Abstract
According to WHO data, about 67 million people worldwide are affected by autism, and this number grows by 14% annually. Among the possible causes of autism are genetic modifications, organic lesions of the central nervous system, metabolic disorders, influence of viral and bacterial infections, chemical influence to the mother’s body during pregnancy, etc. The conducted research shows that research papers published until today do not name any potential protein markers that meet the requirements of the basic parameters for evaluating the efficiency of disease diagnostics, in particular high sensitivity, specificity, and accuracy. Conducting proteomic research on a big scale in order to detect serologic markers of protein nature associated with development of autism spectrum disorders seems to be highly relevant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorders (ASD) are a group of complex disintegrated disorders of psychic development characterized by the absence of capacity for social interaction or communication, stereotyped behavior bringing about social disadaptation. Patients typically suffer from phobia, agitation, and other non-specific syndromes. About 1% of the world population is affected by ASD. It occurs four to five times more often in men than in women. Among possible causes of ASD, there are genetic factors, diseases linked to metabolism disorders, toxic and ecological factors, as well as their combinations. To identify ASD in children, we can only rely on behavioral signs and symptoms that are hard to evaluate on preverbal and prespeech stages of their development. However, psychotherapeutic and pedagogic interventions are particularly efficient at early stages of disease. Nevertheless, the early diagnostics is hurdled by the absence of specific serologic biological markers (Abruzzo et al. 2015).
Diagnostic criteria of ASD are being improved and are a part of frequent discussion on professional and public forums. According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV-TR) published in 2000, autism is diagnosed through detection of disorders in social interaction and communication, as well as identified restricted, repetitive, and stereotyped behavioral models, interests, and activity. Besides that, autism patients fall into four different groups according to disorder type: autism disorder, Asperger syndrome, Pervasive Developmental Disorder-Not Otherwise Specified, and childhood disintegrative disorder. In DSM-V published in 2013, the term “autism spectrum disorder” embraces all the separate diagnoses described in DSM-IV. Thus, individual diagnoses, such as Asperger syndrome, are no longer used. Diagnostics criteria of the ASD are based on two types of disorders: (1) permanent deficiency in social communication and social interaction in different contexts and (2) limited, stereotyped behaviors, actions, and interests (Jiang et al. 2014).
Starting from the first ASD description made at the beginning of the 1940s, a lot of research has been conducted in order to identify serologic markers associated with the ASD development (according to the PubMed science-metrical database www.pubmed.com). Nevertheless, none of the serological protein markers mentioned in various papers up to now has a sufficient sensitivity, specificity, and accuracy to be used in clinical practice.
Genetic Markers of Autism Spectrum Disorder
The first research conducted on the twins affected by autism has shown that ASD is with a high probability an inherited disease (Abruzzo et al. 2015). Taking into account the high rate of inheritance of ASD, a lot of research was aimed at identifying genetic basis of the pathology development and identifying genetic markers that evaluate the risk of disease. In children affected by autism, we can observe both visible through a microscope chromosome anomalies and monogenic mutations. Identifying genes that influence the disease allows to detect the mechanism of development disorder that is the basis of the disease. Many research papers demonstrate the connection between autism and genome variations. Among them, the prevailing are chromosome anomalies that include micro-aberrations and CNVs (copy number variations) (Abruzzo et al. 2015). Betancur (2011) has annotated 103 genes and 44 genome loci detected in subjects affected by ASD. Ninety-nine genes among them are classified as autism syndrome genes because signs of autism appear in the context of a complex syndrome with well-known genetic sources such as fragile X syndrome (Martin-Bell syndrome), tuberous sclerosis, or Rett syndrome. Such one-gene mutations amount to 3–5% of the total number of ASD cases (Betancur 2011). Besides that, achievements in genetic testing, in particular chromosome analysis, have allowed to define de novo copy number variations (CNVs) in 30% of children affected by ASD. About 300 variations of DNA sequence have been defined is association with ASD development. Apart from CNVs, over 500 single-gene mutations have been identified under whole exome and whole genome sequencing (Abruzzo et al. 2015).
First papers dedicated to genetic basis of autism were issued at the end of the 1970s when the research conducted on the twins was published (Folstein and Rutter 1977). Folstein research group identified, already in 1977, 36% concordance in monozygotic twins and the absence of such in dizygotic pairs. However, during further data examination with the analysis of a wide range of cognitive and social anomalies, the concordance level raised up to 82% in monozygotic and 10% in dizygotic groups. Research conducted in the last decades show the concordance in cases of ASD between 0 and 27% in dizygotic twins and between 26 and 96% in monozygotic pairs (Filippova and Barylnik 2014). Identification of gene mutations in several syndrome cases of ASD, such as the fragile X syndrome and Rett syndrome were in the 1990s the first direct proof of genetic etiology of ASD. Results of this later genetic and genome research provide a solid proof not only supporting the role of genetic etiology of ASD but also highlighting the issue of understanding molecular bases that ASD development is based on. In fact, in the majority of ASD cases (70%), the cause of the disease remains unknown (Jiang et al. 2014).
Despite the high degree of inheritance, genetic inheritance involved in the development of the majority of ASD cases remains unknown. Knowledge obtained during the study of syndromic ASD indicates that autism spectrum disorders can be caused by a single-gene defect. Knowledge of syndromic ASDs is the basis for differentiated diagnostics of these diseases (Jiang et al. 2014).
In their overview, Bobylova and Petchatnikova (2013) mention over 200 candidate genes for autism (Attachment 1) and divide them as follows:
-
1.
Rare: in case of monogenic forms, when single-gene mutations cause autism (for example, NRXN1, SHANK3);
-
2.
Syndrome based: genes in case of syndromes with autism symptoms (for example, FMR1 (fragile X syndrome), MECP2 (Rett syndrome));
-
3.
Associated: genes with polymorphism and low risk of autism development, “idiopathic” forms of autism (for example, MET, GABRB1);
-
4.
Functional: autism genes in animals, not found in case of other psychic disorders (for example, CADPS2 causes autism in mice, whereas the result of mutation in people is not known) (Bobylova and Petchatnikova 2013)).
Detection of genetic defects in cases of ASD, including single gene mutations and CNVs has been in the focus of researchers’ attention in the last decades. Although the term designating copy number variations of DNA sequences is a relatively new one, influence of chromosomal deletions and duplications on the ASD development and other nervous system disorders has been known for a long time. ASD cases with deletions and duplications, including Angelman and Prader-Willi, Smith-Magenis, and Potocki-Lupski syndromes, are well described. One of the first and still one of the best examples of a disease linked to a chromosomal rearrangement is a chromosome duplication 15q11-q13 inherited from the mother in case of Angelman and Prader-Willi syndromes, detected using a traditional chromosome analysis. Although in case of such duplication a different phenotype is observed, still autism, retardation, convulsions, and hypotension are common characteristics. This chromosomal rearrangement was confirmed by an extensive posterior research and a large-scale CNV genome research using chromosome analysis (Jiang et al. 2014).
Rare CNVs that include genes participating in synaptic plasticity are believed to be connected with ASD and intellectual disability. Disorders of these biologic ways may lead to psychomotor disturbance. Thus, within a research where 996 ASD-affected patients of European origin were compared with 1287 healthy people of the control group, a significantly higher number of cases of rare CNVs was detected in ASD-affected persons (Jiang et al. 2014).
Up to 10% of sporadic and 2% of family ASD cases were shown to be linked to CNV-type chromosome aberrations. Some CNVs are frequent and occur in certain chromosome regions; 15 (duplications q11-13), 16 (duplication and deletion р11), and 22 (deletion q11-13). Each of such genome rearrangements is detected with a frequency of approximately 0.5–1%. It is probably the increase of risk of CNV occurrence in gametes that comes with aging, which is likely to increase the probability of birth of a child with autism the older the parents are (especially fathers). Genome-wide CNV study with the use of 550,000 markers conducted on 859 individuals with ASD and 1409 healthy children has detected a number of pathogenic changes in genes that code neuron adhesion (NRXN1, CNTN4, NLGN1 ASTN2) and genes that participate in ubiquitination (UBE3A, PARK2, RFWD2, FBXO40) (Yurov et al. 2016).
An overview of genome variations that are probably specific for autism shows their extreme heterogeneity. We should mention once again that autism cases are frequently accompanied by CNVs. However, CNV loci or chromosome anomalies do not usually demonstrate any positive connection with autism development. Nevertheless, a series of research with the use of high-resolution genome scanning techniques has defined a certain connection between specific CNVs and autism (Iourov et al., 2013). This research apparently allows to identify new potential candidate genes for autism disposition. Thus, in case of such clinically and genetically heterogenic disease as autism the necessity of additional large-scale high-resolution research of interindividual and intercellular genome variations taking into account their functional manifestations is evident (Vorsanova et al. 2010; Iourov et al. 2015).
Identification of genes involved in syndromic ASD shows that changing one nucleotide in one gene may lead to a risk of ASD development. One of the best examples is the identification of a mutation in SHANK3 gene. SHANK3 was detected in a critical area of 22q13.3 chromosomes in case of Phelan-McDermid syndrome. Later on, pathogenic one-gene mutations in SHANK3 were identified in idiopathic ASD in numerous independent research papers. Likewise, a number of other genes were detected, such as MBD5, CNTNAP2, neuroligins, and neurexins involved in ASD. Low frequency of occurrence of these mutations has been the reason for a difficulty in research of new candidate genes before the new-generation sequencing (NGS) method appeared. Sequencing with the use of NGS technology enlarged the opportunities to detect new rare mutations in genome in cases of ASD (Jiang et al. 2014).
Yeh and Weiss (2016) suggest that the bases of the ASD are such phenomena as alterations to transcription and translation. Monogenic syndromes with an increased risk of developing ASD and the de novo mutations found in the exome study often have a key effect on RNA and protein expression. Mutations can affect transcription factors, chromatin remodeling, translation, and protein biosynthesis. The question arises as to how mutations of protein factors of transcription can lead to the development of specific behavioral symptoms of ASD. One possible hypothesis suggests that the cause of their appearance is the imbalance between the stability of the transcriptome necessary for the formation of a neural network and the flexibility of the transcriptome necessary for synaptic plasticity. In the emerging human brain, the neural network formed by a network of cells, each of which has specific morphological and complex connections, requires constant expression of neuron-specific genes to maintain stable morphology. On the other hand, the flexible neural network and synaptic plasticity which are important for learning and memory require dynamic changes in the transcriptome (Yeh and Weiss 2016). The level of gene expression, which, as a rule, differs in the frontal and temporal cortex of the brain, significantly decreases in ASD, which indicates that the inadequate maintenance of various stable gene expression profiles, along with synaptic dysfunction, potentially leads to a decrease in dynamic regulation. In addition, the development of the nervous system may be affected by a violation of genomic transcription of neurons and glial cells. Thus, murine remodulating chromatin gene CHD1 is necessary for the development of the ectoderm. Averagely, CHD1 reduces the expression of all coding and non-coding RNA genes by 24% (Yeh and Weiss 2016). Therefore, it is quite possible that a disturbance in the regulation of gene expression in the developing brain leads similarly to ASD by changing the total amount of RNA and/or the protein produced by the cell (Yeh and Weiss 2016).
Along with genetic factors, environmental factors play an important role in ASD development. Susceptibility to ASD is considered to be of a moderate genetic nature and is largely determined by environmental factors. Environmental factors include metabolic diseases, immune disorders, infectious diseases, nutrition, intestinal microflora, and a number of toxic substances, including pesticides, heavy metals, and pollutants in the atmosphere. Evaluation of the contribution of environmental factors to ASD development is particularly difficult, since it is often difficult to distinguish factors among themselves or to determine the correct cause-effect relationships. In addition, we should remember that the contribution of environmental factors can be underestimated for reasons of time. In a recent review, Rossignol et al. (2014) concluded that there is a link between some pollutants (mainly air pollutants and pesticides) and the development of the ASD, indicating at the same time a number of limitations and weaknesses found in the papers dedicated to the influence of environmental factors on the etiology of ASD. In addition, they reviewed a number of studies showing that children with ASD had a number of genetic polymorphisms that could reduce the expression of enzymes, such as PON1 and GST, which can effectively eliminate environmental toxic substances. These results led to the development of a new direction of toxicological studies of the ASD. In fact, the reduced or weakened expression of the enzyme involved in detoxification can be added together with an increase in oxidative stress, whether of ecological or genetic origin, as well as with other traits, such as hormonal factors that make men more susceptible both to pollutants’ effect and to the ASD. These considerations support the concept that the signs of ASD are the result of a variety of genetic and environmental factors (Abruzzo et al. 2015).
Apart from genetic research, the following types of research aimed at searching for potential diagnostic markers are being conducted:
-
1.
Research of structure of MRT that allow to identify differences in brain structures connected with ASD. Such research has been conducted in order to detect diagnostic morphologic markers, such as the increase of the size of brain, change in the correspondence between gray and white substances in the frontal cortex, limbic zone, basal ganglia, and tentorium, which show disorders in brain development in prenatal and postnatal periods, changes in neuronal integration in prefrontal zone, as well as disorders of coupling between different brain regions, changes of synthesis of neurotransmitters and affinity of their receptors. However, none of these changes has been firmly proved. Besides that, this method is quite costly and complicated (Voineagu and Yoo 2013).
-
2.
Electrophysiological research. Extensive research is dedicated to identifying electrophysiological changes connected with ASD. Children’s brain potentials conditioned by events are delayed and this indicator can be normalized in case of early therapy. As another biomarker we can suggest the indicator of delay of acoustically caused potential of the superior temporal gyrus with the sensitivity of 75% and specificity of 81% for differentiating 25 children with ASD and 17 normally developing children (Voineagu and Yoo 2013).
Thus, we can conclude that ASD are not homogeneous (O’Roak et al. 2012). Genes associated with development of such disorders may participate in development of other psychiatric and neurologic disorders (Guilmatre et al. 2009). This points out the fact that genome information is ambiguous and only gives an indirect representation of ASD risk. A better understanding of causes of autism can be reached by detecting functional protein markers along with phenoptic and behavioral reactions (Neale et al. 2012). It is the fact of identifying protein markers that will ensure early diagnostics on preverbal and prespeech stages of development when identifying ASD is especially difficult (Neale et al. 2012). In addition, protein biomarkers will point the objectives for medications, define biological points of reference for monitoring of behavioral changes or efficiency of medical care, will disclose the causes of ASD development.
Potential ASD Protein Markers
In recent years, proteomics have been increasingly developing to find out the pathophysiology of mental disorders. Proteomics facilitates the study of complex network interactions of proteins. Using proteomic methods, we can determine the quantitative and qualitative content of specific prognostic and diagnostic biomarkers (Taurines et al. 2010). Postgenomic transcriptomic, proteomic, metabolic technologies, the so-called OMICS technologies, are high-performance studies of a large number of analytes in one analysis (Anderson and Maes 2014). Transcriptomics, proteomics, and metabolomics are dedicated to studying, on the one hand, of changes in the profile as a whole, and on the other hand, changes in the quantitative content of individual types of RNA, peptides/proteins and low molecular compounds, respectively. OMICS technologies allow us to identify group differences, explain the pattern of observed changes. Two decades ago, proteomic studies of the protein nature of autism were still carried out using two-dimensional gel electrophoresis. But in the last decade, researchers have been increasingly interested in identifying potential protein markers of ASD, which is explained by the qualitative development of the characteristics of chromatographic and mass spectrometric equipment (according to DB PubMed.com). First of all, the development of nanoflow technology in chromatography (high-performance liquid chromatography) and the emergence of high-resolution and high scanning speed mass analyzers have increased the accuracy, sensitivity, and reliability of the analysis.
Further on, we will dwell more on common in scientific research protein groups/peptides and separate proteins that might be associated with ASD development in children.
Peptides
Peptide analysis of blood plasma samples of children with ASD was performed by Momeni et al. (2012), where three differentially expressed peptides with mass-charge characteristics (m/z) of 2020 ± 1, 1864 ± 1, and 1978 ± 1 Da in heparinized plasma of children with ASD, which were significantly different from the peptide composition of the plasma in the control group of children. The authors used the SELDI-TOF mass spectrometry method to compare peptide profiles of blood plasma in 28 children with ASD and in 30 healthy children aged 3 to 12 in order to discover new peptide markers with diagnostic value and to understand the role of these peptides in pathophysiology of ASD. Levels of peptides with m/z 2020 ± 1 and1864 ± 1 Da were elevated in children with ASD, whereas peptide levels characterized by m/z of 1978 ± 1 Da were lowered in children with autism spectrum disorders compared with healthy control group of children. The sequences of amino acids of these peptides were determined. The first peptide with m/z 2020 ± 1 Da consists of 17 amino acid residues and corresponds to a peptide known as the C3f protein of the complement system (C3 protein derivative). The second peptide with m/z 1864 ± 1 Da corresponds to a peptide of 16 amino acid residues with the same sequence as C3f but without C-terminal arginine and is known as C3fdesArg. The third peptide with m/z 1978 ± 1 Da, which is found in a larger concentration in the healthy children group, has the same sequence as C3f, but carries a modified arginine residue that corresponds to ornithine in the C-terminal region. The authors believe that this peptide set represents a diagnostic and prognostic interest (Momeni et al. 2012).
Neuropeptide oxytocin and its receptor regulate the social functioning of animals and humans. Parker et al. (2014) tested a group of children with ASD aged 3–12 (193 participants in total) on the possibility to use the plasma level of oxytocin or its receptor as a protein marker of ASD. The authors found that the concentration of oxytocin in the blood is a highly heritable trait that predicts the social functioning of children with ASD, their healthy siblings, and the control group of healthy children. These data extend to the oxytocin receptor, the level of which is largely related to differences in social functioning regardless of the status of the disease. The authors conclude that there is no strict correlation between the level of oxytocin and its receptor in children with ASD in the blood. These indicators are more likely related to the individual characteristics of the human body (Parker et al. 2014).
Proteins
Proteomic profiling of blood sera from children with ASD was performed by the Taurines research team, where the protein and peptide profile of blood serum of 16 patients with ASD aged 8–18 (male) was determined using the method of time-of-flight mass spectrometry with laser ionization and desorption (MALDI-ToF-MS). Fractionation of whey proteins was carried out using C8 magnetic microparticles. The authors emphasize that non-trypsin-treated peptides and intact protein/peptide molecules were used for the study. Patients with ASD were significantly different from the healthy group in three peaks with a mass/charge ratio (m/z) of 4.40, 5.15, and 10.38 kDa. One peak differed in patients suffering from ASD with concomitant hyperactivity syndrome and ADD from a healthy group and from patients with ASD without hyperactivity syndrome and ADD. The authors conclude that their findings indicate a change in protein levels in the peripheral blood of patients with autism spectrum disorders (Taurines et al. 2010).
In another proteomico-peptide study, Corbett et al. (2007) performed a proteomic analysis of serum samples of 69 children with autism aged 4–6. Serum blood proteins were digested with trypsin followed by panoramic mass spectrometric analysis (LC-ESI-MS/MS). In total, 6348 peptide components were examined. Identified peptide components were assigned to the following proteins: apolipoprotein B-100, a protein similar to the human factor H complement, the C1q complement component, and fibronectin 1. In addition, the level of apolipoprotein B-100 and apolipoprotein A-IV was higher in children with more severe-stage autism compared with children with an easier form of autism. Apolipoproteins are involved in the transport of lipids, cholesterol, and vitamin E and play a key role in maintaining lipid homeostasis. The meaning of the decrease in the level of apolipoproteins in the serum of children with autism is not clear yet. The role of altered protein levels in the complement system is not identified either. The complement system participates in lysis and the removal of infectious agents from the blood. Changes in this system might quite possibly predispose to a viral or other infection of the mother or fetus during pregnancy and contribute to the development of autoimmune reactions. In addition, the complement system may be involved in cell apoptosis in the brain. The authors conclude that the method they use can help when searching for possible differences in protein composition in autistic patients and normally developing children. However, the method has its limitations and requires standardization of techniques, and the results need to be reproduced (Corbett et al. 2007).
The main goal of the study by Ramsey et al. (2013) from the Institute of Cognitive Sciences and Behavior in the city of Nijmegen (the Netherlands) was to study the change in serological parameters in children with ASD of different ages. A total of 37 children with ASD and 37 children without ASD, comparable by gender, age, and other indicators, who were conditionally divided into three age groups of 4–9, 9–13, and 13–18 years, participated in the study. Various laboratory methods (mainly enzyme immunoassays) allowed to detect statistically significant changes in the level of nine proteins in children with ASD compared with the children of the control group, including the Tamm-Horsfall protein-urinary glycoprotein (P = 0.004), interleukin-3 (P = 0.01), Von Willebrand factor (P = 0.023), interleukin-12 subunit p40 (P = 0.025), tyrosine kinase (P = 0.033), tumor necrosis factor-beta (P = 0.034), interleukin-13 (P = 0.038), chemotactic for macrophages protein (P = 0.042), and the stem cell factor (P = 0.05) (Hajiyeva et al. 2014).
In a research conducted by Bailey et al. (2008), a study was made on the possible use of peripheral protein markers in the early diagnosis of autism using the ELISA method. The authors measured the plasma level of the sAPP-α protein (amyloid precursor protein) in 25 children with autism aged 2–4 years and in 25 healthy children of the same age. As a result, 60% of children with autism were proved to have significantly higher levels of sAPP-α protein. In addition, the researchers tested 150 umbilical cord blood samples and found a significant increase in the plasma level of the sAPP-α protein in only 10 of the 150 samples. The authors note that further study of this concept is required (Bailey et al. 2008).
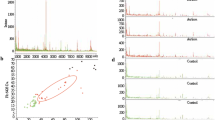
In the course of the last experimental pilot study carried out by the authors of this article, a panoramic proteome profiling was made with the help of high-resolution mass spectrometry of nine depleted serum samples, five of which belonged to children with ASD (Kaysheva et al. 2016). We found out that the depleted samples differed in both the number of proteins identified and the protein composition. When compared with control samples in blood serum samples of autistic children, a small group of common proteins not related functionally or structurally was found. Among these proteins, only the protein LIM-containing protein 1 was detected in four of the five samples, while the other 12 proteins of this group were found in three samples of children with autistic disorders (Kaysheva et al. 2016).
Autoantibodies
An increasing amount of data suggests that ASD may be linked to disorders of reactions of innate immunity and reactions of adaptive immunity. The plasma of ASD patients shows an increase in interleukin IL-1, IL-6, IL-8, and IL-12 percentage, interferon-γ, and macrophage migration inhibition factor, as well as a decrease in TGF-β level. ASD patients also show an increase in IgG levels in plasma and an abnormal level of activation of natural killer cells. Autoanibodies against ganglion cells and brain tissue are found in affected children’s plasma.
Momeni et al. (2012) used SELDI-TOF mass spectrometry method to identify potential peptide biomarkers in children affected by ASD. The research identified peptides associated with C3 protein of complement system, whose levels differed between the group of children with ASD and the control group of children without ASD signs (Momeni et al. 2012). However, the results obtained in this research have not been validated. The research evaluates the potential of proteomic approach to detect immune biomarkers for ASD. Detecting reliable markers of immune system functioning in ASD patients may lead to a better understanding of etiology of the disease, as well as allow to identify a group of patients who may benefit from the treatment oriented at the immune response (Voineagu and Yoo 2013).
Autoimminuty may play the key role in pathogenesis of neurologic disorders including ASD (Elamin and Al-Ayadhi 2014). In children affected by ASD, autoantibodies specific to the brain, lymphocyte function disorders and cytokine regulation disorders are detected; there is a connection with viral infections and immunogenetic factors, as well as neuroglial activation and neuroinflammation in the central nervous system. In addition, a number of genes associated with the immune system are associated with this disorder. These include the null allele of the C4B gene, the complement component, and the extended allele of the HLA gene. The role of maternal antibodies as a pathogenic factor is possible (Elamin and Al-Ayadhi 2014).
Under normal conditions, large molecules, such as IgG and other immune components, are excluded from the CNS through the blood-brain barrier (BBB). However, infectious and environmental factors can increase the permeability of the BBB. Thus, antibodies can penetrate through the BBB into the CNS, and in combination with brain tissue, antigens form immune complexes. Such immune complexes can cause damage to the nervous tissue and behavioral changes and congenital disorders (Elamin and Al-Ayadhi 2014).
Several studies reported the synthesis of autoantibodies specific to brain proteins and brain tissues in autistic children. Autoantibodies specific to brain tissue have also been observed in individuals with other pathological disorders, including attention deficit/hyperactivity disorder (ADHD), Down’s syndrome, Behcet’s disease, Alzheimer’s disease, schizophrenia, and cerebral folate deficiency (CDF). Despite the fact that the production of autoantibodies of the brain in children with ASD has not been fully studied, it is assumed that an autoimmune reaction to neurons can be caused by certain environmental factors. Such factors include food allergens, infectious agents, heavy metals that cause the release of neuronal antigens and trigger autoimmune reactions through the activation of inflammatory cells in genetically predisposed individuals (Elamin and Al-Ayadhi 2014). Circulating autoantibodies specific to brain proteins can affect the functioning of the attacked brain tissue. Autoantibodies react with several proteins, such as MBP (myelin basic protein), frontal cortical cells, endothelial cells, and neurofilaments in autistic children. Several types of myelin proteins associated with the destruction of myelin have been proposed, such as MBP and MAG (myelin-associated glycoprotein), which are responsible for myelination in the CNS (Elamin and Al-Ayadhi 2014).
Thus, among the consequences of circulating anti-MBP and anti-MAG antibodies, there is the formation of abnormal myelin sheaths during brain development, and, as a result, impaired brain functions such as speech, language, communication, and social interaction, as well as other neurological symptoms, manifested in patients with ASD. Elevated serum levels of anti-MBP antibodies in ASD have been reported in several studies (Mostafa and Al-Ayadhi 2013). In addition, Mostafa and AL-Ayadhi (2013) described the association of anti-MBP and anti-MAG with allergic reactions in children with autism. In the tested group of children, the serum level of these autoantibodies was increased due to the induction of autoimmune reaction to the CNS by allergic substances (Elamin and Al-Ayadhi 2014).
The presence of antibodies against M1 gangliosides is observed in some children with autism. Gangliosides can be targeted in a complex autoimmune response due to their localization in the nervous system. In a subgroup of psychoneurological patients with cognitive impairment, psychosis, depression and seizures, elevated levels of antiphospholipid antibodies are detected. In addition, anti-phospholipid antibodies were first detected at high levels in young children with autism spectrum disorders compared with the control group and were associated with behavioral disorders, mainly with cognition, anxiety, and hyperactivity. When antiphospholipid antibodies were injected into mouse models, they caused a number of psychological symptoms, including anxiety and decreased cognition, learning capacity, and memory (Mostafa 2011; Moeller et al. 2013; Careaga et al. 2013).
Autoantibodies against the folate receptor affect the transport of folate across the blood-brain barrier and lead to the development of neurological pathologies. The presence of these antibodies leads to an increase in oxidative stress in some children with ASD and decline in social interaction, attention and behavior (Frye et al. 2013).
Autoantibodies against neuronal and glial protein filaments and antinuclear antibodies have been identified in some children with autism. The serum levels of these autoantibodies were significantly increased in children with autism compared with the control group and had a positive correlation with the severity of the disease (Elamin and Al-Ayadhi 2014).
Elevated levels of neurotrophins and neuropeptides have been proposed as prognostic indicators of intellectual and social development disorders (Hashimoto et al. 2006). One of these factors is BDNF, a small protein found throughout the central nervous system and in the peripheral blood. BDNF is involved in the maturation and differentiation of dopaminergic neurons in the developing brain and can contribute to the pathophysiology of ASD and other mental illnesses. It has been reported recently that levels of IgG and IgM autoantibodies to BDNF have been elevated in children with autism, in contrast with a study conducted by Hashimoto et al. (2006) that reported a significantly lower level of serum antibodies to BDNF in children with ASD compared with healthy volunteers. An explanation for this discrepancy may be the age difference of subjects between different studies or methodological differences (e.g., sampling time and serum collection) (Hashimoto et al. 2006).
The occurrence of autoreactivity in the brain tissues of autistic children may represent a neuroprotective response of the immune system to a previous craniocerebral injury that may occur during neurodevelopment. Antibodies against Purkinje cells and IgM antibodies against brain endothelial cells have been found in children with autism, idiopathic mental retardation, and epilepsy. Dysfunction of neurons or glial cells in the pathogenesis of ASD is observed, since autoantibodies have been found in autistic children (Singer et al. 2006). Singer et al. (2006) investigated the reactivity of autoantibodies to different areas of the human brain in the sera of children with autism spectrum disorders, siblings of children with autism spectrum disorders and healthy people. Children with autism spectrum disorders showed a greater level of immunoreactivity to the basal ganglia and frontal lobe, as well as to the cingulate gyrus and deep cores of the cerebellum, compared with the control group (Singer et al. 2006).
A study conducted by Singh and Rivas (2004) was focused on autoantibodies specific to five different areas of the brain: basal nuclei, cerebral cortex, cerebellum, brain stem, and hippocampus. Brain antibodies against neural proteins of basal nuclei (49%), cerebral cortex (18%), and cerebellum (9%) were detected in the serum of children with autism. However, antibodies against brainstem proteins and hippocampal proteins were not detected (Singh and Rivas 2004).
In connection with the foregoing, we can conclude that autoantibodies are not specific for ASD, since they are found in patients with various neurological disorders, as well as in healthy people. In addition, autoantibodies are not found in all children suffering from ASD. For example, autoantibodies against MBP are found in patients with multiple sclerosis, IgG autoantibodies against brain tissue were present in the sera of healthy people and in patients with depression, IgG autoantibodies to endothelial cells are common in the serum of children with Landau-Cleffner syndrome and ASD. This may lead to the assumption that the accumulation of these antibodies in the body may be secondary to the congenital CNS pathology (Elamin and Al-Ayadhi 2014).
Cytokines
In most cases, the following are considered as potential cytokine markers of ASD: IL-1, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IFN-γ, TNF-α and TGF-β (Rose and Ashwood 2014).
IL-6 is a pro-inflammatory cytokine, a differentiation factor for B cells, IL-6 supports the functioning of hematopoietic stem cells, supports the activation and differentiation of T cells and macrophages, and is involved in neuronal differentiation. IL-6 is also one of the known cytokines that can penetrate the placenta and the blood-brain barrier. Of the nine studies evaluating changes in IL-6 plasma or serum levels in patients with autism spectrum disorders, two showed an increase in IL-6, two showed a reduction in IL-6, and the other five showed no significant deviations in the content IL-6 in patients with ASD from the control group. The contradictory nature of these studies may be due to inappropriate selection of the groups studied. Thus, the control group of normally developing children and the group of children with ASD in these studies could differ in age, the number of participants, gender, etc. Within the framework of these nine studies, the largest study of the scientific group of Prof. Ashwood included 97 children aged 2 to 5 diagnosed with ASD, 87 normally developing children of the same age, and 39 children with developmental delay not related to ASD (Ashwood et al. 2011). The authors report an increase in IL-6 levels, as well as an increase in IL-12 levels of p40, IL-8, and IL-1β in plasma samples of children with ASD compared with normally developing children and those with non-ASD developmental delay (Rose and Ashwood 2014).
We can mention other proinflammatory cytokines IL-1, IL-12, TNF-α, and IFN-γ, whose levels were evaluated in plasma and serum of children with autism spectrum disorders. Out of the published 12 studies where at least one of the cytokines mentioned above was measured, four reported a significant increase in it, another one an equivocal increase, two others showed a significant decrease in these cytokines, and five did not find a significant difference between children with Autistic spectrum disorders and a control group for the level of cytokines studied (Rose and Ashwood 2014).
The two main regulatory cytokines are IL-10 and TGF-β known for their inhibitory effect on the immune system. In two studies that measured levels of TGF-1β in plasma or blood serum, a significant reduction in this parameter was detected in patients with autism spectrum disorders compared with typically developing children (Rose and Ashwood 2014). In a more extensive study, TGF-1β levels in plasma were measured in 75 children with ASD, 32 normally developing children, and 36 children with developmental disorders other than ASD aged 2 to 5, where a decrease in TGF-1β was found in children with ASD in comparison with normally developing children, as well as a significant decrease in comparison with children with a developmental delay other than ASD. Moreover, this study also showed that a decrease in TGF-1β in children with autism spectrum disorders was inversely proportional to a worsening of children’s behavior (Rose and Ashwood 2014).
Among the studies particularly relevant for the search of potential cytokine biomarkers are the studies that analyze not only changes in the level of individual cytokines in plasma in children with autism spectrum disorders but also assess individual patterns of behavior and the severity of the disease. In addition to the above-mentioned inverse dependence of the decrease in plasma TGF-1β level with the severity of the disease, a positive correlation was also found between elevated TGF-1β levels and an improvement in behavioral characteristics of ASD-affected children (Rose and Ashwood 2014). In addition, proinflammatory cytokines are positively associated with impaired behavior.
There are interesting studies aimed at detecting chemokines associated with the development of ASD in children. Chemokines are low molecular weight cytokines involved in the migration and activation of phagocytic cells and lymphocytes; they play a central role in the inflammatory response. In most cases, in patients suffering from ASD, researchers focus on changes in the chemokine content of MCP-1, MIP-1α, MIP-1β, and RANTES in blood samples (plasma/serum) compared with healthy volunteers. Out of the six studies that determined the level of at least one of the above chemokines in serum or plasma, three studies found no significant differences, two found elevated chemokine levels, and one study showed a reduction in chemokine levels in patients with ASD compared with the control group (Rose and Ashwood 2014).
The Suzuki team studied the cytokine / chemokine profile of 28 blood plasma samples of boys with autism aged older than 6, as well as 28 blood samples of boys without ASD signs (Suzuki et al. 2011). The levels of IL-1β, IL-1RA, IL-5, IL-8, IL-12p70, IL-13, IL-17, and GRO-α in blood plasma were found to be elevated in children with ASD compared with the control group. Interestingly, IL-1β and IL-1RA are pro-inflammatory cytokines produced by monocytes, macrophages, dendritic cells, neutrophils, and endothelial cells. IL-5 is mainly produced by T helper cell type 2 and mast cells. IL-5 stimulates the secretion of immunoglobulins in B cells and also mediates the differentiation and activation of eosinophils. IL-12p70 is a heterodimeric cytokine that consists of two subunits, p35 and p40. IL-12p70 is an immunoregulatory cytokine that is produced primarily by B cells and monocytes, and is involved in the differentiation of naive T cells into T helper cell type 1. Chemokines IL-8 and GRO-α are produced by macrophages and other types of cells, such as epithelial and endothelial cells. They participate in chemotaxis of neutrophils and play an important role in the innate immune response (Suzuki et al. 2011).
Thus, it can be concluded that, among the proteins of the immune system (immunoglobulins, cytokines, chemokines, etc.), there is still no factor that could be used as a specific ASD diagnostic marker. However, data on non-specific but associated with ASD factors is now emerging (TGF-1β, IL-1β, IL-1RA, IL-5, IL-8, IL-12p70, IL-13, IL-17, and GRO-α), which could serve as a diagnostic marker of ASD; a study of these indicators can help identify children with ASD, with serious immune system disorders, and adjust their treatment.
Serotonin
Increased serotonin level in the blood is one of the key indicators for a wide range of pathological processes, ASD being no exception. Children with autism demonstrate a 25% increase in serotonin level in the whole blood. The serotonin percentage is determined and regulated by genetic variants in the serotonin SLC6A4 gene receptor and in the ITGB3 beta integrin gene. Interestingly, a recent study describing this model on mice carrying the SLC6A4 variant showed hyperserotoninemia and behavioral changes, including repetitive behavior (Adamsen et al. 2011). The authors suggest that imbalance of serotonin may be the cause of ASD in children and the serotonin way may be therapeutically useful for patients with ASD (Anderson and Maes 2014).
Biomarkers of Oxidative Stress
Oxidative stress is the result of insufficient counteraction of endogenous and exogenous active forms of oxygen, of ineffective antioxidant mechanisms, excessive production of active forms of oxygen, or a combination of these factors. The presence of oxidative stress in patients with ASD has been reported in numerous studies. Reduced plasma levels of glutathione, peroxidase, methionine, and cysteine and an increase in the oxidized glutathione level have been reported in patients with ASD. Measurements of biomarkers of oxidative stress in postmortem brain tissue of ASD subjects showed changes in the balance of reduced and oxidized glutathione and an increase in the level of 3-nitrotirozine and 8-oxo-deoxyguanosine, which are markers of oxidative damage to proteins. A meta-analysis of biomarkers of oxidative stress in ASD patients showed the most significant differences between ASD cases and the indicator of the average level of oxidative stress markers for reduced glutathione and (decrease by 27%) and oxidized glutathione (45% increase). This meta-analysis also highlighted the fact that changes in the level of oxidative stress markers associated with autism spectrum disorders were generally heterogeneous, and all observations are based on small samples and moderate effects, thus indicating the need for standardization of further research (Voineagu and Yoo 2013).
Conclusion
Due to the fact that the number of cases of ASD is steadily growing, and the possibility of a positive effect of therapy depends directly on its application, the issue of objective early diagnosing of autism spectrum disorders remains highly relevant. Numerous studies in this area point only to possible diagnostic and prognostic disease markers of protein nature, which require additional confirmation in large-scale studies. Potential markers mentioned in research papers do not prove a sufficient level of sensitivity and specificity and accuracy. Nevertheless, it is worth noting that the qualitative growth in the characteristics of analytical systems, primarily the growing use of nanotechnology in chromatography and the emergence of mass-analyzers of high resolution and scanning speed, has expanded the possibilities for proteomic analysis of blood samples of children with ASD. This allowed us to obtain new data on the significance of the LIM domain containing protein 1 for serological diagnosing of ASD, as well as a number of other proteins. High sensitivity, reliability, and accuracy of modern proteomic analysis will allow to determine the list of potential serological markers for ASD and possible mechanisms of pathogenesis of the disease in the coming years.
References
Abruzzo PM, Ghezzo A, Bolotta A, Ferreri C, Minguzzi R, Vignini A, Visconti P, Marini M (2015) Perspective biological markers for autism spectrum disorders: advantages of the use of receiver operating characteristic curves in evaluating marker sensitivity and specificity. Disease Markers doi. doi:10.1155/2015/329607
Adamsen D, Meili D, Blau N, Thöny B, Ramaekers V (2011) Autism associated with low 5-hydroxyindolacetic acid in CSF and the heterozygous SLC6A4 gene Gly56Ala plus 5-HTTLPR L/L promoter variants. Mol Genet Metab 102:368–373
Anderson G, Maes M (2014) Redox regulation and the autistic spectrum: role of tryptophan catabolites, immuno-inflammation, autoimmunity and the amygdala. Curr Neuropharmacol 12:148–167. doi:10.2174/1570159X11666131120223757
Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van De Water J (2011) Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25:40–45
Bailey AR, Giunta BN, Obregon D, Nikolic WV, Tian J, Sanberg CD, Sutton DT, Tan J (2008) Peripheral biomarkers in autism: secreted amyloid precursor protein-alpha as a probable key player in early diagnosis. Int J Clin Exp Med 1:338–344
Betancur C (2011) Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res 1380:42–77
Bobylova IYU, Petchatnikova IL (2013) Genetics of autistic disorder (review of foreign literature). Russian Journal of Child Neurology 8:31–45 (in Russ.). doi:10.17650/2073-8803-2013-8-3-31-45
Careaga M, Hansen RL, Hertz-Piccotto I, Van de Water J, Ashwood P (2013) Increased anti-phospholipid antibodies in autism spectrum disorders. Mediators Inflamm. doi:10.1155/2013/935608
Corbett BA, Kantor AB, Schulman H, Walker WL, Lit L, Ashwood P, Rocke DM, Sharp FR (2007) A proteomic study of serum from children with autism showing differential expression of apolipoproteins and complement proteins. Mol Psychiatry 12:292–306
Elamin NE, Al-Ayadhi LY (2014) Brain autoantibodies in autism spectrum disorder. Biomark Med 8:345–352
Filippova NV, Barylnik YB (2014) Genetic factors in etiopathogenesis of autistic spectrum disorders. Russian Society of Psychiatrists 24:96–100 (in Russ)
Folstein S, Rutter M (1977) Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry 18:297–321
Frye RE, Sequeira JM, Quadros EV, James SJ, Rossignol DA (2013) Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry 18:369–381
Guilmatre A, Dubourg C, Mosca AL, Legallic S, Goldenberg A et al (2009) Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Arch Gen Psychiatry 66:947–956
Hajiyeva N, Pavlichenko AV, Kabosnidze K (2014) Advances in autistic spectrum disorder and attention deficit hyperactivity disorder (according to the proceedings of the 26st European College of Neuropsychopharmacology). Psychiatry and Psychopharmacotherapy 16(3):41–44 (in Russian)
Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, Suzuki K, Minabe Y, Takei N, Iyo M, Mori N (2006) Reduced serum levels of brain-derived neurotrophic factor in adult male patients with autism. Prog Neuro-Psychopharmacol Biol Psychiatry 30:1529–1531
Iourov IY, Vorsanova SG, Yurov YB (2013) Somatic cell genomics of brain disorders: a new opportunity to clarify genetic-environmental interactions. Cytogenet Genome Res 139:181–188
Iourov IY, Vorsanova SG, Korostelev SA, Zelenova MA, Yurov YB (2015) Long contiguous stretches of homozygosity spanning shortly the imprinted loci are associated with intellectual disability, autism and/or epilepsy. Mol Cytogenet 8:77
Jiang YH, Wang Y, Xiu X, Choy KW, Pursley AN, Cheung SW (2014) Genetic diagnosis of autism spectrum disorders: the opportunity and challenge in the genomics era. Crit Rev Clin Lab Sci 51:249–262
Ramsey JM, Guest PC, Broek JA, Glennon JC, Rommelse N, Franke B, Rahmoune H, Buitelaar JK, Bahn S (2013) Identification of an age-dependent biomarker signature in children and adolescents with autism spectrum disorders. Mol Autism 4:27. doi:10.1186/2040-2392-4-27
Kaysheva AL, Kopylov AT, Yurov IY, Vorsanova SG, Yurov YB, Galiullin RA, Anashkina AS, Archakov AI, Ivanov YD (2016) Proteomic analysis of blood serum protein profiles in children with autism. Clinical Practice in Pediatrics 11:12–17 (in Russ)
Moeller S, Lau NM, Green PH, Hellberg D, Higgins JJ, Rajadhyaksha AM, Alaedini A (2013) Lack of association between autism and anti-GM1 ganglioside antibody. Neurology 81:1640–1641
Momeni N, Bergquist J, Brudin L, Behnia F, Sivberg B, Joghataei MT, Persson BL (2012) A novel blood-based biomarker for detection of autism spectrum disorders. Transl Psychiatry. doi:10.1038/tp.2012.19
Mostafa GA, AL-Ayadhi LY (2011) Increased serum levels of anti-ganglioside M1 autoantibodies in autistic children: relation to the disease severity. J Neuroinflammation. doi:10.1186/1742-2094-8-39
Mostafa GA, Al-Ayadhi LY (2013) The possible relationship between allergic manifestations and elevated serum levels of brain specific auto-antibodies in autistic children. J Neuroimmunol 261:77–81. doi:10.1016/j.jneuroim.2013.04.003
Neale BM, Kou Y, Stevens L, Wang LS, Makarov V, Polak P (2012) Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485:242–245
O'Roak BJ, Vives L, Girirajan S, Karakoc E, Lee C, Smith JD et al (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485:246–250
Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, Liao CP, Phillips JM, Hallmayer JF, Hardan AY (2014) Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci U S A 111:12258–12263
Rose D, Ashwood P (2014) Potential cytokine biomarkers in autism spectrum disorders. Biomark Med 8:1171–1181
Rossignol DA, Genuis SJ, Frye RE (2014) Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry 4:e360. doi:10.1038/tp.2014.4
Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW (2006) Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol 178:149–155
Singh VK, Rivas WH (2004) Prevalence of serum antibodies to caudate nucleus in autistic children. Neurosci Lett 355:53–56
Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, Yoshihara Y, Wakuda T, Takebayashi K, Takagai S, Matsumoto K, Tsuchiya KJ, Iwata Y, Nakamura K, Tsujii M, Sugiyama T, Mori N (2011) Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One. doi:10.1371/journal.pone.0020470
Taurines R, Dudley E, Conner AC, Grassl J, Jans T, Guderian F, Mehler-Wex C, Warnke A, Gerlach M, Thome J (2010) Serum protein profiling and proteomics in autistic spectrum disorder using magnetic bead-assisted mass spectrometry. Eur Arch Psychiatry Clin Neurosci 260:249–255
Voineagu I, Yoo HJ (2013) Current progress and challenges in the search for autism biomarkers. Dis Markers 35:55–65
Vorsanova SG, Voinova VY, Yurov IY, Kurinnaya OS, Demidova IA, Yurov YB (2010) Cytogenetic, molecular-cytogenetic, and clinical-genealogical studies of the mothers of children with autism: a search for familial genetic markers for autistic disorders. Neurosci Behav Physiol 40:745–756
Yeh E, Weiss LA (2016) If genetic variation could talk: what genomic data may teach us about the importance of gene expression regulation in the genetics of autism. Mol Cell Probes. doi:10.1016/j.mcp.2016.10.007
Yurov IY, Vorsanova SG, Korostelev SA, Vasin KS, Zelenova MA, Kurinnaya OS, Yurov YB (2016) Structural variations of the genome in autistic spectrum disorders with intellectual disability. Zh Nevrol Psikhiatr Im S S Korsakova 116(7):50–54 (in Russian)
Acknowledgments
The study was carried out with the financial support of the Russian Science Foundation (grant no. 14-25-00132).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khramova, T.V., Kaysheva, A.L., Ivanov, Y.D. et al. Serologic Markers of Autism Spectrum Disorder. J Mol Neurosci 62, 420–429 (2017). https://doi.org/10.1007/s12031-017-0950-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-0950-9