Abstract
Multiple sclerosis (MS) is a chronic auto-inflammatory disease of the central nervous system (CNS) and hard to heal. This study aimed to investigate the effect of melatonin on mice with experimental autoimmune encephalomyelitis (EAE), a widely used MS model, and its potential mechanism underlying the action of MT on anti-oxidative stress. Female C57BL/6 mice were injected with MOG35–55 peptide to set up the EAE model, and for detection of the effect of melatonin (10 mg/kg i.p.) on the development and progression of EAE. Combining immunohistochemistry, biochemical technology and western blot approaches, the potential molecular mechanism of melatonin on EAE was evaluated as the levels of oxidative stress and the expression of Nrf2/ARE signal pathway. Our experiments showed a change of oxidative stress and Nrf2/ARE pathway expression in different groups, demonstrating that oxidative stress is associated with the pathophysiology of EAE. The administration of melatonin exerts neuroprotective effects against EAE, notably in suppressing the progression of EAE and pathological changes (lymphocytic infiltration). Furthermore, the effect of melatonin was probably related to decrease of the levels of oxidative stress, by activation of the Nrf2/ARE pathway and increased levels of anti-oxidant enzymes HO-1 and NQO1 expression. So, melatonin may be a promising reagent for intervention for multiple sclerosis in the future, and even for other autoimmune diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic immuno-mediated inflammatory disease in the central nervous system (CNS), which typically affects young adults (Compston and Coles 2008). The etiology of MS is still not completely understood (Ebers 2008). Genetic, environmental, and other viral factors may contribute to the triggering of an aberrant autoimmune attack which may result in damaging the myelin and axons (Bruck and Stadelmann 2005). Notably, inflammation-induced oxidative stress also contributes to the pathogenesis of MS. Activating macrophages and astrocytes can produce large amounts of reactive oxygen species (ROS) or reactive nitrogen species (RNS) (Croxford et al. 2015). These substances are extremely unstable and have strong oxidation activity. Additionally, oxygen consumption in the central nervous system is large, and the cell membranes are rich in polyunsaturated fatty acids (Garg and Smith 2015), but the concentration of antioxidant substances in CNS was lower than that in peripheral blood (Ohl et al. 2016), so it is predisposed to damage from oxidative stress. A number of studies have confirmed that the cerebrospinal fluid, blood samples, urine and/or nervous tissue homogenates in MS (Hammann and Hopf 1986; Koch et al. 2006) and EAE mice (Nikic et al. 2011; van Horssen et al. 2011; Dimitrijevic et al. 2017) presented oxidative stress. Hence, it is not surprising that oxidative stress is a critical factor in both EAE and MS pathogenesis.
The Nrf2/ARE signaling pathway is the key regulator for defense against oxidative stress of the body (Ishii et al. 2000). Under an unstressed situation, transcription factor NF-E2 related factor (Nrf2) and Keap1 are bound together in the cytoplasm (Fukutomi et al. 2014). When exposed to free radical and electrophilic material, Nrf2 evades Keap1, and translocates into the nucleus, combining with antioxidant response elements (ARE), inducing transcription of target genes, such as II phase detoxification enzymes, antioxidant proteins, such as heme oxygenase-1(HO-1), NAD(P)H:quinone oxidoreductase 1 (NQO-1) (Saito et al. 2015). Importantly, a number of studies have identified that Nrf2/ARE activation acts as a central player in MS and EAE. Nrf2 and its target genes including HO-1 and NQO1 are upregulated in MS patient lesions and EAE animals (van Horssen et al. 2006). The induction of Nrf2 and HO-1 can inhibit disease development and progression of EAE (Liu et al. 2014; Higashi et al. 2017), while mice with Nrf2 or HO-1 gene deficiency developed more serious lesions in the CNS than wild type mice (Chora et al. 2007).Furthermore, some recent studies reported that the Nrf2/ARE signaling pathway can regulate immune and inflammatory responses. Nrf2-deficient mice exhibit more severe inflammation in a model of acute pleurisy (Itoh et al. 2004) and the mouse model of sickle cell disease (Keleku-Lukwete et al. 2015). In EAE animals, up-regulation of HO-1 inhibits IL-17 production, while promoting Foxp3 expression and IL-10 production, thus regulating Treg/Th17 balance. Together, the Nrf2/ARE pathway may be a novel therapeutic target for MS.
Melatonin can freely penetrate the blood brain barrier (BBB)(Kutzelnigg and Lassmann 2014), and exhibits multifunctional properties, including antiaging, as an antioxidant, and immunomodulatory effects (Calvo et al. 2013; Hardeland 2013). Several studies have reported alteration in melatonin levels and its main metabolite, 6-sulphatoxymelatonin (6-SMT) in MS patients (Melamud et al. 2012; Damasceno et al. 2015). Melatonin levels are negatively correlated with MS clinical relapses (Farez et al. 2015). Melatonin administration has also been shown to improve sleep and quality of life in MS patients (Adamczyk-Sowa et al. 2014a, b). Melatonin modulates adaptive and innate immunity, which has been confirmed in a variety of both central and peripheral disease conditions involving immune activation (Carrillo-Vico et al. 2005, 2013). In the EAE model, some studies have reported that melatonin have anti-inflammatory effects. Kang and his colleagues (Kang et al. 2001) found MT can ameliorate the clinical signs of EAE and diminished mononuclear infiltrates by suppressing intracellular adhesion molecule 1 (ICAM-1) expression in the spinal cord. Another study found that melatonin protected from EAE by decreasing peripheral and central Th1/Th17 responses and enhancing both the Treg frequency and IL-10 synthesis in the CNS (Alvarez-Sanchez et al. 2015a, b). However, MT is also an efficient free radical scavenger, which can prevent damage from excessive free radicals by clearing the superoxide anion (O2–), NO, ONOO- (Arora and Bhatla 2017). A recent study found that administration of MT is effective in reducing clinical signs and free radical generation in the EAE model of MS(Wen et al. 2016). To our knowledge, there is no study regarding how melatonin modulates oxidative stress in a MOG-induced EAE model. Thus, the objective of this work was to elucidate the therapeutic role of melatonin in EAE and its possible antioxidant stress mechanisms. In the present study, the EAE model was established by MOG35–55 polypeptides. Our findings indicated that treatment with melatonin inhibited the development and progression of EAE, and recovered the oxidation and antioxidant balance, through activating the Nrf2/ARE signal pathway, thereby promoting anti-oxidant enzyme expression.
Materials and Methods
Animals
Female C57BL/6 mice (weighing 18-20 g, aged 8–10 weeks) were purchased from the Chengdu Dashuo laboratory animal Company (Number: 17–199 SPFC57), and reared in the animal room of Southwest Medical University at a room temperature of 24 ± 2 ° C, and maintained on a 12 h light/dark cycle. Animals were given enough food and drinking water. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Luzhou Medical College.
Induction and Assessment of EAE and Treatment
EAE was induced as described previously (Li et al. 2013a, b). Briefly, mice were injected subcutaneously at one side of the flank with 250 μg MOG35–55 peptide (Beijing Keya Light Biotech Corp., Beijing, China) in complete Freund’s adjuvant (CFA, Sigma, St. Louis, MO, USA) and 64 mg/ml of heat-killed Mycobacterium tuberculosis (Difco Laboratories, Detroit, MI, USA). At 0 h and 48 h after MOG injection, 500 ng pertussis toxin in 100 uL PBS was injected intraperitoneally (Chengdu Biological Products, Chengdu, China). The severity of neurological deficit was measured daily by two independent experienced observers blind to animal treatment. Neurological deficit was scored as follows:0 signified no paralysis, 1 signified loss of tail tone, 2 signified hindlimb weakness, 3 signified hindlimb paralysis, 4 signified hindlimb and forelimb paralysis; and 5 signified moribund and death.
Melatonin intervention mice were treated with melatonin(Selleck,USA) 10 mg / kg (10 mg / ml) daily, with treatment on day 0 post immunization of EAE, by intraperitoneal injection, and the control mice only used vehicle saline 1 ml / kg. Animals in accordance with the requirements were injected with the above substances successively to the peak period of disease, then the mice were sacrificed. The criterion for judging the peak period of the disease is that the clinical symptoms of mice did not worsen for three consecutive days or the mice died. Mice in the healthy control group or not diseased were sacrificed at 28 days after modeling.
Histology and Immunohistochemistry
Mice were anesthetized with 10% chloral hydrate, and were perfused through the left cardiac ventricle with saline until the liver turned white; then were irrigated with 4% poly formaldehyde (PFA) until the four limbs and tail stiffened and then decapitated. The brain and spinal cord were removed carefully with a pair of tweezers. Finally the tissues were soaked in 4% PFA for 24 h, and processed for paraffin embedding. The non-serial sections of lumbar spinal cord (5um/each, interval of 50 μm) of each mouse were used for HE staining to detect inflammatory infiltrates. Another three non-serial sections of each mouse were used to detect the levels of Nrf2, HO-1 and NQO1 expression by means of immunohistochemistry(IHC). Primary antibodies were rabbit anti Nrf2 polyclonal antibody(Abcam), anti HO-1 polyclonal antibody(Abcam), and anti-NQO1 (Abcam). The dilution ratio was 1:50. Secondary antibodies were goat anti-mouse IgG antibody as HRP labeled, and with a dilution ratio of 1:100. Lastly, each slice was randomly selected for five fields, and the positive cells were counted under a light microscope.
Biochemical Assays
The degrees of oxidative stress in the brain were assessed for the contents of Thiobarbituric Acid Reactive Substance (TBARS), ROS and the activity of superoxide dismutase (SOD), and catalase (CAT). Briefly, mice were decapitated at the peak stage of EAE, and periventricular brain tissue of 0.5 g was obtained. The tissues were washed in ice cold normal saline, then pieces of brain tissue were cut rapidly, and then diluted ten times with normal saline, and ground in a glass homogenizer on ice to make a brain tissue homogenate. Then the homogenate was centrifuged at 3000 rpm for 15 min to collect supernatant. The supernatant was stored at −80 °C for further analysis. Commercially available assay kits were used to assess the concentration of SOD, CAT, TBARS, and ROS (Nanjing Institute of Biological Engineering, Nanjing, China). All analyses were performed according to kit instructions.
Western Blot Analysis
Western blot was performed as previously described. Tissue samples of spinal cord were homogenized and protein concentration was determined by BCA protein assay kit (Beyotime Biotechnology). Then, equal amounts of protein were applied to 10% SDS-PAGE gels, and electrophoretically transferred onto PVDF membranes (Millipore). The membranes were blocked in blocking buffer and probed with indicated primary antibodies overnight at 4 °C and followed by secondary antibodies conjugated to HRP with one of the following antibodies:anti-Nrf2 antibody (anti-rabbit, 1:1000;Cell Signaling Technology); anti-HO-1 antibody (anti-rabbit, 1:1000; Cell Signaling Technology), and anti-NQO1 antibody (anti-rabbit, 1:1000; Cell Signaling Technology). Enhanced chemiluminescence was performed with a BeyoECL Plus Kit (Beyotime). The density of bands was quantified using Fuion software.
Statistical Analysis
All data analyses were performed using GraphPad Prism 6.00 or SPASS 17.0. All continuous data are present as the mean ± SD or mean ± SEM. Statistical analysis was performed by two-tailed unpaired Student’s t-tests, chi-square test or one-way ANOVA with post-hoc analysis by Tukey’s multiple comparison test. P-value <0.05 was considered statistically significant.
Results
Treatment with Melatonin Reduced the Severity of EAE in Mice
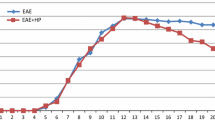
To determine the potential protective effect of melatonin on EAE, C57BL/6 mice were immunized with MOG35–55 in CFA to induce EAE, and the mice were randomly treated with melatonin (10 mg/kg,ip.) (EAE-MT treated group) or vehicle (1 ml/kg,ip.) (EAE-untreated group) alone. The incidence and severity of clinical signs in the different groups of mice are presented in Table 1. In both of the EAE-untreated groups and the EAE-MT treated group, mice had a different morbidity. The incidence of EAE was higher in the EAE-untreated group than in the EAE-MT treated group (81.3% and 56.3%, respectively) (Table 1). But the difference was not statistically significant (P > 0.05). Early onset symptom mainly manifested as listlessness, reduction of eating and drinking, and loss of body weight; and the site of injection antigen appeared in duration, swelling, loss of hair or even ulceration. Mice subsequently developed symptoms of neurological dysfunction, such as tail tension decline, which were usually the first symptoms of EAE, and gradually there appeared paralysis of the double hind limb or forelimb, and there even appeared tetraplegia and incontinence. There were no dead mice in both groups. No adverse effects associated with the melatonin treatment were observed. The neurological deficit scores at the peak stage of EAE (the maximum clinical scores) in the EAE-untreated group was greater than that of the EAE-MT treated group (P < 0.05) (Table 1). The first neurological signs of EAE (latent period) appeared later in the EAE-MT treated group than in the EAE-untreated group mice (p < 0.05) (Fig.1). In addition, the clinical symptomatic stage of the EAE (advanced stage) was of shorter duration in the EAE-MT treated group when compared with the EAE-untreated group (p < 0. 05) (Table 1).
Melatonin treatment mitigates pathological changes of EAE. C57BL/6 mice were immunized with MOG35–55 to induce EAE, and the mice were randomly treated with melatonin or vehicle on day 0 post immunization. HE staining of spinal cords in EAE -untreated group (A) and EAE-MT treated group (B). The degree of inflammatory cells infiltration in the melatonin treated mice was significantly lower than that in the EAE mice. H & E stain, Scale bar = 50um
Treatment with Melatonin Mitigates Inflammatory Infiltrates in the Spinal Cord
We examined the pathological changes in the spinal cords from the EAE-untreated and EAE-MT treated mice at the peak stage of EAE. There was no detectable inflammatory infiltration in the spinal cord of healthy control group mice; while a mass of inflammatory cells infiltrated in the spinal cord in EA -untreated group, especially around the small venous, accompanied by small vascular congestion. The main clustered cells were single nucleus. The typical change was inflammatory cells gathering around small blood vessels, which were described as “vascular cuff”. Compared with the EAE-untreated mice, the number of infiltrating inflammatory cells was obviously reduced in the EAE-MT treated group (Fig 1).
Melatonin Treatment Suppresses Oxidative Stress of EAE
Oxidative stress contributed to the pathogenesis of MS or EAE. We next measured the TBARS, ROS, SOD and CAT expression in the brain of different groups to detect the level of oxidative stress of EAE. At the peak stage of EAE, the mean TBARS and ROS concentrations were significantly higher in either EAE-untreated or EAE-MT treated groups than in the healthy control group (P < 0.05, Fig. 2). Besides, the levels of SOD and CAT were significantly lower in either EAE-untreated or MT-treated groups than that in the healthy control group. But we observed that compared with the EAE-untreated mice, treatment with melatonin decreased the concentration of TBARS and ROS and increased the level of SOD and CAT (Fig. 2).
Melatonin Treatment can Up-Regulate Nrf2 /ARE Pathway
The Nrf2/ARE signal pathway and its related antioxidant enzymes, such as HO-1,NQO1 has been identified as an important role in EAE or MS to inhibit the oxidative stress damage. To explore the mechanism of melatonin on EAE, we next examined the Nrf2, HO-1 and NQO1 expression in different groups by immunohistochemistry and Western blot assays. The number of Nrf2,HO-1 and NQO1 positive cells of the spinal cord in different groups at the peak stage of disease are showed in Table 2. Compared with the control mice, the expression of Nrf2, HO-1 and NQO1 in the spinal cord of EAE increased at the peak stage of disease (Fig 3). But the level of Nrf2, NQO1 and HO-1 increased notably in the melatonin treated mice compared with EAE-untreated mice (P < 0.05). In the healthy mice, the Nrf2 were mainly detected in the cytoplasm of cells in the brain, but in the EAE mice, it was observed in both nuclei and cytoplasm of cells (Fig. 3A-C). A similar change of Nrf2, HO-1 and NAQ1 expression was detected in different groups of mice by Western blot assays. Collectively, treatment with melatonin can up-regulate Nrf2, HO-1 and NQO1 expression in the spinal cord and then contribute to its antioxidant activity.
Melatonin treatment activated the Nrf2/ARE pathway to reduce oxidative stress in EAE mice.(a-c) Immunohistochemical detection of Nrf2, HO-1 and NQO1 in lumber enlargement of mice from different groups at the peak stage of EAE or on day 28 post immunization.(d,e) Western blot analysis of Nrf2, HO-1 and NQO1 in the lumbar enlargement of EAE mice in different groups of mice. β-actin was used as the internal standard of cytosolic protein. The expression of Nrf2, HO-1 and NQO1 in spinal cords increased in melatonin intervention groups (n = 5 per group). Scale bar = 50um.*compared with Control group, P < 0.05,# compared with EAE-untreated group, P < 0.05
Discussion
In this study, we found that melatonin treatment can reduce the neurological dysfunction score, prolong the onset, and shorten the progress of EAE. Additionally, melatonin treatment can reduce the numbers of inflammatory infiltrates in the CNS of EAE. So, melatonin can have a neuroprotective effect on EAE. Our results are consistent with previous studies (Kashani et al. 2014; Chen et al. 2016a, b). We found the main concern of these studies is the immunosuppressive effects of melatonin in the EAE. In addition, melatonin and its metabolites effectively scavenge antioxidants. This study found another role of melatonin on EAE, which can regulate the oxidative imbalance. Then we assumed how did melatonin play this role?
The etiology and pathogenesis of MS are still unknown. The imbalance of T cell responses (including Th1, Th2, Treg,or Th17) in MS has been widely recognized,which is crucial for the development and progression of MS. Extensive studies have found that oxidative stress was involved in the pathogenesis of MS or EAE. Inflammatory mediators, activated macrophages and astrocytes will produce a large number of ROS or RNS in pathogenesis of MS (Dutta et al. 2006). High levels of ROS ,RNS can cause demyelination, shaft axonal injury and neuronal degeneration (van Meeteren et al. 2004). In particular, the injury of neurons and axons is an important factor leading to MS progression, these changes can occur both in the early and late stages of MS. Furthermore, high levels of ROS and RNS also can disrupt the BBB and aggravate inflammatory cell infiltration, so to enhance autoimmune inflammation. Previous studies have shown that there was a large amount of lipid peroxidation in the cerebrospinal fluid and plasma of MS patients (Haider et al. 2011), and the serum ROS levels were significantly increased (Langemann et al. 1992), especially in the active phase of disease. Another study directly detecting the intracranial lesions plaque in MS patients found that ROS and RNS concentration was significantly enhanced. So, it is not surprising that anti-oxidant stress is a promising treatment for MS, and even for other neurodegenerative diseases.
In order to observe the changes of oxidative stress in the pathogenesis of EAE, we used SOD and CAT to indicate the content of antioxidant enzymes. TBARS and ROS represent the stress level of lipid peroxidation and total oxidative levels. We found that the contents of ROS and TBARS in brain homogenates of EAE mice were significantly higher than those in normal control mice and the activities of SOD and CAT were significantly lower than those of the normal group. These results further confirmed that there is an imbalance between oxidation and antioxidation in the pathogenesis of EAE (Li et al. 2013a, b). High levels of ROS can further exhaust the antioxidant enzymes SOD and CAT, which further reduce the ability to defend against oxidative stress damage.
Melatonin is an indole substance, which is periodically secreted by the pineal gland. Melatonin is used as a nonprescription drug and dietary supplements for the treatment of PD and HD(Jung et al. 2010). As early as 1993, Tan and his colleagues found that melatonin and its metabolites can effectively remove free radicals and up regulate the expression of certain antioxidant enzymes, such as CAT and SOD (Poeggeler et al. 1993). By comparing the serum concentration of melatonin from 30 MS patients and 30 normal healthy people, Farhadi found that the rhythm of melatonin secretion was disturbed, and the levels of serum melatonin decreased significantly in MS patients (Farhadi et al. 2014).These findings were consistent with Maestroni’s (Maestroni 2001). Furthermore, melatonin binds to its receptor (MT1,MT2), which is located on the surface of lymphocytes and monocytes, to increase phagocytosis, alter lymphocyte migration, and reduce the production of proinflammatory cytokines, such as interleukin −12 (IL-12) and tumor necrosis factor alpha (TNF-α). So melatonin also has anti-inflammatory properties(Vriend and Reiter 2016). Several studies have reported that melatonin protects against EAE by controlling peripheral and central T effector/regulatory responses, suppresses the expression of pro-inflammatory cytokines and chemokines in the CNS and inhibits antigen-specific T cell proliferation (Farez et al. 2015; Alvarez-Sanchez et al. 2015a, b; Chen et al. 2016b).
We found that treatment with melatonin significantly reduced the content of ROS and TBARS of the mice, while the activity in CAT and SOD was markedly enhanced. It is possible that melatonin has the effect of antioxidative stress damage by scavenging free radicals and increasing the antioxidant enzyme activity. Inhibition of oxidative stress can inhibit the development and progression of EAE, which is consistent with previous findings. Meanwhile, treatment with melatonin can reduce the number of inflammatory infiltrates and inhibit the inflammatory reaction in the CNS of EAE. It is possible that, on the one hand, oxidative stress can aggravate inflammation response, by up-regulating MMP9 and disrupting the BBB. On the other hand, melatonin is claimed to act as an anti-inflammatory agent, and thus alter the lymphocyte migration.
Previous studies have shown that melatonin can activate the JAK2/STAT3 (Yang et al. 2013), AMPK/PGC-1α (Yu et al. 2017), and Nrf2/ARE signal pathway. Researchers found that melatonin intervention can activate the Nrf2/ARE signaling pathway to relieve the symptoms of the respective disease animal model in some diseases, in which oxidative stress plays a dominant role, such as traumatic brain injury and acute pancreatitis. Johnson and his colleagues (Johnson et al. 2010) used Nrf2 knockout mice (Nrf2−/−) to induce an EAE model. Then they found the incidence and neurological scores were higher than that in the wild type group, and demyelination and pathological damage also were more severe. In this study, we found that treatment with melatonin enhanced the activation of Nrf2 and increased the levels of HO-1 and NQO1 expression in the spinal cord of EAE mice. More importantly, the enhanced Nrf2/ARE activation was associated with low levels of oxidative stress and the neuroprotective effect of melatonin. Meanwhile, the increased activity of SOD and CAT of melatonin, on the one hand, may be related to the activation of the Nrf2/ARE pathway, then up-regulation of the expression of SOD and CAT of antioxidant enzymes; on the other hand, it may be due to melatonin directly removed some free radicals, and then reduced the consumption of SOD and CAT. In addition ,it is notable that the levels of Nrf2, HO-1 and NQO1 expression in the EAE mice were higher than that in the healthy control mice. This data were consistent with a previous study (Nguyen et al. 2009). The possible reason is that oxidative stress can activate the Nrf2/ARE pathway, and induce the Nrf2, HO-1, and NQO1 expression in EAE mice (van Horssen et al. 2008).Therefore, the effect of melatonin may via activating the Nrf2/ARE signaling pathway reduce ROS or RNS -induced cellular damage.
Conclusion
Oxidative stress may be involved in the pathogenesis of EAE and may be an important factor leading to the onset and progression of EAE. Treating with melatonin (10 mg/kg/day) inhibited the development and progression of EAE and lessened the infiltration of lymphocytes of mice. Furthermore, melatonin played an important role in anti-oxidative stress via activating the Nrf2/ARE signaling pathway, then up-regulating the activities of antioxidant enzymes, and inhibiting the degree of lipid peroxidation. Our findings may aid in finding new therapies in the clinic, and the Nrf2/ARE pathway may be a novel therapeutic target for MS.
References
Adamczyk-Sowa M, Pierzchala K, Sowa P, Mucha S, Sadowska-Bartosz I, Adamczyk J, Hartel M (2014a) Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem Res 39:1585–1593
Adamczyk-Sowa M, Pierzchala K, Sowa P, Polaniak R, Kukla M, Hartel M (2014b) Influence of melatonin supplementation on serum antioxidative properties and impact of the quality of life in multiple sclerosis patients. J Physiol Pharmacol 65:543–550
Alvarez-Sanchez N, Cruz-Chamorro I, Lopez-Gonzalez A, Utrilla JC, Fernandez-Santos JM, Martinez-Lopez A, Lardone PJ, Guerrero JM, Carrillo-Vico A (2015a) Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav Immun 50:101–114
Alvarez-Sanchez N, Cruz-Chamorro I, Lopez-Gonzalez A, Utrilla JC, Fernandez-Santos JM, Martinez-Lopez A, Lardone PJ, Guerrero JM, Carrillo-Vico A (2015b) Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav Immun 50:101–114
Arora D, Bhatla SC (2017) Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of cu/Zn SOD and Mn SOD. Free Radic Biol Med 106:315–328
Bruck W, Stadelmann C (2005) The spectrum of multiple sclerosis: new lessons from pathology. Curr Opin Neurol 18:221–224
Calvo JR, Gonzalez-Yanes C, Maldonado MD (2013) The role of melatonin in the cells of the innate immunity: a review. J Pineal Res 55:103–120
Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ (2005) A review of the multiple actions of melatonin on the immune system. Endocrine 27:189–200
Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N, Rodriguez-Rodriguez A, Guerrero JM (2013) Melatonin: buffering the immune system. Int J Mol Sci 14:8638–8683
Chen SJ, Huang SH, Chen JW, Wang KC, Yang YR, Liu PF, Lin GJ, Sytwu HK (2016a) Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int Immunopharmacol 31:169–177
Chen SJ, Huang SH, Chen JW, Wang KC, Yang YR, Liu PF, Lin GJ, Sytwu HK (2016b) Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int Immunopharmacol 31:169–177
Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP (2007) Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest 117:438–447
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517
Croxford AL, Spath S, Becher B (2015) GM-CSF in Neuroinflammation: licensing myeloid cells for tissue damage. Trends Immunol 36:651–662
Damasceno A, Moraes AS, Farias A, Damasceno BP, Dos SL, Cendes F (2015) Disruption of melatonin circadian rhythm production is related to multiple sclerosis severity: a preliminary study. J Neurol Sci 353:166–168
Dimitrijevic M, Kotur-Stevuljevic J, Stojic-Vukanic Z, Vujnovic I, Pilipovic I, Nacka-Aleksic M, Leposavic G (2017) Sex difference in oxidative stress parameters in spinal cord of rats with experimental autoimmune encephalomyelitis: relation to neurological deficit. Neurochem Res 42:481–492
Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, Rudick R, Mirnics K, Trapp BD (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59:478–489
Ebers GC (2008) Environmental factors and multiple sclerosis. Lancet Neurol 7:268–277
Farez MF, Mascanfroni ID, Mendez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, Balbuena AM, Patel B, Ysrraelit MC, Zhu C, Kuchroo VK, Rabinovich GA, Quintana FJ, Correale J (2015) Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162:1338–1352
Farhadi N, Oryan S, Nabiuni M (2014) Serum levels of melatonin and cytokines in multiple sclerosis. Biom J 37:90–92
Fukutomi T, Takagi K, Mizushima T, Ohuchi N, Yamamoto M (2014) Kinetic, thermodynamic, and structural characterizations of the association between Nrf2-DLGex degron and Keap1. Mol Cell Biol 34:832–846
Garg N, Smith TW (2015) An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav 5:e362
Haider L, Fischer MT, Frischer JM, Bauer J, Hoftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H (2011) Oxidative damage in multiple sclerosis lesions. Brain 134:1914–1924
Hammann KP, Hopf HC (1986) Monocytes constitute the only peripheral blood cell population showing an increased burst activity in multiple sclerosis patients. Int Arch Allergy Appl Immunol 81:230–234
Hardeland R (2013) Melatonin and the theories of aging: a critical appraisal of melatonin’s role in antiaging mechanisms. J Pineal Res 55:325–356
Higashi C, Kawaji A, Tsuda N, Hayashi M, Saito R, Yagishita Y, Suzuki T, Uruno A, Nakamura M, Nakao K, Furusako S, Yamamoto M (2017) The novel Nrf2 inducer TFM-735 ameliorates experimental autoimmune encephalomyelitis in mice. Eur J Pharmacol 802:76–84
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M (2000) Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275:16023–16029
Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M (2004) Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2). Mol Cell Biol 24:36–45
Johnson DA, Amirahmadi S, Ward C, Fabry Z, Johnson JA (2010) The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci 114:237–246
Jung KH, Hong SW, Zheng HM, Lee HS, Lee H, Lee DH, Lee SY, Hong SS (2010) Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J Pineal Res 48:239–250
Kang JC, Ahn M, Kim YS, Moon C, Lee Y, Wie MB, Lee YJ, Shin T (2001) Melatonin ameliorates autoimmune encephalomyelitis through suppression of intercellular adhesion molecule-1. J Vet Sci 2:85–89
Kashani IR, Rajabi Z, Akbari M, Hassanzadeh G, Mohseni A, Eramsadati MK, Rafiee K, Beyer C, Kipp M, Zendedel A (2014) Protective effects of melatonin against mitochondrial injury in a mouse model of multiple sclerosis. Exp Brain Res 232:2835–2846
Keleku-Lukwete N, Suzuki M, Otsuki A, Tsuchida K, Katayama S, Hayashi M, Naganuma E, Moriguchi T, Tanabe O, Engel JD, Imaizumi M, Yamamoto M (2015) Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation. Proc Natl Acad Sci U S A 112:12169–12174
Koch M, Ramsaransing GS, Arutjunyan AV, Stepanov M, Teelken A, Heersema DJ, De Keyser J (2006) Oxidative stress in serum and peripheral blood leukocytes in patients with different disease courses of multiple sclerosis. J Neurol 253:483–487
Kutzelnigg A, Lassmann H (2014) Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol 122:15–58
Langemann H, Kabiersch A, Newcombe J (1992) Measurement of low-molecular-weight antioxidants, uric acid, tyrosine and tryptophan in plaques and white matter from patients with multiple sclerosis. Eur Neurol 32:248–252
Li B, Cui W, Liu J, Li R, Liu Q, Xie XH, Ge XL, Zhang J, Song XJ, Wang Y, Guo L (2013a) Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp Neurol 250:239–249
Li B, Cui W, Liu J, Li R, Liu Q, Xie XH, Ge XL, Zhang J, Song XJ, Wang Y, Guo L (2013b) Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp Neurol 250:239–249
Liu N, Kan QC, Zhang XJ, Xv YM, Zhang S, Zhang GX, Zhu L (2014) Upregulation of immunomodulatory molecules by matrine treatment in experimental autoimmune encephalomyelitis. Exp Mol Pathol 97:470–476
Maestroni GJ (2001) The immunotherapeutic potential of melatonin. Expert Opin Investig Drugs 10:467–476
Melamud L, Golan D, Luboshitzky R, Lavi I, Miller A (2012) Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci 314:37–40
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284:13291–13295
Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Bruck W, Bishop D, Misgeld T, Kerschensteiner M (2011) A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 17:495–499
Ohl K, Tenbrock K, Kipp M (2016) Oxidative stress in multiple sclerosis: central and peripheral mode of action. Exp Neurol 277:58–67
Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC (1993) Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res 14:151–168
Saito R, Suzuki T, Hiramoto K, Asami S, Naganuma E, Suda H, Iso T, Yamamoto H, Morita M, Baird L, Furusawa Y, Negishi T, Ichinose M, Yamamoto M (2015) Characterizations of three major Cysteine sensors of Keap1 in stress response. Mol Cell Biol 36:271–284
van Horssen J, Schreibelt G, Bo L, Montagne L, Drukarch B, van Muiswinkel FL, de Vries HE (2006) NAD(P)H:quinone oxidoreductase 1 expression in multiple sclerosis lesions. Free Radic Biol Med 41:311–317
van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, de Vries HE (2008) Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med 45:1729–1737
van Horssen J, Witte ME, Schreibelt G, de Vries HE (2011) Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta 1812:141–150
van Meeteren ME, Hendriks JJ, Dijkstra CD, van Tol EA (2004) Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochem Pharmacol 67:967–975
Vriend J, Reiter RJ (2016) Melatonin, bone regulation and the ubiquitin-proteasome connection: a review. Life Sci 145:152–160
Wen J, Ariyannur PS, Ribeiro R, Tanaka M, Moffett JR, Kirmani BF, Namboodiri AM, Zhang Y (2016) Efficacy of N-Acetylserotonin and melatonin in the EAE model of multiple sclerosis. J NeuroImmune Pharmacol 11:763–773
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S, Wang N, Liang Z, Li Y, Chen W, Yi D, Yu S (2013) JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res 55:275–286
Yu L, Gong B, Duan W, Fan C, Zhang J, Li Z, Xue X, Xu Y, Meng D, Li B, Zhang M, Bin Z, Jin Z, Yu S, Yang Y, Wang H (2017) Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1alpha-SIRT3 signaling. Sci Rep 7:41337
Acknowledgments
We appreciate it very much that Professor Jiying Zhou of Chongqing Medical University has provided useful information and suggestions for this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Long, T., Yang, Y., Peng, L. et al. Neuroprotective Effects of Melatonin on Experimental Allergic Encephalomyelitis Mice Via Anti-Oxidative Stress Activity. J Mol Neurosci 64, 233–241 (2018). https://doi.org/10.1007/s12031-017-1022-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-1022-x