Abstract
To investigate the role of miR-372/Beclin-1 on nerve cell apoptosis induced by spinal cord ischemia/reperfusion injury (SCII). We established in vivo and in vitro SCII model. MiR-372 and Beclin-1 expressions in spinal cord tissues of SCII rats and SCII nerve cells were measured. The cell apoptosis was detected by flow cytometry. MiR-372 inhibitor was used to reduce miR-372 expression. Dual luciferase reporter assay was used to confirm the interaction between miR-372 and Beclin-1. MiR-372 expression in spinal cord tissues of SCII rats and SCII nerve cells was increased, while Beclin-1 expression was decreased. Knockdown of miR-372 could inhibit SCII nerve cell apoptosis. In addition, MiR-372 could negatively regulate Beclin-1 expression. Autophagy inhibitor could inhibit autophagy to promote the apoptosis of SCII nerve cells through decreasing Beclin-1, while interference of miR-372 changed the effect of autophagy inhibitor. Interference of miR-372 could reduce nerve cell apoptosis in SCII via increasing autophagy by up-regulating Beclin-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord ischemia/reperfusion injury (SCII) is a common complication in vascular surgery, thoracic surgery, and spinal surgery, which can cause limb movement and sensory dysfunction in the injured spinal cord segment and seriously threatens human health and life (Wang et al. 2018b). SCII is also a serious nervous system injury, and nerve cell apoptosis is one of the pathological features of SCII (Liu et al. 2015). Therefore, exploring the mechanism of nerve damage in SCII may provide new ideas for the treatment of SCII.
Autophagy, also known as type II programmed cell death, can remove redundant or damage organelles in the process of cell growth and aging, which plays an important role in maintaining cell homeostasis (Fan et al. 2014). Recent studies have shown that autophagy is closely related to the apoptosis of nerve cells induced by SCII (Baba et al. 2009). However, the mechanism remains to be elucidated. Beclin-1 is the homolog of yeast ATG6 and is also called BECN1 (Glover et al. 2017). Beclin-1 is a key regulator of autophagy and plays an important role in autophagy activation by interacting with the class III-type phosphoinositide 3-kinase (Maejima et al. 2016). For example, Chang et al. (2017a) showed that lutein could induce autophagy by up-regulating Beclin-1 in intestinal epithelial cells. In addition, a previous study revealed that overexpression of Beclin-1 reduced spinal cord neurons apoptosis induced by mechanical injury through increasing autophagy (Wang et al. 2014), which indicated that Beclin-1-mediated autophagy might be associated with nerve cell apoptosis.
MicroRNAs (miRNAs) are small non-coding RNAs with 17–24 nucleotides in length (Chang et al. 2017b) that play important roles in cell proliferation, apoptosis, and differentiation (Ge et al. 2018). Recently, miRNAs have been shown to be closely regulated with autophagy in ischemia/reperfusion (I/R) injury. For instance, miR-17 up-regulated autophagy to aggravate hepatic I/R injury via suppressing Stat3 expression (Li et al. 2016); hydrogen sulfide protected spinal cord and induced autophagy via down-regulating miR-30c in SCII rat model (Li et al. 2015). MiR-372, a regulator of cancers, is also involved in autophagy. Chen et al. (2017) found that miR-372 could inhibit the autophagy of human pancreatic adenocarcinoma cells. However, little is known about the effect of miR-372 on autophagy in SCII. Zhou et al. (2017) showed an increased expression of miR-372 in spinal cord tissues of SCI and overexpression of miR-372 could inhibit SCI recovery. In addition, Bioinformatics software (TargetScan) predicts that miR-372 has binding sites with Beclin-1. Therefore, we suspected that miR-372 and Beclin-1 might have a certain regulatory relationship with autophagy of SCII.
In the current study, we investigated the role of miR-372/Beclin-1 in the nerve cell apoptosis and autophagy in SCII, aiming to provide therapeutic insights for SCII.
Methods
Animals and Groups
Adult Sprague-Dawley (SD) rats (200–220 g) were obtained from Shanghai Experimental Animal Research Center. All experimental procedures complied with the Animal Care and Use Committee of The First Affiliated Hospital, Zhejiang University. Rats were divided into sham group, SCII group, negative control (NC; the control of miR-372 inhibitor) group, and miR-372 inhibitor group. Each group had 12 rats. Rats in NC group and miR-372 inhibitor group: 5 min after SCII, the bilateral injections (100 nmol/l, 2 injections/side, 1 μl/injection, total 4 μl) of the NC or miR-372 inhibitor were made intrathecally at 1 mm from the mid-line. There was a 2-mm distance between the two injections on each side.
SCII Model
The SCII model was established as described previously (Li et al. 2015). Briefly, rats were anesthetized by intraperitoneal injection of 10% chloral hydrate. The aortic arch was then exposed through a cervicothoracic approach and cross-clamped between the left common carotid artery and the left subclavian artery for 14 min to induce ischemia. A 90% decrease in the blood flow measured at the femoral artery was considered as ischemia and confirmed using a Doppler monitor (Moor Instruments, Axminster, UK). Then, the clamps were removed, and reperfusion was allowed to continue for 60 h. The rats in sham group underwent the same procedure without aortic arch occlusion.
Assessment of Neurologic Function
The hind limb motor function of rats at five time points (1, 6, 12, 24, and 48 h) after the SCII was scored by the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale. The BBB scores range from 0 (complete paralysis) to 21 (normal hind limb motor locomotion). After the BBB score evaluation, rats were sacrificed and the spinal cord tissues (between L2 and L5 levels) were obtained and stored at − 80 °C. Spinal cord tissues in each rat were divided into parts for different purposes.
SCII Cell Model
Nerve cell line (PC12) was purchased from American Type Culture Collection (Shanghai, China). PC12 cells were cultured in hypoxia condition to establish an in vitro SCII cell model (Wang et al. 2018a). Cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco, USA) containing 5% fetal bovine serum (Gibco), 10% horse serum (Gibco), penicillin (100 U/ml), and streptomycin (100 μg/ml). After incubated at 37 °C in a humidified atmosphere of 5% CO2 for 24 h, PC12 cells were cultured in an oxygen-free incubator (Thermo Fisher Scientific, USA) with an atmosphere of 95% N2 and 5% CO2.
Transient Transfection
MiR-372 mimic, miR-372 inhibitor, si-Beclin-1, and the corresponding negative controls were synthesized by GenePharma (Shanghai, China). PC12 cells were transiently transfected with them using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. After transfection for 24 h, cells were processed for further analysis.
Quantitative Real-Time PCR (qRT-PCR)
According to the manufacturer’s protocol, TRIzol reagent (Invitrogen) was used to extract total RNA from spinal cord tissues or PC12 cells. Then, cDNA was synthesized by using a cDNA Synthesis Kit (Takara, Shiga, Japan). SYBR Green Reagents of ABI Prism 7700 System (Takara) was used to analyze the data. The 2−ΔΔCt method was used to calculate the relative expression of miR-372.
Western Blot
Total proteins were extracted from spinal cord tissues or PC12 cells using RIPA buffer (Beyotime, Shanghai, China) on the ice for 15 min and centrifuged at 12,000 rpm for 15 min. The concentrations of total proteins were then determined by BCA Protein Assay kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Samples contained same amount of proteins were separated on 10% SDS-PAGE and transferred to PVDF membranes. Afterward, the membranes were blocked with 5% skim milk for 1 h at room temperature and incubated overnight at 4 °C with the primary antibodies, including anti-microtubule-associated protein 1 light chain 3 (LC3-I) antibody, anti-LC3-II antibody, anti-Beclin-1 antibody, anti-sequestosome-1 (p62) antibody, anti-Bcl-2-associated X protein (Bax) antibody, and anti-β-actin antibody (all in 1:1000, Cell Signaling Technology). Then, the membranes were incubated with horseradish peroxidase-conjugate anti-human or anti-rabbit secondary antibody (1:2000, Santa Cruz) at room temperature for 1 h. Protein-antibody complexes were detected by the enhanced chemiluminescence system (ECL, Roche Molecular Biochemicals).
Cell Apoptosis Assay
The cell apoptotic was detected by flow cytometry. Briefly, PC12 cells were collected and centrifuged at 1500 rpm for 5 min. Then, cells were washed with PBS for three times and stained in PI/Annexin-V at room temperature for 15 min. The apoptotic cells were measured using flow cytometry. Percentage of apoptosis rate (%) = (number of apoptotic cells/number of all cells) × 100%.
Dual Luciferase Reporter Assay
According to the binding sites predicted by bioinformatics software (TargetScan), PCR amplification primers were designed and the wild type (WT) or mutant (Mut) of Beclin-1 3′-UTR fragment containing miR-372 sequence was constructed. PC12 cells were transfected with Beclin-1-WT (or Beclin-1-Mut) and miR-372 mimics (or miR-372 inhibitor) using Lipofectamine 2000 reagent (Invitrogen). After transfection for 24 h, luciferase activity was detected by dual Luciferase detection system (Promega).
Statistical Analysis
SPSS 20.0 software was used to for data analysis. All experiments were performed at least three times, and all data are expressed as mean ± standard deviation (SD). Student’s t test was performed to compare the difference between two groups. One-way ANOVA followed by the Bonferroni post hoc test was used to evaluate the statistical significance between different groups. P < 0.05 was considered statistically significant.
Results
MiR-372 Expression Was Increased and Beclin-1 Expression Was Decreased in Both In Vivo and In Vitro SCII Model
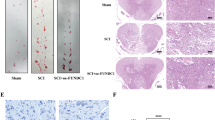
SCII rat model was established, and the hind limb motor function of rats was assessed via the BBB score at five time points (1, 6, 12, 24, and 48 h) after operation. Compared to the sham group, the SCII group showed a significant decreased BBB scores in each time points (Fig. 1a). The sham and SCII model group were sacrificed at 48 h post-operation, and the spinal cord tissues were obtained for analyzing the miRNA level via qRT-PCR. The miR-372 level of spinal cord tissues from SCII rat models was greatly increased (Fig. 1b). And increased miR-372 level was accompanied by decreased protein level of Beclin-1 (Fig. 1c). Then, to investigate the interplay between miR-372 and nerve cell apoptosis in SCII, we established SCII cell model. Being consistent with the previous finding in SCII rat model, the miR-372 level was greatly up-regulated while the Beclin-1 protein level was greatly down-regulated in SCII group (Fig. 1d, e).
The expression of miR-372 and Beclin-1 in both in vivo and in vitro SCII model. Rats were divided into two groups, namely sham group (n = 12) and SCII group (n = 12). In SCII group, in vivo SCII rat models were established. The hind limb motor function of rats was assessed via the BBB score at five time points (1, 6, 12, 24, and 48 h) after operation. The rats were sacrificed at 48 h post-operation, and the spinal cord tissues were collected for qRT-PCR and western blot. PC12 cells were divided into sham group and SCII group. In SCII group, PC12 cells were treated with hypoxia to establish an in vitro SCII cell model. a BBB scores in each time points. b The miR-372 level in the spinal cord tissues of rats. c Beclin-1 expression in the spinal cord tissues of rats. d MiR-372 expression in PC12 cells. e Beclin-1 expression in PC12 cells. *P < 0.05 vs sham group
Interference of miR-372 Ameliorated Nerve Cell Apoptosis in SCII
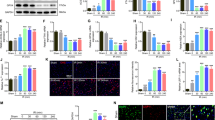
In previous data, we found that miR-372 level was significantly increased in both in vivo and in vitro SCII models. Thus, we doubted that if miR-372 might be a cell apoptosis mediating factor in the post-SCII pathology. We inhibited miR-372 via transfecting miR-372 inhibitor into PC12 SCII cell model (Fig. 2a), and the cell apoptosis were accessed by flow cytometry. Results showed that the protein level of Bax (a pro-apoptosis regulator) was decreased after miR-372 inhibition (Fig. 2b). Moreover, interference of miR-372 reduced the apoptosis of PC12 cells after SCII (Fig. 2c).
The role of miR-372 on nerve cell apoptosis in SCII. PC12 cells were divided into NC group and miR-372 inhibitor group. MiR-372 inhibitor was used to reduce miR-372 expression in PC12 cells. a MiR-372 expression in PC12 cells. b Beclin-1 expression in PC12 cells. c The apoptosis of PC12 cells. *P < 0.05 vs NC group
MiR-372 Regulated Beclin-1 Expression
In this experiment, we investigated whether miR-372 would be a regulator of Beclin-1. To study the relationship between miR-372 and Beclin-1, we first utilized the computational analyze the nucleotide sequences and result showed that miR-372 could potentially bind to the 3′-UTR of Beclin-1 (Fig. 3a). Next, to confirm this, we performed dual luciferase reporter assay. The results showed that miR-272 mimic inhibited the luciferase activity of Beclin-1-WT in PC12 cells, while miR-372 inhibitor increased the luciferase activity of Beclin-1-WT (Fig. 3b). Finally, we further confirmed the regulatory role of miR-372 on Beclin-1 by assessing Beclin-1 protein expression after PC12 cells transfected with miR-372 mimic or miR-372 inhibitor. The results showed that miR-272 mimic down-regulated Beclin-1 expression, and miR-372 inhibitor up-regulated Beclin-1 expression (Fig. 3c).
Interference of miR-372 Enhanced Autophagy to Reduce Nerve Cell Apoptosis by Up-regulating Beclin-1
In this experiment, we explored the mechanism of miR-372/Beclin-1 in nerve cell apoptosis. PC12 SCII cell models were treated with 10 mM 3-methyladenine (3-MA; a common inhibitor of PI3K mediated autophagy) for 24 h after transfected with miR-372 inhibitor and si-Beclin-1. Autophagy protein (LC3I, LC3II, p62) expression and apoptosis protein (Bax) expression was measured using western blot. As shown in Fig. 4a, b, miR-372 inhibitor reversed the decrease of Beclin-1 expression induced by 3-MA, the decrease of LC3-II/LC3-I ratio, and the increase expression of Bax and p62, while si-Beclin-1 countered its effect. In addition, interference of miR-372 inhibited PC12 cell apoptosis, and knockdown of Beclin-1 promoted the cell apoptosis (Fig. 4c).
Interference of miR-372 enhanced autophagy to reduce nerve cell apoptosis by up-regulating Beclin-1. SCII cell models were treated with 10 mM 3-MA (a common inhibitor of PI3K-mediated autophagy) for 24 h after transfected with miR-372 inhibitor and si-Beclin-1. a Beclin-1 expression in PC12 cells. b The expression of LC3-II, LC3-I, Bax, and p62 in PC12 cells. c The apoptosis of PC12 cells. *P < 0.05 vs control group; #P < 0.05 vs 3-MA+NC group; &P < 0.05 vs 3-MA+miR-372 inhibitor+si-control group
Interference of miR-372 Enhanced Autophagy to Inhibit Nerve Cell Apoptosis Induced by SCII In Vivo via Increasing Beclin-1
The rescuing effects of miR-372 inhibition in in vitro experiments may imply a possible strategy to relieve SCII. We then further confirmed the protective role of miR-372 inhibition in animal models. MiR-372 inhibitor was injected into the spinal cord injury region of the rat SCII models. Compared to NC group, injecting miR-372 inhibitor could lead better functional recovery after SCII (Fig. 5a). After the BBB score evaluation, the rats were sacrificed and the spinal cord tissues were obtained to measure the miR-372 level as well as the targeting protein levels. The miR-372 level was greatly decreased in the miR-372 inhibitor injection group (Fig. 5b). Moreover, both p62 and Bax were decreased after miR-372 inhibitor injection (Fig. 5c).
Interference of miR-372 enhanced autophagy to inhibit nerve cell apoptosis induced by SCII in vivo via increasing Beclin-1. Rats were divided into NC group and miR-372 inhibitor group. Each group had 12 rats. SCII rat models were established, NC or miR-372 inhibitor (100 nmol/l) was injected in injury site of the spinal cord of rats. The hind limb motor function of rats was assessed via the BBB score at five time points (1, 6, 12, 24, and 48 h) after operation. The rats were sacrificed at 48 h post-operation, and the spinal cord tissues were collected for qRT-PCR and western blot. a BBB scores in each time points. b The miR-372 level in the spinal cord tissues of rats. c The expression of Beclin-1, LC3-II, LC3-I, Bax, and p62 in the spinal cord tissues. *P < 0.05 vs NC group
Discussion
The nerve cell apoptosis is one of the main causes of nerve injury during spinal cord I/R (Gokce et al. 2016). Unfortunately, there is no effective therapy for neuroprotection following SCII. To our knowledge, miRNAs play key roles in nerve cells apoptosis induced by SCII. MiR-221 relieved the inflammatory response and neuronal cell apoptosis by targeting TNFAIP2 in SCII (Zhao et al. 2018). Knockdown of miR-448 inhibited the apoptosis of nerve cells by up-regulating SIRT1 in SCII (Wang et al. 2018b). MiR-372, a novel miRNA, is closely related with various cancers. Cheng et al. (2018) found that miR-372 promoted the proliferation of breast cancer cell by targeting LATS2. Wang et al. (2017) reported that miR-372 induced cell growth and metastasis in lung squamous cell carcinoma by targeting FGF9. In neuronal damage following SCI, previous study revealed that knockdown of miR-372 might provide benefits for SCI treatments. However, the relationship between miR-372 and SCII has not been studied.
In this study, we found a significantly increased expression of miR-372 in spinal cord tissues of SCII rats and SCII nerve cells, and the nerve cell apoptosis was inhibited after down-regulating miR-372 expression using miR-372 inhibitor. These results indicated a relation between miR-372 and nerve cells apoptosis induced by SCII. To understand the specific mechanism of miR-372 in the process, we conducted further research.
Bioinformatics software (TargetScan) results showed that miR-372 and Beclin-1 had binding sites. Beclin-1 is the key autophagy gene (Valente and Morani 2014). It has been reported that knockdown of Beclin-1 could inhibit autophagy (Wang et al. 2014). Our results also showed that interfering with Beclin-1 inhibited autophagy. These results indicated that si-Beclin-1 might be an autophagy inhibitor. As a form of programmed cell death, autophagy plays a very important role in balancing cell energy and promoting cell survival (Divac Rankov et al. 2017). Fang et al. (2017) revealed that electroacupuncture suppressed neurocyte apoptosis and neuroinflammation induced by SCII through enhancing autophagy in rats. In the current study, we found that Beclin-1 expression was markedly reduced in spinal cord tissues of SCII rats and SCII nerve cells, and 3-MA (an inhibitor of autophagy) could inhibit autophagy to promote the apoptosis of SCII nerve cells through decreasing Beclin-1. As expected, knockdown of miR-372 could change the effect of autophagy inhibitor on Beclin-1 expression and SCII nerve cells apoptosis. In addition, the mechanism of miR-372 on SCII has been verified in in vivo experiment.
In summary, our data demonstrated that interference of miR-372 could increase autophagy to reduce nerve cell apoptosis in SCII by up-regulating Beclin-1. This is the first time to report the effect of miR-372 on SCII, which might provide a novel insight for SCII treatments. However, this study had limitations. First, there is a lack of detection of clinical samples. Second, only one cell line was studied. But we also did in vivo studies to verify the mechanism. In further studies, these limitations need to be determined.
References
Baba H, Sakurai M, Abe K, Tominaga R (2009) Autophagy-mediated stress response in motor neuron after transient ischemia in rabbits. J Vasc Surg 50:381–387. https://doi.org/10.1016/j.jvs.2009.03.042
Chang CJ, Lin JF, Hsiao CY, Chang HH, Li HJ, Chang HH, Lee GA, Hung CF (2017a) Lutein induces autophagy via Beclin-1 upregulation in IEC-6 rat intestinal epithelial cells. Am J Chin Med 45:1273–1291. https://doi.org/10.1142/s0192415x17500707
Chang Z, Zhong Y, Jia Y (2017b) The role of microRNAs in the occurrence and development of esophageal squamous cell carcinoma. Clin Surg Res Commun 1:1–9
Chen H, Zhang Z, Lu Y, Song K, Liu X, Xia F, Sun W (2017) Downregulation of ULK1 by microRNA-372 inhibits the survival of human pancreatic adenocarcinoma cells. Cancer Sci 108:1811–1819. https://doi.org/10.1111/cas.13315
Cheng X, Chen J, Huang Z (2018) miR-372 promotes breast cancer cell proliferation by directly targeting LATS2. Exp Ther Med 15:2812–2817. https://doi.org/10.3892/etm.2018.5761
Divac Rankov A, Ljujic M, Petric M, Radojkovic D, Pesic M, Dinic J (2017) Targeting autophagy to modulate cell survival: a comparative analysis in cancer, normal and embryonic cells. Histochem Cell Biol 148:529–544. https://doi.org/10.1007/s00418-017-1590-4
Fan J, Zhang Z, Chao X, Gu J, Cai W, Zhou W, Yin G, Li Q (2014) Ischemic preconditioning enhances autophagy but suppresses autophagic cell death in rat spinal neurons following ischemia-reperfusion. Brain Res 1562:76–86. https://doi.org/10.1016/j.brainres.2014.03.019
Fang B, Qin M, Li Y, Li X, Tan W, Zhang Y, Ma H (2017) Electroacupuncture preconditioning and postconditioning inhibit apoptosis and neuroinflammation induced by spinal cord ischemia reperfusion injury through enhancing autophagy in rats. Neurosci Lett 642:136–141. https://doi.org/10.1016/j.neulet.2017.02.010
Ge WQ, Hao P, Huang YH, Hou JQ, Pu JX, Wang LL (2018) miR-539 inhibits inflammation in renal transplant iscemia-reperfusion injury via blocking the MyD88/NF-κB pathway. Clin Surg Res Commun 2:14–21
Glover K, Li Y, Mukhopadhyay S, Leuthner Z, Chakravarthy S, Colbert CL (2017) Structural transitions in conserved, ordered Beclin 1 domains essential to regulating autophagy. J Biol Chem 292:16235–16248. https://doi.org/10.1074/jbc.M117.804195
Gokce EC et al (2016) Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J Neurosurg Spine 24:949–959. https://doi.org/10.3171/2015.10.spine15612
Li L, Jiang HK, Li YP, Guo YP (2015) Hydrogen sulfide protects spinal cord and induces autophagy via miR-30c in a rat model of spinal cord ischemia-reperfusion injury. J Biomed Sci 22:50. https://doi.org/10.1186/s12929-015-0135-1
Li S, Zhang J, Wang Z, Wang T, Yu Y, He J, Zhang H, Yang T, Shen Z (2016) MicroRNA-17 regulates autophagy to promote hepatic ischemia/reperfusion injury via suppression of signal transductions and activation of transcription-3 expression. Liver Transpl 22:1697–1709. https://doi.org/10.1002/lt.24606
Liu B, Huang W, Xiao X, Xu Y, Ma S, Xia Z (2015) Neuroprotective effect of ulinastatin on spinal cord ischemia-reperfusion injury in rabbits. Oxidative Med Cell Longev 2015:624819. https://doi.org/10.1155/2015/624819
Maejima Y, Isobe M, Sadoshima J (2016) Regulation of autophagy by Beclin 1 in the heart. J Mol Cell Cardiol 95:19–25. https://doi.org/10.1016/j.yjmcc.2015.10.032
Valente G, Morani F (2014) Expression and clinical significance of the autophagy proteins BECLIN 1 and LC3 in ovarian cancer. Biomed Res Int 2014:462658. https://doi.org/10.1155/2014/462658
Wang ZY, Lin JH, Muharram A, Liu WG (2014) Beclin-1-mediated autophagy protects spinal cord neurons against mechanical injury-induced apoptosis. Apoptosis 19:933–945. https://doi.org/10.1007/s10495-014-0976-1
Wang Q, Liu S, Zhao X, Wang Y, Tian D, Jiang W (2017) MiR-372-3p promotes cell growth and metastasis by targeting FGF9 in lung squamous cell carcinoma. Cancer Med 6:1323–1330. https://doi.org/10.1002/cam4.1026
Wang P et al (2018a) AMP-activated protein kinase-dependent induction of autophagy by erythropoietin protects against spinal cord injury in rats CNS. Neurosci Ther. https://doi.org/10.1111/cns.12856
Wang Y, Pang QJ, Liu JT, Wu HH, Tao DY (2018b) Down-regulated miR-448 relieves spinal cord ischemia/reperfusion injury by up-regulating SIRT1. Braz J Med Biol Res 51:e7319. https://doi.org/10.1590/1414-431x20177319
Zhao D, Deng SC, Ma Y, Hao YH, Jia ZH (2018) miR-221 alleviates the inflammatory response and cell apoptosis of neuronal cell through targeting TNFAIP2 in spinal cord ischemia-reperfusion. Neuroreport 29:655–660. https://doi.org/10.1097/wnr.0000000000001013
Zhou W, Yuan T, Gao Y, Yin P, Liu W, Pan C, Liu Y, Yu X (2017) IL-1beta-induces NF-kappaB and upregulates microRNA-372 to inhibit spinal cord injury recovery. J Neurophysiol 117:2282–2291. https://doi.org/10.1152/jn.00936.2016
Funding
This study was supported by the Natural Science Foundation of China (No. 81401004 and No. 81402862).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Lou, X., Xu, S. et al. Knockdown of miR-372 Inhibits Nerve Cell Apoptosis Induced by Spinal Cord Ischemia/Reperfusion Injury via Enhancing Autophagy by Up-regulating Beclin-1. J Mol Neurosci 66, 437–444 (2018). https://doi.org/10.1007/s12031-018-1179-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1179-y