Abstract
Chaperone-rich cell lysates (CRCLs) may play an important role in the development of anti-tumor vaccines. Tumor-derived CRCLs have been reported to activate dendritic cells (DCs) to elicit potent anti-tumor activity. However, the role of DC-derived exosomes (DEXs) secreted from DCs loaded with CRCLs in the treatment of tumors has not been clearly determined. In the present study, DEXs were generated from DCs loaded with CRCLs derived from GL261 glioma cells. These DEXs, designated DEX (CRCL-GL261), were then used to treat DCs to create DEX (CRCL-GL261)-DCs. The DEX (CRCL-GL261)-DCs were found to promote cell proliferation and cytotoxic T lymphocyte (CTL) activity of CD4+ and CD8+ T cells in vitro compared with DEX (GL261)-DCs, which were loaded with DEXs derived from DCs loaded with GL261 tumor cell lysates. DEX (CRCL-GL261)-DCs significantly prolonged the survival of mice with tumors and inhibited tumor growth in vivo. In addition, DEX (CRCL-GL261)-DCs induced enhanced T cell infiltration in intracranial glioma tissues compared with other treatments. DEX (CRCL-GL261)-DCs induced strong production of anti-tumor cytokines, including interleukin-2 and interferon-γ. Moreover, depletion of CD4+ and CD8+ T cells significantly impaired the anti-tumor effect of DEX (CRCL-GL261)-DCs. Finally, DEX (CRCL-GL261)-DCs were found to negatively regulate Casitas B cell lineage lymphoma (Cbl)-b and c-Cbl signaling, leading to the activation of phosphatidyl inositol 3-kinase (PI3K)/Akt and extracellular signal-regulated kinase (ERK) signaling in T cells. In summary, we present evidence that DEX (CRCL-GL261)-DCs induce more potent and effective anti-tumor T cell immune responses and delineate the underlying mechanism by which DEX (CRCL-GL261)-DCs exerted their anti-tumor activity through modulating Cbl-b and c-Cbl signaling. These results provide novel and promising insight for the development of an anti-tumor vaccine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma is one of the most common malignant brain tumors, with high morbidity and mortality worldwide (Mao et al. 2009). Despite the advances in current therapy for intracranial glioma, including surgical resection with adjuvant radio- or chemotherapy (Maher et al. 2001), the disease continues to have a poor prognosis and frequent recurrence, with a median survival time of 14 months and a 5-year survival rate of less than 2 % (Sanai and Berger 2008). Recently, many oncologists have highlighted immunotherapy for the treatment of tumors. It has been suggested that the blood–brain barrier is opened up under pathological conditions, permitting lymphocytes to enter the central nervous system (Parajuli et al. 2007). Thereby, the immune system becomes involved in the central nervous system disease, which provides a possibility for treating intracranial glioma by immunotherapy.
Dendritic cells (DCs) are considered to be the most effective antigen-presenting cells. Such cells induce a strong immune response in vivo, and DC-based immunotherapy has become increasingly attractive for tumor therapy (Sabel 2009; Steinman 2012). The DC vaccine has been clinically evaluated for tumor therapy, and it shows considerable efficiency and safety (Ramanathan et al. 2014). However, it retains some defects and needs improvement. Various agents, including tumor cell lysates and tumor-derived DNA/messenger RNA and cytokines, have been used to modify DCs (Okada et al. 2011).
Recently, exosomes secreted by immune cells or tumor cells have been investigated for their potential in tumor immunotherapy (Navabi et al. 2005; Wolfers et al. 2001). Exosomes are extracellular vesicles with a diameter of 30–100 nm; with ultracentrifugation, they settle at a density between 1.13 and 1.19 g/mL on a linear sucrose gradient (Ailawadi et al. 2015; Buzas et al. 2014; Sluijter et al. 2014). Various cells including tumor cells and immune cells have been reported to secrete exosomes (Yang and Robbins 2011), and exosomes secreted from different parental cells have been demonstrated to have various enriched proteins with differing effects on immune activation (Stoorvogel et al. 2002). DC-derived exosomes (DEXs) contain abundant co-stimulatory molecules and major histocompatibility complex (MHC) class I and II molecules and directly activate T cell immunity (Mignot et al. 2006; Raposo et al. 1996; Zitvogel et al. 1998). Loaded DEX by indirectly or directly modifying DCs has been demonstrated to elicit an effective immune response in vivo (Escudier et al. 2005; Morse et al. 2005). Therefore, DEXs have been proposed to be a useful and effective agent for inducing anti-tumor immunity (Luketic et al. 2007; Zitvogel et al 1998).
Chaperone-rich cell lysates (CRCLs) generated by a free-solution isoelectric focusing (FS-IEF) technique have drawn considerable attention in the development of effective tumor therapies (Zeng et al. 2006b). CRCLs have been demonstrated to contain at least four chaperone proteins, including heat shock protein (Hsp) 70, Hsp 90, calreticulin, and glucose-regulated protein 94 (GRP94) (Graner et al. 2003). Although CRCLs also contain many unidentified proteins, depletion of these four specific proteins significantly abrogates the CRCL-induced immune response (Zeng et al. 2003). Chaperone proteins are able to interact with all peptides in cells; thereby, chaperone–tumor antigen peptide complexes are multivalent and can prevent immune escape. Hence, tumor-derived CRCLs have potential in the development of a vaccine.
To date, the underlying mechanism of exosomes in regulating immune cells remains poorly understood. The Casitas B cell lineage lymphoma (Cbl) proteins, including Cbl-b and c-Cbl, have been suggested to play a critical role in setting a signaling threshold for T cell activation (Lin and Mak 2007; Rangachari and Penninger 2004). Cbl-b may negatively regulate T cell activation, and T cells lacking Cbl-b have been reported to exhibit hyperproliferation with co-stimulation (Bachmaier et al. 2000; Chiang et al. 2000). Cbl-b is overexpressed in T cell anergy-promoting conditions (Jeon et al. 2004), and it acts as a negative regulator of T cell receptor (TCR) signaling (Liu 2004; Murphy et al. 1998; Thien and Langdon 2005). However, whether Cbl is involved in regulating exosome-mediated T cell activation remains unknown. DEXs have been suggested to more effectively activate naive CD8+ proliferation and differentiation compared with exosomes from tumor cells (Hao et al. 2006). A DC vaccine generated from DCs treated with tumor cell lysates was shown to have potent anti-tumor immunity in vivo, prolong survival, and improve quality of life (Lund-Johansen and Olweus 1999). Notably, Hao et al. demonstrated that DCs loaded with DEXs provide more potent anti-tumor immunity than either DEXs or DCs applied alone (Hao et al. 2007), implying that DCs loaded with DEXs may offer a novel and effective vaccine for tumor therapy. In the present study, DEXs were generated by adding CRCLs to DCs and were then used to treat DCs. The current study is the first to evaluate the anti-tumor effect of DCs treated with DEXs derived from CRCL-loaded DCs in vitro and in vivo, and it was intended to delineate the underlying molecular mechanisms for these activities.
Materials and Methods
Animals
Six-week-old female C57BL/6 mice weighing 25–30 g were obtained from the Experimental Animal Center (College of Medicine, Xi’an Jiaotong University, Xi’an, China). Mice were raised in pathogen-free cages (five per cage) under standard conditions of dark–light cycles, room temperature, and humidity, with free access to water and food. The animal experimental procedures were conducted under a protocol that was reviewed and approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University.
Cell Cultures
Mouse glioma cells (GL261 cells) purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) were grown in Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10 % fetal bovine serum (FBS) plus 2 mM l-glutamine and 1 % penicillin and streptomycin. Normal mouse fibroblast cells (L929) were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal calf serum and 1 % penicillin and streptomycin. The cell culture was incubated in a humidified incubator at 37 °C containing 5 % CO2.
Preparation of Glioma Cell Lysate and CRCL
GL261 cells cultured in RPMI-1640 medium containing inactive FBS were frozen in liquid nitrogen for 10 min and defrosted at 4 °C followed by centrifugation at 12,000 g for 15 min. The supernatants were collected as glioma cell lysate and stored at −80 °C. The protein concentration was measured using the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA). CRCLs were extracted and purified by FS-IEF as previously reported (Graner et al. 2000a), with some modifications. In brief, cells were homogenized in 1 mL of radioimmunoprecipitation assay (RIPA) lysis buffer plus 0.1 % detergents and 10 μL phenylmethylsulfonyl fluoride. The homogenate was centrifuged at 10,000 g at 4 °C for 30 min. The supernatants were collected and re-centrifuged at 100,000 g at 4 °C for 1 h. Thereafter, the high-speed supernatants were collected and dialyzed. The protein concentration was measured, and the samples were frozen in 25-mg aliquots. For isoelectric focusing, the sample was subjected to FS-IEF in a Bio-Rad Rotofor cell (Bio Rad Laboratories, Hercules, CA, USA) at 15 W constant power for 5 h. Twenty fractions were collected and detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis. Fractions containing proteins including GRP94, Hsp90, Hsp70, and calreticulin were collected and dialyzed into phosphate-buffered saline (PBS). The detergents were cleared by passage over an Extracti-Gel matrix. Endotoxin concentration was less than 0.01 endotoxic units/μg detected by the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD, USA).
Generation of DCs
DCs were generated from bone marrow cells of C57BL/6 mice as described previously (Guo et al. 2002). In brief, mice were sacrificed, and bone marrow cells were harvested and cultured in RPMI-1640 medium containing 10 % FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, and 1 % penicillin and streptomycin, supplemented with 20 ng/mL granulocyte macrophage colony-stimulating factor and 10 ng/mL interleukin (IL)-4 (R&D Systems, Minneapolis, MN, USA). On the third day, floating cells were removed and equal volumes of fresh medium were added. On the sixth day, loosely adherent and nonadherent cells were collected and further enriched by CD11c MicroBeads (Miltenyi Biotec, Auburn, CA, USA) according to the supplier’s instructions.
Preparation and Isolation of DEXs
A total of 25 mL of RPMI-1640 medium was added to the culture compartment of CELLine 1000 bioreactor flasks (INTEGRA Biosciences, Zürich, Switzerland). Until the semipermeable membrane was fully infiltrated, DCs resuspended in 15 mL of medium (2.0 × 106 cells/mL) containing 10 % FBS were added to the culture compartment and 975 mL of fresh medium was added to a volume of 1000 mL, after which the culture was continued for 7 days. GL261 cell lysate or CRCLs (50 μg/mL) were added, and the cultures were incubated for another 48 h. Cell culture supernatants were harvested and centrifuged at 800 g for 5 min followed by centrifugation at 12,000 g for 20 min at 4 °C. The supernatants were collected and filtered through a 0.22-μm-pore filter (Millipore, Boston, MA, USA) followed by ultracentrifugation at 110,000 g for 3 h at 4 °C. The pellets at the bottom of the tube were collected, resuspended in PBS, and ultracentrifuged at 110,000 g for 2 h. Finally, the pellets containing exosomes were collected and stored at −80 °C until use. The protein concentrations were quantified using the BCA protein assay kit.
Preparation of DCs Loaded with DEX
The cultured DCs were divided into three groups (5 × 105 DCs per group): DEX (GL261)-DCs, DCs treated with DEXs (10 μg/mL) derived from DCs loaded with GL261 cell lysate; DEX(CRCL-GL261), DCs treated with DEX (10 μg/mL) derived from DCs loaded with CRCL extracted from GL261 cells; and L929-DCs, DCs treated with L929 cell lysate. After 24 h incubation, all DCs in the three groups were treated with tumor necrosis factor-α (10 ng/mL)and cultured for another 48 h.
Intracranial Tumor Implantation
Mice were anesthetized by subcutaneous injection of sodium pentobarbital (40 mg/kg), and DL216 cells (1 × 104) diluted in 2.5 μL of PBS were stereotactically implanted into the right striatum, as reported previously (Ehtesham et al. 2002). Three days after tumor cell implantation, 1 × 105 DEX (GL261)-DCs, DEX (CRCL-GL261)-DCs, or L929-DCs diluted in 2.5 μL of PBS was injected intrathecally. The injection was performed every 3 days during the experiment. Survival time was calculated as the time elapsed from the tumor implantation until the death of the mice. The tumor volume was assessed when the mice were dead, after being euthanized by a subcutaneous injection of pentobarbital sodium (100 mg/kg).
Cytotoxic T Lymphocyte Assay
Splenocytes were harvested from the normal or immunized mice, and CD4+ and CD8+ T cells in splenocytes were further purified by the mouse CD4+ T and CD8+ cell isolation kit (Miltenyi Biotec). The cultured CD4+ and CD8+ T cells were diluted to 1.5 × 106 cells/mL. Treated DCs, including L929-DCs, DEX (GL261)-DCs, and DEX (CRCL-GL261)-DCs, were added to T cells at a ratio of 1:3 (T/DCs) and incubated for 3 days. The cultured cells were then used as effector cells (E). After incubation, the stimulated effector cells were diluted to 1 × 106 cells/mL and 100 μL per well was added to a 96-well plate. GL261 glioma cells as target cells (T) were added at a ratio of 10:1, 25:1, or 50:1 (E/T) and incubated at 37 °C for 5 h. The cytolytic activity was examined by a cytotoxicity detection kit (Promega, Madison, WI, USA) according to the manufacturer’s instruction.
Cell Proliferation Assay
CD4+ and CD8+ T cells were cultured with treated DCs (L929-DCs, DEX (GL261)-DCs, DEX (CRCL-GL261)-DCs) in a ratio of 1:3 (T/DCs) and incubated for 72 h. Then, 0.5 μCi of [3H]-thymidine (3HTdR) was added to each reaction, and the culture was continued for 16 h. The uptake of 3HTdR was measured by a microBeta counter (Beckmen, Krefeld, Germany).
Immunohistochemistry Staining
Brain tissues harvested from treated mice were frozen in dry ice and cut into 10-μm sections. The sections were pretreated with 5 % FBS (Sigma, St. Louis, MO, USA) for 30 min. The sections were then stained with primary rat antibodies for CD4 and CD8 (Abcam, Cambridge, UK) and incubated for 1 h at 37 °C. The secondary antibody, horseradish peroxidase (HRP)-conjugated goat anti-rat IgG, was added and incubated for 30 min at 37 °C. The sections were developed with the DAB Color Development Kit (Beyotime, Haimen, China) before being mounted on slides.
Evaluation of Cytokines
Intracranial tumor tissue was harvested, weighed, and homogenized in 1 mL of RIPA lysis buffer. The mixtures were centrifuged to remove cellular debris, and the supernatants were collected. The levels of IL-2 and interferon (IFN)-γ in the supernatants were measured by using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA). The cytokine levels were normalized to tumor weight.
Depletion of CD4+ and CD8+ T Cells
For the depletion of CD4+ and CD8+ T cells, 0.5 mg of rat anti-mouse CD4 (GK1.5) and CD8 (2.43) monoclonal antibodies (Abcam, Cambridge, UK) diluted in 200 μL of PBS were injected intraperitoneally into each mouse. Normal rat IgG was used as the control. The first injection was performed before tumor implantation, followed by once daily treatment for the next three consecutive days and then twice a week. Depletion efficiency was detected by flow cytometry assay using fluorescein isothiocyanate (FITC)-labeled anti-CD4 and phycoerythrin (PE)-labeled anti-CD8 (BD Biosciences, San Diego, CA, USA).
Western Blot Analysis
A total of 20 μg of proteins from cell lysis was separated by electrophoresis on 12 % SDS-PAGE followed by transferring to a nitrocellulose membrane (Bio-Rad). Nonfat milk (2.5 %) was used as a buffer to block the nonspecific binding on the membrane for 1 h at 37 °C. Primary antibodies diluted in block buffer were then added, and membranes were incubated at 4 °C overnight. The primary antibodies were as follows: anti-Cbl-b, anti-Akt, and anti-β-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-p-Akt and anti-p-extracellular signal-regulated kinase (ERK)1/2 were purchased from Cell Signaling Technology (Danvers, MA, USA); anti-c-Cbl was purchased from Transduction Laboratories (Lexington, KY, USA); and anti-ERK was purchased from Abcam (Abcam, Cambridge, UK). After three washes with Tris-buffered saline and Tween 20 (TBST), HRP-conjugated secondary antibody was added and incubation continued for 4 h. Finally, the protein band was detected by an enhanced chemiluminescence (ECL) detection system (Amersham, Little Chalfont, UK).
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Significant comparisons in different groups were analyzed by one-way ANOVA followed by Bonferroni post hoc test. The survival curve was analyzed by the Kaplan–Meier method. A p value less than 0.05 was considered statistically significant.
Results
DCs Loaded with DEX (CRCL-GL261) Promote the Proliferation and Cytotoxic T Lymphocyte of T Cells In Vitro
In our previous study, we have demonstrated that DEX (CRCL-GL261) has more abundant expression of MHC class I and II molecules, intercellular adhesion molecule-1, and other immune molecules than DEX (GL261), implying that it possesses more potent immune activity (Bu et al. 2014). Therefore, we sought to further investigate the effect of DEX (CRCL-GL261) on immune activation. First, we examined the effect of DCs loaded with DEX (CRCL-GL261) on the proliferation of T cells according to a previously described method (Hao et al 2007). CD4+ and CD8+ T cells were purified from the spleens of mice and treated with DCs loaded with DEX (GL261) or DEX (CRCL-GL261), respectively. The DCs loaded with L929 cell lysate were used as the control, which yielded no obvious effect on the proliferation and cytotoxic T lymphocyte (CTL) activity of T cells. Both DEX (GL261)-DCs and DEX (CRCL-GL261)-DCs affected the proliferation of CD4+ (Fig. 1a) and CD8+ (Fig. 1b) T cells, but DEX (CRCL-GL261)-DCs exhibited more significant motivating effect compared with DEX (GL261)-DCs. Furthermore, DEX (CRCL-GL261)-DCs upregulated the cytotoxicity of CD4+ (Fig. 1c) and CD8+ (Fig. 1d) T cells against GL261 glioma cells more highly than DEX (GL261)-DCs.
Effect of DCs loaded with DEX (CRCL-GL261) on the proliferation and CTL of T cells. Detection of the proliferation of CD4+ (a) and CD8+ (b) T cells in response to treated DCs. L929, cells treated with DCs pretreated with PBS; DEX (GL261), cells treated with DCs pretreated with DEX derived from GL261 cell lysate-loaded DCs; DEX (CRCL-GL261), cells treated with DCs pretreated with DEX derived from purified CRCL-loaded DCs. T cells and DCs were incubated at a ratio of 1:3 (T/DCs) and co-cultured for 72 h. Then 0.5 μCi of 3HTdR per reaction was added, and the cells were continuously cultured for another 16 h. Cell proliferation was evaluated by measuring the uptake of 3HTdR (cpm). Detection of the CTL of CD4+ (c) and CD8+ (d) T cells stimulated by treated DCs. Treated T cells, as already mentioned, were co-incubated with GL261 cells as targeted cells at a ratio of 10:1, 25:1, or 50:1 (E/T) at 37 °C for 5 h before the cytolytic activity was determined by a cytotoxicity detection kit. *p < 0.05; **p < 0.01
Injection of DCs Loaded with DEX (CRCL-GL261) Prolongs Survival and Inhibits Intracranial Glioma Growth in a Mouse Model
To determine whether DCs treated with DEX (CRCL-GL261) provided a therapeutic benefit against intracranial glioma growth, DEX (GL261)-DCs or DEX (CRCL-GL261)-DCs were delivered into established intracranial gliomas in C57BL/6 mice. DEX (CRCL-GL261)-DC treatment was found to significantly prolong survival compared with other treatments (Fig. 2a). L929-DCs exhibited no protective effect, showing the same effect as the PBS control treatment. Both DEX (GL261)-DC and DEX (CRCL-GL261)-DC treatment groups were significantly different from the L929-DC or PBS treatment groups (p < 0.01, DEX (GL261)-DCs versus L929-DCs or PBS group; p < 0.001, DEX (GL261)-DCs versus L929-DCs or PBS group). A significant difference also existed between the DEX (CRCL-GL261)-DC treatment group and the DEX (GL261)-DC treatment group (p < 0.01). In addition, the effect of DEX (CRCL-GL261)-DCs on tumor volume was evaluated. DEX (CRCL-GL261)-DC treatment significantly decreased tumor growth in comparison with the L929-DC treatment and the PBS control (p < 0.001; Fig. 2b). DEX (CRCL)-DCs yielded significant inhibition of tumor growth compared with L929-DCs or PBS (p < 0.01), but DEX (CRCL-GL261)-DCs showed greater inhibition of tumor growth in comparison with DEX (GL261)-DCs (p < 0.05).
Effect of DCs loaded with DEX (CRCL-GL261) on the experimental glioma in C57BL/6 mice. a Kaplan–Meier survival curve of intracranial glioma-bearing mice injected intrathecally with PBS, L929-DCs, DEX (GL261)-DCs, or DEX (CRCL-GL261)-DCs. N = 20 per group. b Volume of tumors of the groups mentioned above. *p < 0.05; **p < 0.01; ***p < 0.001
DCs Loaded with DEX (CRCL-GL261) Induce Enhanced T Cell Infiltration in Intracranial Glioma
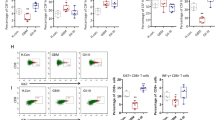
To determine whether the delivery of DCs loaded with DEX (CRCL-GL261) triggered a more dramatic T cell response in vivo in mice, we used immunohistochemistry to assess the infiltration of CD4+ and CD8+ in intracranial gliomas. Negligible CD4+ and CD8+ T cell infiltration in tumors was observed in mice in the PBS and L929-DC treated groups. DEX (GL261)-DCs resulted in a certain degree of intracranial T cell infiltration, but DEX (CRCL-GL261)-DCs induced more robust CD4+ and CD8+ T cell infiltration than DEX (GL261)-DCs (Fig. 3). These results suggest that DCs loaded with DEX (CRCL-GL261) exhibited a strong protective effect against intracranial tumors by inducing enhanced T cell infiltration.
T Cells from Mice Treated with DEX (CRCL-GL261)-DCs Exhibit an Enhanced Tumor-Specific CTL Against GL261
To determine whether DEX (CRCL-GL261)-DCs elicited a tumor-specific immunity, mice that survived tumor implantation for 8 weeks after DC treatments were subjected to CTL detection. CD4+ and CD8+ T cells isolated from splenocytes were re-stimulated with mitomycin C-treated GL261 cells that were used as effector cells. Glioma cells GL261 and mouse mastocytoma cells P815 were used as target cells. Compared with the L929-DC-treated group, both the DEX (CRCL-GL261)-DC- and DEX (GL261)-DC-treated groups showed a significant increase in CTL activity of CD4+ and CD8+ T cells (p < 0.001). Nonetheless, mice treated with DEX (CRCL-GL261)-DCs showed significantly higher CTL activity compared with those treated with DEX (GL261)-DCs (p < 0.01). Furthermore, CD4+ and CD8+ T cells from DEX (CRCL-GL261)-DCs showed GL261-specific CTL activity, whereas no cytolytic effect was observed on P815 cells (p < 0.001) (Fig. 4a, b).
Tumor-specific CTL activity of T cells. Spleen CD4+ (a) and CD8+ (b) T cells from L929-DC-, DEX (GL261)-DC-, or DEX (CECL-GL261)-DC-treated mice 8 weeks after tumor implantation were re-stimulated with mitomycin C-treated GL261 cells for 5 days, and the CTL activity was measured against GL261 or mouse mastocytoma cells P815 at a ratio of 10:1, 25:1, and 50:1 (E/T)
Depletion of T Cells Weakens the Anti-tumor Effect of DEX (CRCL-GL261)-DCs
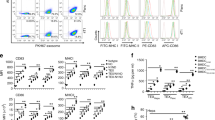
To further validate T cells being involved in anti-tumor activity induced by DEX (CRCL-GL261)-DCs, CD4+ and CD8+ T cells were depleted by the injection of anti-CD4 or anti-CD8 monoclonal antibody (mAb) before and after DEX (CRCL-GL261)-DC treatment. The results showed that depletion of CD8 CD4+ and CD8+ T cells significantly impaired anti-tumor effects of DEX (CRCL-GL261)-DCs in mice with intracranial tumors, and long-term survival was not observed in either group (p < 0.01, DEX (CRCL-GL261)-DCs + anti-CD4 vs. DEX (CRCL-GL261)-DCs + IgG; p < 0.001, DEX (CRCL-GL261)-DCs + anti-CD8 vs. DEX (CRCL-GL261)-DCs + IgG). It is noteworthy that the depletion of CD8+ T cells had a more significant negative impact on the survival of tumor-bearing mice (p < 0.01; DEX (CRCL-GL261)-DCs + anti-CD8 vs. DEX (CRCL-GL261)-DCs + anti-CD4). These data imply that the anti-tumor immunity of DEX (CRCL-GL261)-DCs is CD4+ and CD8+ T cell dependent, with CD8+ T cells playing a more important role in the anti-tumor immunity induced by DEX (CRCL-GL261)-DCs (Fig. 5a). The depletion efficiency of anti-CD4 or anti-CD8 mAb was validated by flow cytometry assay in which CD4+ and CD8+ T cell populations were rarely detected in mice treated with anti-CD4 or anti-CD8 mAb, respectively (Fig. 5b).
Effect CD4+ and CD8+ T cell depletion on DEX (CRCL-GL261)-DC-mediated anti-tumor immunity. a Kaplan–Meier survival curve of intracranial glioma-bearing mice. Intracranial glioma-bearing mice were randomly divided into four groups. One group was treated with L929-DCs and intraperitoneally injected normal rat IgG; the other three groups were treated with DEX (CRCL-GL261)-DCs plus injection with anti-CD4+ T cell-depleting antibody, anti-CD8+ T cell-depleting antibody, or normal rat IgG. N = 12 per group. b Flow cytometry assay of CD4+ and CD8+ T cell population in mice treated with different antibodies. Peripheral blood (100 μL) was collected and mixed with 20 μL of FITC-labeled anti-CD4 and PE-labeled anti-CD8 and incubated for 15 min in darkness. Then, 500 μL of hemolysin was added, and the samples were incubated for 10 min followed by centrifugation at 1500 g for 5 min. The cell precipitate was resuspended in PBS and detected by flow cytometry
DEX (CRCL-GL261)-DCs Induce Strong Production of IL-2 and IFN-γ
To further verify that DEX (CRCL-GL261)-DCs potentiated strong anti-tumor immunity, we detected the expression levels of cytokines IL-2 and IFN-γ in brain tumor tissues, using the ELISA method. We found that tumor tissues from DEX (GL261)-DC-treated mice exhibited high levels of IL-2 and IFN-γ compared to mice treated with PBS or L929-DCs. There were also significant differences between mice treated with DEX (CRCL-GL261)-DCs and DEX (GL216)-DCs (Fig. 6a). We also assessed the IL-2 and IFN-γ secretion ability of T cells from mice treated with DEX (CRCL-GL261)-DCs that survived tumor implantation for 8 weeks. CD4+ and CD8+ T cells isolated from splenocytes were re-stimulated with mitomycin C-treated GL261 cells, and the secretion levels of IL-2 and IFN-γ were examined. We found that IL-2 (Fig. 6b) and IFN-γ (Fig. 6c) production was significantly potentiated in both CD4+ and CD8+ T cells in the group treated with DEX (CRCL-GL261)-DCs compared with the other groups. These results indicate that DEX (CRCL-GL261)-DCs induced potent anti-tumor immunity in vivo by the upregulation of IL-2 and IFN-γ, which were both closely related to anti-tumor immunity (Chen et al. 2006).
Effect of DEX (CRCL-GL261)-DCs on IL-2 and IFN-γ expression. Detection of IL-2 (a) and IFN-γ (b) levels in tumors from glioma-bearing mice treated with PBS, L929-DCs, DEX (GL261)-DCs, or DEX (CRCL-GL261)-DCs. Examination of IL-2 (c) and IFN-γ (d) production levels in spleen CD4+ and CD8+ T cells. Spleen CD4+ and CD8+ T cells were isolated from mice that survived for 8 weeks after tumor implantation and were re-stimulated with mitomycin C-treated GL261 cells for 5 days. *p < 0.05; **p < 0.01
DEX (CRCL-GL261)-DCs Maintain T Cell Activation by Regulating Cbl-b and c-Cbl Signaling
To explore the underlying mechanism of DEX (CRCL-GL261)-DCs in inducing potent anti-tumor activity, we examined their effects on Cbl-b and c-Cbl, which play an essential role in setting the signaling threshold for T cell activation (Lin and Mak 2007; Rangachari and Penninger 2004). In the splenocytes isolated from mice, DEX (CRCL-GL261)-DC treatments had lower Cbl-b and c-Cbl expression compared with other groups. To further confirm the increased expression of Cbl-b and c-Cbl, we detected the expression of their downstream genes. We found that the p85 regulatory subunit of PI3K and phosphorylation of Akt were highly upregulated by DEX (CRCL-GL261)-DCs in splenocytes. Furthermore, the phosphorylation of ERK1/2 was also increased by DEX (CRCL-GL261)-DC treatment (Fig. 7a). To further validate that PI3K/Akt and ERK1/2 signaling was involved in regulating the potent immunity induced by DEX (CRCL-GL261)-DCs, we treated splenocytes with DEX (CRCL-GL261)-DCs in the presence of PI3K inhibitor LY294002 and ERK1/2 inhibitor PD98059 and determined their proliferation and CTL activity. We found that inhibition of P13K or ERK1/2 significantly decreased the ability of DEX (CRCL-GL261)-DCs to enhance cell proliferation (Fig. 7b) and CTL activity (Fig. 7c). Taken together, these results suggest that DEX (CRCL-GL261)-DCs induced potent T cell activation by negatively regulating Cbl-b and c-Cbl signaling, leading to the activation of PI3K/Akt and ERK1/2 signaling.
Effect of DEX (CRCL-GL261)-DCs on Cbl-b and c-Cbl signaling. a Detection of protein levels using indicated antibodies in splenocytes treated with DCs. b Detection of the effects of PI3K inhibitor LY294002 and ERK1/2 inhibitor PD98059 on cell proliferation (b) and CTL (c) activity induced by DEX (CRCL-GL261)-DCs. Splenocytes were pretreated with LY294002 (10 μM) or PD98059 (10 μM) for 1 h before treated DCs were added. For the detection of cell proliferation, splenocytes were co-incubated with DCs for 72 h, then 0.5 μCi of 3HTdR per reaction was added, and the cells were continuously cultured for another 16 h. Cell proliferation was evaluated by measuring the uptake of 3HTdR (cpm). For CTL activity detection, splenocytes were simulated by treated DCs for 3 days. Then, GL261 cells were added at a ratio of 50:1 (E/T) and incubated at 37 °C for 5 h before the cytolytic activity was determined by a cytotoxicity detection kit. *p < 0.05
Discussion
In the current study, we gathered evidence that DCs treated with DEXs derived from CRCL-loaded DCs have a substantial anti-tumor effect against glioma in vitro and in vivo compared with those treated with DEXs derived from DCs loaded with tumor cell lysates. DCs and DEXs have been separately used as vaccines for tumor treatment, but the efficiency needs to be improved. DCs loaded with tumor cell lysates have potent anti-tumor immunity in vivo, prolong survival, and improve life quality (Lund-Johansen and Olweus 1999). In particular, DCs loaded with tumor-derived exosomes have been reported to significantly improve survival in malignant mesothelioma-bearing mice in comparison with DCs loaded with tumor cell lysate (Mahaweni et al. 2013). DCs loaded with exosomes derived from glioma tissues elicit potent tumor-specific CD8+ CTL activity (Bu et al. 2011). Notably, Hao et al. demonstrated that DCs loaded with DEXs provide more potent anti-tumor immunity than either DEXs or DCs applied alone (Hao et al 2007). Here, we found that DEX (CRCL-GL261)-DCs elicited potent cell proliferation and CTL activity in CD4+ and CD8+ T cells, implying that DEX (CRCL-GL261)-DCs induced a T cell immune response. In vivo immunohistochemical experiments demonstrated that CD4+ and CD8+ T cell infiltration was markedly enhanced in intracranial mouse glioma tissues by treatment with DEX (CRCL-GL261)-DCs as compared with other treatments. Furthermore, depletion of CD4+ or CD8+ T cells significantly impaired the treatment effects of DEX (CRCL-GL261)-DCs, implying that DEX (CRCL-GL261)-DCs elicited anti-tumor immunity through the induction of T cell immune response.
Chaperone proteins such as Hsp70, Hsp90, calreticulin, and GPR94 purified from tumors have been reported to individually exhibit effective, specific anti-tumor activity (Basu and Srivastava 1999; Graner et al. 2000b; Nair et al. 1999; Tamura et al. 1997; Udono and Srivastava 1994). In particular, vaccines based on tumor-derived GRP94 have been used in clinical trials (Caudill and Li 2001; Janetzki et al. 2000; Srivastava and Amato 2001). Since Graner et al. (2000a) first used the FS-IEF method to purify and identify CRCLs, a multi-protein complex including Hsp70, Hsp90, calreticulin, GRP94, and many unidentified proteins, their role in anti-tumor agent development has been widely studied (Zeng et al 2006b). CRCLs exhibit more effective anti-tumor activity than an equivalent total quantity of any individual chaperone proteins such as Hsp70, Hsp90, or GPR94 (Graner et al 2000a). CRCLs are a multivalent tumor antigen that may enable DCs to effectively present antigens to T cells, thus leading to potent T cell immunity. Importantly, depletion of the four specific proteins significantly abrogates the CRCL-induced immune response (Zeng et al 2003). CRCL-loaded DCs induced more potent maturation of DCs and significantly prolonged the survival of tumor-bearing mice in a CD4+ and CD8+ T cell-dependent manner (Li et al. 2007; Zeng et al 2003). Here, we found that DEX (CRCL-GL261)-DCs also induced an anti-tumor immune response in a CD4+ and CD8+ T cell-dependent manner, although CRCLs have also been reported to exert an anti-tumor effect through natural killer cells (Zeng et al. 2006a). Interestingly, CRCL-loaded DCs are more resistant to a tumor-induced regulatory T cell suppression effect (Larmonier et al. 2008). (Guntinas-Lichius 2008) and have demonstrated that CRCLs induce both T cell-specific and B cell-specific responses against breast cancer. However, the role of CRCLs in the induction of anti-tumor immunity is in doubt. The combination of imatinib with DC-loaded tumor cell-derived CRCLs acquired high activation-specific T cell and potent anti-tumor activity (Zeng et al. 2004). In the present study, the DCs loaded with DEXs derived from CRCL-loaded DCs were found to markedly prolong the survival of tumor-bearing mice in comparison with other treatments, implying that the generation of a DC vaccine based on our methods would have potent anti-tumor activity.
The precise underlying molecular mechanism of potent exosome-mediated anti-tumor activity remains unclear. Here, we demonstrated that both Cbl-b and c-Cbl expression levels in T cells were downregulated upon treatment with DEX (CRCL-GL261)-DCs, implying that Cbl-b and c-Cbl signaling was involved in regulating T cell activation induced by them. Overexpression of c-Cbl decreases TCR-induced zeta-associated protein of 70 kDa (ZAP-70) activation, which initiates kinase activity in various downstream signaling pathways (Chan et al. 1995; Fournel et al. 1996; Rao et al. 2000). Co-stimulatory signals such as CD43 were found to abrogate the interaction between c-Cbl and ZAP-70 (Pedraza-Alva et al. 2011). Co-stimulation was also found to reduce Cbl-b activity (Zhang et al. 2002). Therefore, one can speculate that vaccines based on CRCLs, such as DEX (CRCL-GL261)-DCs in the present study, may provide extensive co-stimulatory signals that block c-Cbl and Cbl-b signals, thus favoring sustained TCR signaling and T cell activation. Both c-Cbl and Cbl-b are involved in decreasing PI3K activity by their E3 ubiquitin ligase activity (Fang and Liu 2001; Yingchun et al. 2011). In addition, ERK was also negatively regulated by Cbl-b and c-Cbl in the activation of Jurkat T cells (Zhao et al. 2013). As expected, we demonstrated that DEX (CRCL-GL261)-DCs not only inhibit the expression of Cbl-b and c-Cbl but also increase the protein levels of p85-PI3K and the phosphorylation of Akt and ERK. These results demonstrate the underlying molecular mechanism of DEX (CRCL-GL261)-DCs in triggering potent anti-tumor immunity. Furthermore, the inhibition of PI3K or ERK by specific inhibitors significantly abrogated the potent effect of DEX (CRCL-GL261)-DCs on T cell proliferation and CTL activity, further confirming that DEX (CRCL-GL261)-DCs enhanced T cell activation by negatively regulating Cbl-b and c-Cbl, leading to the increased activation of PI3K/Akt and ERK. Interestingly, Cantrell et al. (2010) found that tumor-derived CRCLs promote the activation of DCs and macrophages by inducing mitogen-activated protein kinase, signal transducer and activator of transcription, and nuclear factor-kappa B activation both in vitro and in vivo.
In summary, our study demonstrated for the first time that DCs loaded with DEX from glioma cell-derived CRCL-loaded DCs exhibited potent anti-tumor activity against intracranial mouse glioma by sustaining T cell activation. Furthermore, these results also provided a potential mechanism by which CRCL-based vaccines exert their anti-tumor activity. In the present study, the manner of generating DCs loaded with DEX derived from CRCL-loaded DCs provided novel and promising insight for developing anti-tumor vaccines based on CRCLs. Further development and investigation are warranted.
Abbreviations
- CRCL:
-
Chaperone-rich cell lysate
- DC:
-
Dendritic cell
- DEX:
-
Dendritic cell-derived exosome
- CTL:
-
Cytotoxic T lymphocyte
- Cbl:
-
Casitas B cell lineage lymphoma
- PI3K:
-
Phosphatidyl inositol 3-kinase
- ERK:
-
Extracellular signal-regulated kinase
- FS-IEF:
-
Free-solution isoelectric focusing
- Hsp:
-
Heat shock protein
- GRP94:
-
Glucose-regulated protein 94
- HTdR:
-
[3H]-thymidine
References
Ailawadi S, Wang X, Gu H, Fan GC (2015) Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta 1852:1–11
Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A et al (2000) Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403:211–216
Basu S, Srivastava PK (1999) Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med 189:797–802
Bu N, Wu H, Sun B, Zhang G, Zhan S, Zhang R, Zhou L (2011) Exosome-loaded dendritic cells elicit tumor-specific CD8+ cytotoxic T cells in patients with glioma. J Neurooncol 104:659–667
Bu N, Wu H, Zhang G, Ma X, Zhao P, Zhai N, Xiang L, Cao H, Yang X, Liu J (2014) Exosome from chaperone-rich cell lysates-loaded dendritic cells produced by CELLine 1000 culture system exhibits potent immune activity. Biochem Biophys Res Commun 6:02159–02157
Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S (2014) Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol 10:356–364
Cantrell J, Larmonier C, Janikashvili N, Bustamante S, Fraszczak J, Herrell A, Lundeen T, LaCasse JC, Situ E, Larmonier N et al (2010) Signaling pathways induced by a tumor-derived vaccine in antigen presenting cells. Immunobiology 215:535–544
Caudill MM, Li Z (2001) HSPPC-96: a personalised cancer vaccine. Expert Opin Biol Ther 1:539–547
Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R, Kurosaki T (1995) Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J 14:2499–2508
Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X (2006) Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol 36:1598–1607
Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H (2000) Cbl-b regulates the CD28 dependence of T-cell activation. Nature 403:216–220
Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS (2002) The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res 62:5657–5663
Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S et al (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med 3:10
Fang D, Liu YC (2001) Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol 2:870–875
Fournel M, Davidson D, Weil R, Veillette A (1996) Association of tyrosine protein kinase Zap-70 with the protooncogene product p120c-cbl in T lymphocytes. J Exp Med 183:301–306
Graner M, Raymond A, Akporiaye E, Katsanis E (2000a) Tumor-derived multiple chaperone enrichment by free-solution isoelectric focusing yields potent antitumor vaccines. Cancer Immunol Immunother 49:476–484
Graner M, Raymond A, Romney D, He L, Whitesell L, Katsanis E (2000b) Immunoprotective activities of multiple chaperone proteins isolated from murine B-cell leukemia/lymphoma. Clin Cancer Res 6:909–915
Graner MW, Zeng Y, Feng H, Katsanis E (2003) Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother 52:226–234
Guntinas-Lichius O (2008) Cetuximab in head and neck cancer. N Engl J Med 359:2725, author reply 2726
Guo J, Wang B, Zhang M, Chen T, Yu Y, Regulier E, Homann HE, Qin Z, Ju DW, Cao X (2002) Macrophage-derived chemokine gene transfer results in tumor regression in murine lung carcinoma model through efficient induction of antitumor immunity. Gene Ther 9:793–803
Hao S, Bai O, Yuan J, Qureshi M, Xiang J (2006) Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol 3:205–211
Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J (2007) Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology 120:90–102
Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK (2000) Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer 88:232–238
Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L et al (2004) Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 21:167–177
Larmonier N, Cantrell J, Lacasse C, Li G, Janikashvili N, Situ E, Sepassi M, Andreansky S, Katsanis E (2008) Chaperone-rich tumor cell lysate-mediated activation of antigen-presenting cells resists regulatory T cell suppression. J Leukoc Biol 83:1049–1059
Li G, Zeng Y, Chen X, Larmonier N, Sepassi M, Graner MW, Andreansky S, Brewer MA, Katsanis E (2007) Human ovarian tumour-derived chaperone-rich cell lysate (CRCL) elicits T cell responses in vitro. Clin Exp Immunol 148:136–145
Lin AE, Mak TW (2007) The role of E3 ligases in autoimmunity and the regulation of autoreactive T cells. Curr Opin Immunol 19:665–673
Liu YC (2004) Ubiquitin ligases and the immune response. Annu Rev Immunol 22:81–127
Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, Gauldie J, Bramson J, Wan Y (2007) Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J Immunol 179:5024–5032
Lund-Johansen F, Olweus J (1999) Dendritic cells—strong candidates for immunotherapy. Tidsskr Nor Laegeforen 119:2510–2514
Mahaweni NM, Kaijen-Lambers ME, Dekkers J, Aerts JG, Hegmans JP (2013) Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J Extracell Vesicles 24
Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15:1311–1333
Mao XG, Zhang X, Zhen HN (2009) Progress on potential strategies to target brain tumor stem cells. Cell Mol Neurobiol 29:141–155
Mignot G, Roux S, Thery C, Segura E, Zitvogel L (2006) Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med 10:376–388
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A et al (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 3:9
Murphy MA, Schnall RG, Venter DJ, Barnett L, Bertoncello I, Thien CB, Langdon WY, Bowtell DD (1998) Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol 18:4872–4882
Nair S, Wearsch PA, Mitchell DA, Wassenberg JJ, Gilboa E, Nicchitta CV (1999) Calreticulin displays in vivo peptide-binding activity and can elicit CTL responses against bound peptides. J Immunol 162:6426–6432
Navabi H, Croston D, Hobot J, Clayton A, Zitvogel L, Jasani B, Bailey-Wood R, Wilson K, Tabi Z, Mason MD et al (2005) Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol Dis 35:149–152
Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK et al (2011) Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 29:330–336
Parajuli P, Mathupala S, Mittal S, Sloan AE (2007) Dendritic cell-based active specific immunotherapy for malignant glioma. Expert Opin Biol Ther 7:439–448
Pedraza-Alva G, Merida LB, del Rio R, Fierro NA, Cruz-Munoz ME, Olivares N, Melchy E, Igras V, Hollander GA, Burakoff SJ et al (2011) CD43 regulates the threshold for T cell activation by targeting Cbl functions. IUBMB Life 63:940–948
Ramanathan P, Ganeshrajah S, Raghanvan RK, Singh SS, Thangarajan R (2014) Development and clinical evaluation of dendritic cell vaccines for HPV related cervical cancer—a feasibility study. Asian Pac J Cancer Prev 15:5909–5916
Rangachari M, Penninger JM (2004) Negative regulation of T cell receptor signals. Curr Opin Pharmacol 4:415–422
Rao N, Lupher ML Jr, Ota S, Reedquist KA, Druker BJ, Band H (2000) The linker phosphorylation site Tyr292 mediates the negative regulatory effect of Cbl on ZAP-70 in T cells. J Immunol 164:4616–4626
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172
Sabel MS (2009) Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 58:1–11
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–764, discussion 264-756
Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA (2014) Microvesicles and exosomes for intracardiac communication. Cardiovasc Res 102:302–311
Srivastava PK, Amato RJ (2001) Heat shock proteins: the ‘Swiss Army Knife’ vaccines against cancers and infectious agents. Vaccine 19:2590–2597
Steinman RM (2012) Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 30:1–22
Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G (2002) The biogenesis and functions of exosomes. Traffic 3:321–330
Tamura Y, Peng P, Liu K, Daou M, Srivastava PK (1997) Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science 278:117–120
Thien CB, Langdon WY (2005) c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J 391:153–166
Udono H, Srivastava PK (1994) Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol 152:5398–5403
Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T et al (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7:297–303
Yang C, Robbins PD (2011) The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol 2011:842849
Yingchun L, Xiujuan Q, Jinglei Q, Ye Z, Jing L, Yuee T, Xuejun H, Kezuo H, Yunpeng L (2011) E3 ubiquitin ligase Cbl-b potentiates the apoptotic action of arsenic trioxide by inhibiting the PI3K/Akt pathway. Braz J Med Biol Res 44:105–111
Zeng Y, Feng H, Graner MW, Katsanis E (2003) Tumor-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood 101:4485–4491
Zeng Y, Graner MW, Feng H, Li G, Katsanis E (2004) Imatinib mesylate effectively combines with chaperone-rich cell lysate-loaded dendritic cells to treat bcr-abl+ murine leukemia. Int J Cancer 110:251–259
Zeng Y, Chen X, Larmonier N, Larmonier C, Li G, Sepassi M, Marron M, Andreansky S, Katsanis E (2006a) Natural killer cells play a key role in the antitumor immunity generated by chaperone-rich cell lysate vaccination. Int J Cancer 119:2624–2631
Zeng Y, Graner MW, Katsanis E (2006b) Chaperone-rich cell lysates, immune activation and tumor vaccination. Cancer Immunol Immunother 55:329–338
Zhang J, Bardos T, Li D, Gal I, Vermes C, Xu J, Mikecz K, Finnegan A, Lipkowitz S, Glant TT (2002) Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J Immunol 169:2236–2240
Zhao MF, Qu XJ, Qu JL, Jiang YH, Zhang Y, Hou KZ, Deng H, Liu YP (2013) The role of E3 ubiquitin ligase Cbl proteins in interleukin-2-induced Jurkat T-cell activation. Biomed Res Int 2013:430861
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594–600
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81201983) and the Special Research Foundation of Personnel Training of the Second Affiliated Hospital of Xi’an Jiaotong University (New Talents of Technology) (No. RC(XM)201201).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bu, N., Wu, H., Zhang, G. et al. Exosomes from Dendritic Cells Loaded with Chaperone-Rich Cell Lysates Elicit a Potent T Cell Immune Response Against Intracranial Glioma in Mice. J Mol Neurosci 56, 631–643 (2015). https://doi.org/10.1007/s12031-015-0506-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0506-9