Abstract
Schizophrenia is a severe chronic neuropsychiatric disorder, and it negatively affects individuals’ quality of life, but the pathogenesis of schizophrenia remains unclear. This study aimed to explore whether the administration of ketamine in rats causes changes in mTOR (mechanistic/mammalian target of rapamycin) expression in the hippocampus and prefrontal cortex. Ketamine was used to establish an animal model of schizophrenia. Rats were randomly divided into four groups: control group (normal saline), low-dose group (15 mg/kg ketamine), middle-dose group (30 mg/kg ketamine), and high-dose group (60 mg/kg ketamine). The rats were intraperitoneally injected with ketamine or normal saline twice a day (9 AM and 9 PM) for 7 consecutive days. Immunohistochemistry was used to detect mTOR protein expression in the hippocampus and prefrontal cortex from rats at 13 h after the last treatment. Using immunohistochemistry, the expression of the mTOR protein was localized exclusively in the CA3 region of the hippocampus and in the Cg1 region of the prefrontal cortexes. Ketamine at 60 mg/kg decreased the expression of mTOR protein in the brain of rats. Ketamine successfully established a rat model of schizophrenia. This study helps elucidate the mechanisms of ketamine-induced schizophrenia and provides novel insights for drug discovery and development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ketamine, a non-competitive and non-selective N-methyl-d-aspartate (NMDA) receptor antagonist, has been widely used as an anesthetic for inducing and maintaining anesthesia during surgeries (Oh and Kingsley 2018; Hulsman et al. 2018; Forget and Cata 2017; Bonhomme et al. 2016). As a fast-acting antidepressant, a single subanesthetic dose of ketamine produces a therapeutic response within a few hours that lasts for about 7 days (Zarate Jr. et al. 2006; Berman et al. 2000). As a new-type drug, ketamine innately possesses strong mental activity and is illegally consumed as a recreational drug in order to seek for euphoria (Liu et al. 2016; Sassano-Higgins et al. 2016; Li and Vlisides 2016). It has been commonly reported that long-term abusive usage of ketamine could produce symptoms of schizophrenia in rodent models and humans (Matuszko et al. 2017; Haaf et al. 2018). In addition, other negative features of exposure to ketamine, including hair loss (Favretto et al. 2016), inhibition of human sperm function (He et al. 2016), and induction of DNA damage (Leffa et al. 2016), have been reported.
Schizophrenia is a severe neuropsychiatric disorder, mainly characterized by three categories of symptoms: positive (e.g., hallucinations and delusions), negative (e.g., social withdrawal), and cognitive impairments (e.g., impaired executive functions and visual memory) (Skodlar and Henriksen 2019; Seeman 2019; Loch 2019; Tandon et al. 2013). Both negative symptoms and cognitive impairments are also strongly associated with social functioning, which obviously influences independent living skills and quality of life (Skodlar and Henriksen 2019; Seeman 2019; Loch 2019; Tandon et al. 2013). The pathogenesis of schizophrenia still remains unclear (Kuo and Liu 2019; Focking et al. 2019; Krajcovic et al. 2019; Zhuo et al. 2019). Thus, there is an urgent need for identifying the predictors of disease progression and treatment biomarkers of schizophrenia.
As a serine/threonine-protein kinase, the mechanistic/mammalian target of rapamycin (mTOR) is a master regulator of protein synthesis (Saxton and Sabatini 2017) and also controls several neuronal functions (e.g., neural plasticity) (Switon et al. 2017; Lee 2015; Huang et al. 2018; Yoon et al. 2008). Aberrant changes in mTOR signaling are associated with some neurological disorders such as schizophrenia (Ryskalin et al. 2018; Pham et al. 2016; Gururajan and van den Buuse 2014). Ketamine has been demonstrated to activate the mTOR pathway rapidly and to lead to increased synaptic signaling proteins and increased number and function of new spine synapses in the prefrontal cortex of rats, while blockade of mTOR signaling could completely block ketamine induction of synaptogenesis and behavioral responses in models of depression (Li et al. 2010). Similarly, ketamine can also activate the mTOR signaling in rat brain in vivo (Wesseling et al. 2015) and rat hippocampal neurons in vitro (Liu et al. 2019). These reports have revealed that abnormal mTOR expression is identified in rat brain tissues after an acute ketamine administration. Nevertheless, how mTOR expression is affected under the condition of a chronic ketamine treatment remains largely unknown.
In this study, we established an animal model of schizophrenia through the administration of ketamine in rats for 7 consecutive days. To explore whether ketamine causes changes in brain mTOR expression, we used immunohistochemistry to localize and assess mTOR expression in rat hippocampus, and prefrontal cortex tissues from rats received different doses of ketamine.
Materials and Methods
Animals
Sprague-Dawley (SD) rats (male, 3 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). They were maintained in the experimental animal center of Kunming Medical University (Kunming, China) at 22–24 °C with 45–55% humidity and a 12-h alternating light-dark cycle. Standard diet and water were given ad libitum. The rats were euthanized after anesthesia with ether.
All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals 8th Edition. The study was approved by the Ethics Committee of Kunming Medical University (Kunming, China).
Drug Administration
The SD rats were acclimatized for 1 week. Subsequently, 24 male SD rats at the age of 4 weeks were randomly divided into four experimental groups: control group, low-dose group, middle-dose group, and high-dose group (n = 6 per group). The rats in three drug-treatments groups were intraperitoneally administered with ketamine (ketamine hydrochloride injection; cat. no. H35020148, Fujian Gutian Pharmaceutical Co., Ltd., Fujian, China), with doses of 15 (low-dose group), 30 (middle-dose group), and 60 (high-dose group) mg/kg. The rats in the control group received an equal volume of normal saline (0.9% sodium chloride saline). The injection frequency was twice (9 AM and 9 PM) per day, and all the groups were continuously treated for 7 days.
Tissue Preparation
All the rats were euthanized at 13 h after the last administration of ketamine or saline. They were perfused with normal saline until the livers became white, and the effluent was clear. After head dissection, the brain tissues were obtained and kept on ice. The tissues were fixed in 4% paraformaldehyde (PFA). The hippocampal and prefrontal cortex tissues were collected, embedded in paraffin, and prepared for immunohistochemistry.
Immunohistochemistry
The paraffin-embedded tissues were cut at 5 μm as serial coronal sections. The coronal sections were respectively placed on slides and were dried at 70 °C for 3 h. The sections were deparaffinized and rehydrated through a graded series of ethanol. Antigen retrieval was performed using 1% (w/w) citrate buffer (pH 6.0). Endogenous peroxidase was blocked using hydrogen peroxide. The sections were incubated at room temperature (RT) for 10 min and washed three times for 2 min each time with phosphate-buffered saline (PBS). After blocking with 5% normal goat serum in PBS at 37 °C for 10 min, the sections were incubated with rabbit monoclonal anti-mTOR antibody (1:400 dilution; cat. no. ab32028, Abcam, Cambridge, UK) at 4 °C overnight. The next day, the sections were incubated at 37 °C for 30 min, followed by three washes in PBS, 2 min each time. The sections were stained with reaction enhanced reagent and incubated at 37 °C for about 20 min at RT. After washing three times, the sections were stained with goat anti-mouse/rabbit IgG polymer marked horseradish peroxidase and then incubated at 37 °C for about 30 min. Finally, after washing three times, the sections were stained with 3′-diaminobenzidine (DAB; DAB Detection kit, cat. no. PV-9000, Beijing Zhong Shan Jin Qiao Biotechnology Co. Ltd., Beijing, China) for 5 min before they were washed with tap water. The sections were rapidly dehydrated using a graded series of ethanol, and dimethylbenzene was used to clear them. The sections were sealed with neutral gum. Images were acquired under a microscope (Bx53; Olympus, Tokyo, Japan) and analyzed using the Image-pro plus 6.0 software (Media Cybernetics, Rockville, MD, USA). The gray value of mTOR protein was measured by the analysis system and converted into optical density (OD) value. The ratio of optical density value to the area, namely OD/area, was calculated, and the expression of mTOR protein was quantified by the value of the OD/area ratio.
Statistical Analysis
All data were presented as mean ± standard error of the mean (SEM). The statistical analysis and graphs were conducted using the GraphPad Prism 5 software (San Diego, CA, USA). Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by the Newman-Keuls post hoc test. A P value < 0.05 was considered statistically significant.
Results
Localization and Expression of mTOR Protein in Rat Hippocampus After Ketamine Administration
We treated the SD rats with ketamine at various doses (15, 30, and 60 mg/kg) and observed their behavior phenotype daily. Only the rats in the ketamine high-dose group displayed symptoms of schizophrenia and apparent aggressive behavior on the third day of ketamine administration. In order to investigate the influence of ketamine on the localization and expression level of brain mTOR protein, we carried out immunohistochemistry in rat hippocampus and prefrontal cortex tissues.
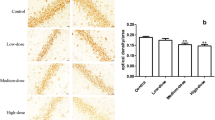
The results showed that the mTOR-positive cells were localized predominately in the CA3 region of the hippocampus, and the mTOR expression was significantly decreased in the high-dose group in comparison with the control group (Fig. 1). Compared with the control group, the low-dose and middle-dose groups displayed slightly lower expression of mTOR, although the differences were not statistically significant (one-way ANOVA, Newman-Keuls post hoc test). Nevertheless, there was a significant difference in mTOR expression levels in hippocampus tissues between the high-dose and control groups (Fig. 2).
The expression of mTOR in rat hippocampus tissues was detected by immunohistochemistry. Male Sprague-Dawley (SD) rats at the age of 4 weeks were randomly assigned into four groups (n = 6/group): the control group (treated with equal volume of normal saline), low-dose group (ketamine at 15 mg/kg body weight), middle-dose group (ketamine at 30 mg/kg), and high-dose group (ketamine at 60 mg/kg). The rats were intraperitoneally administered ketamine or normal saline twice (9 AM and 9 PM) per day for 7 days. The rats were sacrificed at 13 h after the last injection of ketamine or saline. The hippocampal tissues were subjected to immunohistochemistry. Images are a representative of staining for mTOR protein in the CA3 region of the hippocampus tissues. Scale bar, 20 μm
Quantitation on the mTOR protein expression levels in the CA3 region of the hippocampus tissues from rats treated with normal saline or different doses of ketamine. Experimental procedures were as described in Fig. 1. The relative expression levels of mTOR protein in the CA3 region of the hippocampus tissues from the indicated groups were quantified by OD/area. n = 6 for each group; *P < 0.05, compared with the control group
Localization and Expression of mTOR Protein in Rat Prefrontal Cortex After Ketamine Administration
The immunohistochemistry results of prefrontal cortex tissues revealed that the mTOR-positive cells were exclusively localized in the Cg1 region of the prefrontal cortex, and mTOR expression was markedly decreased upon ketamine administration (Fig. 3). Compared with the control group, the low-dose and middle-dose groups displayed slightly lower expression of mTOR in the prefrontal cortex, although the differences were not statistically significant (one-way ANOVA, Newman-Keuls post hoc test). Nevertheless, there was a significant difference in mTOR expression levels in prefrontal cortex tissues between the high-dose and control groups (Fig. 4).
The expression of mTOR in the prefrontal cortex from rats by immunohistochemistry. Experimental procedures were as described in Fig. 1, except that the mTOR protein expression was examined in the prefrontal cortex. Images are a representative of staining for mTOR protein in the Cg1 region of the prefrontal cortex. Scale bar, 20 μm
Quantification of the mTOR protein expression levels in the Cg1 region of the prefrontal cortex from rats treated with normal saline or different doses of ketamine. Experimental procedures were as described in Fig. 1. The relative expression levels of mTOR protein in the Cg1 region of the prefrontal cortex from the indicated groups were quantified by OD/area. n = 6 for each group; *P < 0.05, compared with the control group
Taken together, ketamine at 60 mg/kg in rats seemed to cause a downregulation of mTOR expression in the CA3 region of the hippocampus and the Cg1 region of the prefrontal cortex. Ketamine at 60 mg/kg could result in significantly lower mTOR expression in those brain regions after 7 days of treatment in rats.
Discussion
Similarity between the cognitive and behavioral effects of ketamine in animals and humans has suggested that abnormalities in the NMDA receptor function might contribute to the pathogenesis of schizophrenia (Abi-Saab et al. 1998; Javitt et al. 2012; Krystal et al. 2003; Lahti et al. 2001). Individuals with persisting ketamine-induced psychosis demonstrate a more similar symptom factor structure of schizophrenia than the individuals receiving a single dose of ketamine (Xu et al. 2015). The mTOR signaling cascade is involved in the intracellular regulation of protein synthesis, specifically for proteins involved in controlling neuronal morphology and facilitating synaptic plasticity (Gururajan and van den Buuse 2014). In this study, we investigated the effects of a relatively long-term administration of ketamine in rats on the expression of the mTOR protein in rat hippocampus and prefrontal cortex tissues. This is of importance since mTOR holds a central role in neurons, astrocytes, microglia, and various cells of the central nervous system by regulating cell growth, gene expression and translation, and metabolic balance. Of note, brain mTOR dysfunction has been implicated in neurological diseases such as epilepsy, autism, and neurodegenerative conditions (Lipton and Sahin 2014). Therefore, mTOR might be involved in schizophrenia.
As a highly potent amphetamine derivative, methamphetamine (N-methyl-alpha-methylphenethylamine) is a strong psychostimulant that can induce psychosis among recreational and chronic users (Wearne and Cornish 2018; Li et al. 2014). Some users of methamphetamine can develop a persistent psychotic syndrome that shows similarities to schizophrenia (Wearne and Cornish 2018; Li et al. 2014). Although methylamphetamine has been reported to be used to establish a kind of animal model of schizophrenia, this model could only reflect the positive symptoms of schizophrenia (Atkins et al. 2001; Arai et al. 2008; Ma and Leung 2000). Therefore, in this study, ketamine was used to establish an animal model that can reflect both the positive and negative symptoms of schizophrenia. In the high-dose group (60 mg/kg), we could observe symptoms of schizophrenia, including apparent aggressive behavior, starting on the third day of ketamine administration. On the other hand, the rats receiving lower doses of ketamine (15 and 30 mg/kg) did not exhibit the evident symptoms of schizophrenia even after completing the scheduled treatments, suggesting that higher doses of ketamine is a prerequisite in chronic ketamine exposure-induced rat models of schizophrenia.
Corresponding to the eliciting of symptoms of schizophrenia only in the rats treated with high-dose ketamine, immunohistochemistry showed that significant downregulation of the expression levels of the mTOR protein in the hippocampus and prefrontal cortex tissues were only observed in rats receiving high-dose ketamine, compared with the saline-treated rats. Aberrant changes in mTOR signaling in brain tissues are associated with neurological disorders such as schizophrenia (Ryskalin et al. 2018; Pham et al. 2016; Gururajan and van den Buuse 2014). Using selected reaction monitoring mass spectrometry (SRM-MS) profiling of rat brain tissues, Wesseling et al. reported that the mTOR protein levels in the frontal cortex and the hippocampus tissues of rat were significantly increased at 2 h after 10 mg/kg ketamine treatment (Wesseling et al. 2015). A previous study showed that the fast antidepressant actions of ketamine are associated with the transient and dose-dependent activation of mTOR signaling in rat prefrontal cortex (Li et al. 2010). A low dose of ketamine (10 mg/kg) was able to activate mTOR signaling in the rat prefrontal cortex starting 30 min after ketamine administration (Li et al. 2010). Moreover, a recent report from Liu et al. indicated that ketamine could dose-dependently promote the apoptosis of in vitro cultured rat hippocampal neurons with the upregulation of p-mTOR and its downstream regulators (p-4E-BP-1 and p-p70S6K), which can be reversed by the administration of mTOR inhibitor rapamycin (Liu et al. 2019).
Previous studies reported a dose-dependent effect of ketamine on mTOR (Li et al. 2010; Liu et al. 2019), which was not observed here in the low- and middle-dose groups. This might be associated with the detrimental effects of long-term treatment with ketamine on cell proliferation, survival, and metabolism (Wu et al. 2014; Weckmann et al. 2014; Cheung and Yew 2019).
This study has limitations. The number of animals in each group was small. In addition, no ketamine dose higher than 60 mg/kg was used, preventing the determination of the optimal dose for modeling of schizophrenia in rats. Third, only one animal model was used, and it was not compared with other known models of schizophrenia. Finally, the pharmacological modulation of mTOR might be a treatment of neurological conditions associated with mTOR dysfunction (Lipton and Sahin 2014), but such interventions were not examined in the present study.
In future studies, we will examine a wider range of ketamine doses on the occurrence of schizophrenia in rats. This should allow the optimization of the model and potentially examine a dose-dependent response. Future studies will also include other rat models of schizophrenia (Jones et al. 2011; Winship et al. 2019). Finally, future studies will examine the effects of mTOR pharmacological modulation in those animal models.
Conclusions
First, the in vivo administration of ketamine in rats affects the expression of the mTOR protein in rat hippocampus and prefrontal cortex. Second, ketamine at 60 mg/kg could be used for establishing a rat model of schizophrenia. Third, the mTOR-positive cells seem to be localized in the CA3 region in the hippocampus and the Cg1 region in the prefrontal cortex. The expression of mTOR is decreased upon high-dose ketamine treatment in rats. This study might provide novel insights on the mTOR protein distribution and expression in the encephalic regions of rats that are chronically treated with ketamine, and provides novel insights for drug discovery and development for the therapy of schizophrenia.
References
Abi-Saab W, D’souza D, Moghaddam B, Krystal J (1998) The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry 31(S 2):104–109
Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Takahashi K et al (2008) Involvement of pallidotegmental neurons in methamphetamine- and MK-801-induced impairment of prepulse inhibition of the acoustic startle reflex in mice: reversal by GABAB receptor agonist baclofen. Neuropsychopharmacology. 33(13):3164–3175
Atkins AL, Helms ML, O’Toole LA, Belknap JK (2001) Stereotypic behaviors in mice selectively bred for high and low methamphetamine-induced stereotypic chewing. Psychopharmacology 157(1):96–104
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4):351–354
Bonhomme V, Vanhaudenhuyse A, Demertzi A, Bruno MA, Jaquet O, Bahri MA, Plenevaux A, Boly M, Boveroux P, Soddu A, Brichant JF, Maquet P, Laureys S (2016) Resting-state network-specific breakdown of functional connectivity during ketamine alteration of consciousness in volunteers. Anesthesiology. 125(5):873–888
Cheung HM, Yew DTW (2019) Effects of perinatal exposure to ketamine on the developing brain. Front Neurosci 13:138
Favretto D, Vogliardi S, Tucci M, Simoncello I, El Mazloum R, Snenghi R (2016) Occupational exposure to ketamine detected by hair analysis: a retrospective and prospective toxicological study. Forensic Sci Int 265:193–199
Focking M, Doyle B, Munawar N, Dillon ET, Cotter D, Cagney G (2019) Epigenetic factors in schizophrenia: mechanisms and experimental approaches. Mol Neuropsychiatry 5(1):6–12
Forget P, Cata J (2017) Stable anesthesia with alternative to opioids: are ketamine and magnesium helpful in stabilizing hemodynamics during surgery? A systematic review and meta-analyses of randomized controlled trials. Best Pract Res Clin Anaesthesiol 31(4):523–531
Gururajan A, van den Buuse M (2014) Is the mTOR-signalling cascade disrupted in schizophrenia? J Neurochem 129(3):377–387
Haaf M, Leicht G, Curic S, Mulert C (2018) Glutamatergic deficits in schizophrenia - biomarkers and pharmacological interventions within the ketamine model. Curr Pharm Biotechnol 19(4):293–307
He Y, Zou Q, Li B, Chen H, Du X, Weng S et al (2016) Ketamine inhibits human sperm function by Ca(2+)-related mechanism. Biochem Biophys Res Commun 478(1):501–506
Huang S-H, Wu W-R, Lee L-M, Huang P-R, Chen J-C (2018) mTOR signaling in the nucleus accumbens mediates behavioral sensitization to methamphetamine. Prog Neuro-Psychopharmacol Biol Psychiatry 86:331–339
Hulsman N, Hollmann MW, Preckel B (2018) Newer propofol, ketamine, and etomidate derivatives and delivery systems relevant to anesthesia practice. Best Pract Res Clin Anaesthesiol 32(2):213–221
Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D (2012) Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull 38(5):958–966
Jones CA, Watson DJ, Fone KC (2011) Animal models of schizophrenia. Br J Pharmacol 164(4):1162–1194
Krajcovic B, Fajnerova I, Horacek J, Kelemen E, Kubik S, Svoboda J, et al. (2019) Neural and neuronal discoordination in schizophrenia: from ensembles through networks to symptoms. Acta Physiol (Oxford):e13282
Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003) NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 169(3–4):215–233
Kuo HY, Liu FC (2019) Synaptic wiring of corticostriatal circuits in basal ganglia: insights into the pathogenesis of neuropsychiatric disorders. eNeuro;6(3)
Lahti AC, Weiler MA, Michaelidis BT, Parwani A, Tamminga CA (2001) Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 25(4):455–467
Lee DY (2015) Roles of mTOR signaling in brain development. Exp Neurobiol 24(3):177–185
Leffa DD, Bristot BN, Damiani AP, Borges GD, Daumann F, Zambon GM, Fagundes GE, de Andrade VM (2016) Anesthetic ketamine-induced DNA damage in different cell types in vivo. Mol Neurobiol 53(8):5575–5581
Li L, Vlisides PE (2016) Ketamine: 50 years of modulating the mind. Front Hum Neurosci 10:612
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 329(5994):959–964
Li H, Lu Q, Xiao E, Li Q, He Z, Mei X (2014) Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatr 59(2):107–113
Lipton JO, Sahin M (2014) The neurology of mTOR. Neuron. 84(2):275–291
Liu Y, Lin D, Wu B, Zhou W (2016) Ketamine abuse potential and use disorder. Brain Res Bull 126(Pt 1):68–73
Liu FF, Zhao S, Liu P, Huo SP (2019) Influence of mTOR signaling pathway on ketamine-induced injuries in the hippocampal neurons of rats. Neurol Res 41(1):77–86
Loch AA (2019) Schizophrenia, not a psychotic disorder: Bleuler revisited. Front Psych 10:328
Ma J, Leung LS (2000) Relation between hippocampal gamma waves and behavioral disturbances induced by phencyclidine and methamphetamine. Behav Brain Res 111(1–2):1–11
Matuszko G, Curreli S, Kaushik R, Becker A, Dityatev A (2017) Extracellular matrix alterations in the ketamine model of schizophrenia. Neuroscience. 350:13–22
Oh S, Kingsley K (2018) Efficacy of ketamine in pediatric sedation dentistry: a systematic review. Compend Contin Educ Dent 39(5):e1–e4
Pham X, Song G, Lao S, Goff L, Zhu H, Valle D, Avramopoulos D (2016) The DPYSL2 gene connects mTOR and schizophrenia. Transl Psychiatry 6(11):e933
Ryskalin L, Limanaqi F, Frati A, Busceti CL, Fornai F (2018) mTOR-related brain dysfunctions in neuropsychiatric disorders. Int J Mol Sci;19(8)
Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M (2016) A review of ketamine abuse and diversion. Depress Anxiety 33(8):718–727
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell. 168(6):960–976
Seeman MV (2019) Schizophrenia mortality: barriers to progress. Psychiatr Q
Skodlar B, Henriksen MG (2019) Toward a phenomenological psychotherapy for schizophrenia. Psychopathology.:1–9
Switon K, Kotulska K, Janusz-Kaminska A, Zmorzynska J, Jaworski J (2017) Molecular neurobiology of mTOR. Neuroscience. 341:112–153
Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, Malaspina D, Owen MJ, Schultz S, Tsuang M, van Os J, Carpenter W (2013) Definition and description of schizophrenia in the DSM-5. Schizophr Res 150(1):3–10
Wearne TA, Cornish JL (2018) A comparison of methamphetamine-induced psychosis and schizophrenia: a review of positive, negative, and cognitive symptomatology. Front Psych 9:491
Weckmann K, Labermaier C, Asara JM, Muller MB, Turck CW (2014) Time-dependent metabolomic profiling of ketamine drug action reveals hippocampal pathway alterations and biomarker candidates. Transl Psychiatry 4:e481
Wesseling H, Rahmoune H, Tricklebank M, Guest PC, Bahn S (2015) A targeted multiplexed proteomic investigation identifies ketamine-induced changes in immune markers in rat serum and expression changes in protein kinases/phosphatases in rat brain. J Proteome Res 14(1):411–421
Winship IR, Dursun SM, Baker GB, Balista PA, Kandratavicius L, Maia-de-Oliveira JP, Hallak J, Howland JG (2019) An overview of animal models related to schizophrenia. Can J Psychiatr 64(1):5–17
Wu YQ, Liang T, Huang H, Zhu YZ, Zhao PP, Xu CM et al (2014) Ketamine inhibits proliferation of neural stem cell from neonatal rat hippocampus in vitro. Cell Physiol Biochem 34(5):1792–1801
Xu K, Krystal JH, Ning Y, He H, Wang D, Ke X et al (2015) Preliminary analysis of positive and negative syndrome scale in ketamine-associated psychosis in comparison with schizophrenia. J Psychiatr Res 61:64–72
Yoon SC, Seo MS, Kim SH, Jeon WJ, Ahn YM, Kang UG, Kim YS (2008) The effect of MK-801 on mTOR/p70S6K and translation-related proteins in rat frontal cortex. Neurosci Lett 434(1):23–28
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8):856–864
Zhuo C, Hou W, Li G, Mao F, Li S, Lin X, Jiang D, Xu Y, Tian H, Wang W, Cheng L (2019) The genomics of schizophrenia: shortcomings and solutions. Prog Neuro-Psychopharmacol Biol Psychiatry 93:71–76
Funding
This study was supported by the National Natural Sciences Foundation of China [Grant No. 81373239], the Yunnan Applied Basic Research/Kunming Medical University Union Project [Grant Nos. 2017FE467-(166) and 2018FE001-(011)], and the Kunming Medical University Innovation Group Project [Grant Nos. CXTD201604 and CXTD201803].
Author information
Authors and Affiliations
Contributions
R-F Xie and L-C Liao: study concept and design, analysis and interpretation of data, drafting of the manuscript, and obtained funding. R-F Xie and J-M Xie contributed equally to this work. R-F Xie, J-M Xie, Y Ye, X-Y Wang: the acquisition of samples or data, statistical analysis, review of the manuscript, and obtained funding. F Chen, L Yang, Y-Y Yan: statistical analysis, review of the manuscript, and material supports.
Corresponding author
Ethics declarations
All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals 8th Edition. The study was approved by the Ethics Committee of Kunming Medical University (Kunming, China).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
All authors declare that the submitted manuscript does not contain previously published data, and it is also not being under consideration for publication elsewhere.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Runfang Xie and Jiming Xie are joint first authors.
Rights and permissions
About this article
Cite this article
Xie, R., Xie, J., Ye, Y. et al. mTOR Expression in Hippocampus and Prefrontal Cortex Is Downregulated in a Rat Model of Schizophrenia Induced by Chronic Administration of Ketamine. J Mol Neurosci 70, 269–275 (2020). https://doi.org/10.1007/s12031-019-01476-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01476-9