglia coordinate the inflammatory response to injury in the retina is complex, varies with injury type, and changes in the acute and chronic period (Subirada et al. 2018). Therefore, an important question for future studies is which cell types in addition to microglia contribute to the neuroprotective effect of blocking MyD88.
We also noted that the protective effect of the MyD88 inhibitor was not dose-dependent. The optimal dose was 2 mg/kg, which is within the range of previous studies using MyD88 inhibitors (Van Tassell et al. 2010; Dishon et al. 2018), but a higher dose was not protective (the 3 mg/kg MI dose showed a mild but not statistically significant increase ERG responses compared with the control). The reason for the narrow effective range will require analysis of bioavailability within the retina and peptide stability. Unfortunately, for technical reasons, we were not able to measure MyD88 peptides within the retina or quantify changes in MyD88 signaling proteins in retina tissues. Therefore, we had to rely on substitute markers of inflammatory changes, including microglia/macrophage phenotypes and cytokine levels. Importantly, our results indicate that the MyD88 inhibitor, which we postulate is reducing innate immunity signaling, decreased the severity of photoreceptor death. This conclusion is supported by our previous studies showing that activation of TLR4 promoted photoreceptor death (Yi et al. 2012) and lack of MyD88 reduced photoreceptor death in the rapidly degenerating MyD88-deficient rd1 mouse line (Syeda et al. 2015). Although the half-life of the MyD88 inhibitor has not been reported, a recently published study in a rodent model of epilepsy demonstrated long-lasting effects (28 days) after a single dose of the inhibitor, which the authors attributed to inhibition by the peptide during the acute phase after injury (Liu et al. 2018). Studies using other peptide inhibitors have demonstrated long-lasting effects due to the peptide binding to albumin. In our study, we performed multiple injections to extend the inhibitory effect past the initial injury period and future studies will be necessary to characterize the half-life and kinetics of the inhibitor in the retina and systemic circulation.
Retinal degeneration in rd10 mice and other animal models shows initial primary rod death due to signaling defects caused by the mutant protein expressed in rods, followed by secondary non-cell autonomous cone degeneration. The majority of studies have focused on the role of microglia in early phases of rod degeneration, and it is unclear if activated microglia have equivalent effects on cone death (Narayan et al. 2016). Interestingly, the light-evoked ERG responses in our study indicated that blocking MyD88 only rescued rod photoreceptor function and not cone function. Quantification of cone photoreceptors by immunohistochemistry also indicated no changes in cone survival among the treated mice (data not shown). Other reports have noted similar rod-specific effects. Inhibition of microglia activation with minocycline led to significant rescue of rod survival and rod-driven light responses, but had only minor protective effects on cone survival and no effect on cone-driven light responses (Peng et al. 2014). Although activated microglia can be found in proximity to dying cones, depletion of microglia in a cone degeneration model did not substantially modify cone survival (Tang et al. 2017). Dying rods are believed to release DAMPS that specifically stimulate TLR/MyD88 signaling, leading to microglial recruitment and further rod death (Hua et al. 2007; Kaczorowski et al. 2008; Ko et al. 2011; Kohno et al. 2013), whereas numerous routes of cone death have been proposed in addition to inflammation, such as elevated oxidative stress, starvation, and lack of trophic factors derived from rods (Narayan et al. 2016). Therefore, MyD88-mediated innate immunity pathways may contribute primarily to rod photoreceptor death, whereas possibly cone photoreceptor degeneration is mediated by other pathways.
Non-MyD88 mediated pro-inflammatory pathways are also upregulated in rd10 retinas and may contribute to degeneration in the absence of MyD88 signaling, including complement, non-MyD88 TLR pathways (TLR3/TRIF, some TLR4 signaling), other DAMP receptors (such as RAGE), and NLRP3 inflammasome activation (Akhtar-Schafer et al. 2018). These pathways are not expected to be significantly inhibited by the MyD88 inhibitor. Consistent with this idea is that microglia deficient in MyD88 were only transiently reduced in rd1 retinal degeneration mice (Syeda et al. 2015), and mice lacking the MyD88 gene have no phenotype, suggesting that other pathways were active later in disease that promoted their migration. Inhibiting other major inflammatory pathways along with MyD88 may lead to more photoreceptor rescue.
In conclusion, inhibiting MyD88 led to reduced migration of microglia to the photoreceptor layer and increased polarization to the neuroprotective subtype of those microglia that did enter the ONL, which was associated with rod photoreceptor rescue. Therefore, enhancing reparative neuroprotective microglia using MyD88 inhibitors may be a potential new therapeutic strategy for retinal degenerations.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- Arg1:
-
Arginase 1
- CNS:
-
Central nervous system
- ERG:
-
Electroretinogram
- IL-1R:
-
Interleukin 1 receptors
- IP:
-
Intraperitoneally
- MI:
-
MyD88 inhibitor peptide
- MyD88:
-
Myeloid differentiation factor 88
- ONL:
-
Outer nuclear layer
- rd10:
-
Retinal degeneration 10
- TLR:
-
Toll-like receptors
- UT:
-
Untreated
References
Aikawa T, Mogushi K, Iijima-Tsutsui K, Ishikawa K, Sakurai M, Tanaka H, Mizusawa H, Watase K (2015) Loss of MyD88 alters neuroinflammatory response and attenuates early Purkinje cell loss in a spinocerebellar ataxia type 6 mouse model. Hum Mol Genet 24:4780–4791
Akhtar-Schafer I, Wang L, Krohne TU, Xu H, Langmann T (2018) Modulation of three key innate immune pathways for the most common retinal degenerative diseases. EMBO Mol Med 10(10):e8259
Arango-Gonzalez B, Trifunovic D, Sahaboglu A, Kranz K, Michalakis S, Farinelli P, Koch S, Koch F, Cottet S, Janssen-Bienhold U, Dedek K, Biel M, Zrenner E, Euler T, Ekstrom P, Ueffing M, Paquet-Durand F (2014) Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS One 9:e112142
Barhoum R, Martinez-Navarrete G, Corrochano S, Germain F, Fernandez-Sanchez L, de la Rosa EJ, de la Villa P, Cuenca N (2008) Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience 155:698–713
Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I (2007) Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 115:1599–1608
Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, Nusinowitz S, Rengarajan K, Boyd AP, Sidney SS, Phillips MJ, Stewart RE, Chaudhury R, Nickerson JM, Heckenlively JR, Boatright JH (2007) Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vis Res 47:624–633
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399
Dishon S, Schumacher A, Fanous J, Talhami A, Kassis I, Karussis D, Gilon C, Hoffman A, Nussbaum G (2018) Development of a novel backbone cyclic peptide inhibitor of the innate immune TLR/IL1R signaling protein MyD88. Sci Rep 8:9476
Gargini C, Terzibasi E, Mazzoni F, Strettoi E (2007) Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol 500:222–238 PMC2590657
Geloso MC, Corvino V, Marchese E, Serrano A, Michetti F, D'Ambrosi N (2017) The dual role of microglia in ALS: mechanisms and therapeutic approaches. Front Aging Neurosci 9:242
Hackam AS, Strom R, Liu D, Qian J, Wang C, Otteson D, Gunatilaka T, Farkas RH, Chowers I, Kageyama M, Leveillard T, Sahel JA, Campochiaro PA, Parmigiani G, Zack DJ (2004) Identification of gene expression changes associated with the progression of retinal degeneration in the rd1 mouse. Invest Ophthalmol Vis Sci 45:2929–2942
Hanke ML, Angle A, Kielian T (2012) MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PLoS One 7:e42476
Hayakawa K, Okazaki R, Morioka K, Nakamura K, Tanaka S, Ogata T (2014) Lipopolysaccharide preconditioning facilitates M2 activation of resident microglia after spinal cord injury. J Neurosci Res 92:1647–1658
Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH, Li C (2007) Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol 190:101–111 PMC2453597
Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR (2008) Early events in the recognition of danger signals after tissue injury. J Leukoc Biol 83:546–552
Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T (2015) Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res 45:30–57
Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM (2008) TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis 31:33–40
Kleinman ME, Kaneko H, Cho WG, Dridi S, Fowler BJ, Blandford AD, Albuquerque RJ, Hirano Y, Terasaki H, Kondo M, Fujita T, Ambati BK, Tarallo V, Gelfand BD, Bogdanovich S, Baffi JZ, Ambati J (2012) Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol Ther 20:101–108 PMC3255577
Ko MK, Saraswathy S, Parikh JG, Rao NA (2011) The role of TLR4 activation in photoreceptor mitochondrial oxidative stress. Invest Ophthalmol Vis Sci 52:5824–5835 PMC3176080
Kohno H, Chen Y, Kevany BM, Pearlman E, Miyagi M, Maeda T, Palczewski K, Maeda A (2013) Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J Biol Chem 288:15326–15341 PMC3663552
Kumar A, Shamsuddin N (2012) Retinal Muller glia initiate innate response to infectious stimuli via toll-like receptor signaling. PLoS One 7:e29830
Liu JT, Wu SX, Zhang H, Kuang F (2018) Inhibition of MyD88 signaling skews microglia/macrophage polarization and attenuates neuronal apoptosis in the hippocampus after status epilepticus in mice. Neurotherapeutics 15:1093–1111
Nakano Y, Shimazawa M, Ojino K, Izawa H, Takeuchi H, Inoue Y, Tsuruma K, Hara H (2017) Toll-like receptor 4 inhibitor protects against retinal ganglion cell damage induced by optic nerve crush in mice. J Pharmacol Sci 133:176–183
Narayan DS, Wood JP, Chidlow G, Casson RJ (2016) A review of the mechanisms of cone degeneration in retinitis pigmentosa. Acta Ophthalmol 94:748–754
Patel AK, Hackam AS (2014) A novel protective role for the innate immunity Toll-like receptor 3 (TLR3) in the retina via Stat3. Mol Cell Neurosci 63:38–48
Patel AK, Akinsoji E, Hackam AS (2016) Defining the relationships among retinal function, layer thickness and visual behavior during oxidative stress-induced retinal degeneration. Curr Eye Res 41:977–986
Peng B, Xiao J, Wang K, So KF, Tipoe GL, Lin B (2014) Suppression of microglial activation is neuroprotective in a mouse model of human retinitis pigmentosa. J Neurosci 34:8139–8150
Roque RS, Rosales AA, Jingjing L, Agarwal N, Al-Ubaidi MR (1999) Retina-derived microglial cells induce photoreceptor cell death in vitro. Brain Res 836:110–119
Subirada PV, Paz MC, Ridano ME, Lorenc VE, Vaglienti MV, Barcelona PF, Luna JD, Sanchez MC (2018) A journey into the retina: Muller glia commanding survival and death. Eur J Neurosci 47:1429–1443
Syeda S, Patel AK, Lee T, Hackam AS (2015) Reduced photoreceptor death and improved retinal function during retinal degeneration in mice lacking innate immunity adaptor protein MyD88. Exp Neurol 267C:1–12
Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP (2007) Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A 104:13798–13803 PMC1959462
Tang PH, Pierson MJ, Heuss ND, Gregerson DS (2017) A subpopulation of activated retinal macrophages selectively migrated to regions of cone photoreceptor stress, but had limited effect on cone death in a mouse model for type 2 Leber congenital amaurosis. Mol Cell Neurosci 85:70–81
Templeton JP, Freeman NE, Nickerson JM, Jablonski MM, Rex TS, Williams RW, Geisert EE (2013) Innate immune network in the retina activated by optic nerve crush. Invest Ophthalmol Vis Sci 54:2599–2606
Van Tassell BW, Seropian IM, Toldo S, Salloum FN, Smithson L, Varma A, Hoke NN, Gelwix C, Chau V, Abbate A (2010) Pharmacologic inhibition of myeloid differentiation factor 88 (MyD88) prevents left ventricular dilation and hypertrophy after experimental acute myocardial infarction in the mouse. J Cardiovasc Pharmacol 55:385–390
Voet S, Prinz M, van Loo G (2019) Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med 25:112–123
Wang M, Ma W, Zhao L, Fariss RN, Wong WT (2011) Adaptive Muller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J Neuroinflammation 8:173
Yang Y, Zhang R, Xia F, Zou T, Huang A, Xiong S, Zhang J (2013) LPS converts Gr-1(+)CD115(+) myeloid-derived suppressor cells from M2 to M1 via P38 MAPK. Exp Cell Res 319:1774–1783
Yao X, Liu S, Ding W, Yue P, Jiang Q, Zhao M, Hu F, Zhang H (2017) TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J Neuroimmunol 310:38–45
Yi H, Patel AK, Sodhi C, Hackam DJ, Hackam AS (2012) Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis. PLoS One 7(5):e36560 1-15
Zeiss CJ, Johnson EA (2004) Proliferation of microglia, but not photoreceptors, in the outer nuclear layer of the rd-1 mouse. Invest Ophthalmol Vis Sci 45:971–976
Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, Qian H, Parkhurst CN, Gan WB, Wong WT (2015) Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Molec Med 7:1179–1197
Acknowledgements
We thank Dr. Oliver Umland of the Flow Cytometry Core facility at the University of Miami Diabetic Research Institute, and Joeli Roth and Kristy Hamlin for technical assistance.
Funding
Financial support for this study for ASH was from the Foundation Fighting Blindness and NEI R01 EY026546. RB was supported by NIH-NINDS (grant 1R01NS094522-01), the Italian Multiple Sclerosis Foundation (grant FISM 2015/R/7), the US National Multiple Sclerosis Society (grant NMSS PP-1804-30716), and The Miami Project To Cure Paralysis. PI was supported by the Italian Multiple Sclerosis Foundation (grant FISM 2017/B/4). Institutional support to BPEI was from a Research to Prevent Blindness Unrestricted Grant and an NEI Center Core Grant EY014801.
Author information
Authors and Affiliations
Contributions
ASH designed the experiments, performed experiments, analyzed data, and wrote the paper; RB designed experiments, analyzed data, and edited the paper; and KG, TC, and PI performed experiments and edited the paper.
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures involving mice were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmology and Vision Research and were approved by the Institutional Animal Care and Use Committee at the University of Miami (protocol no. 16-073).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garces, K., Carmy, T., Illiano, P. et al. Increased Neuroprotective Microglia and Photoreceptor Survival in the Retina from a Peptide Inhibitor of Myeloid Differentiation Factor 88 (MyD88). J Mol Neurosci 70, 968–980 (2020). https://doi.org/10.1007/s12031-020-01503-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01503-0