Abstract
Parkinson’s disease (PD) is an obstinate progressive neurodegenerative disease and characterized by locomotor impairment and dopaminergic neuronal degeneration in the substantia nigra pars compacta (SNc). We examined in here the dietary effect of nucleoprotein (NP) extracted from salmon soft roe on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-injected PD-like mice model to prevent the symptom as an alternative medicine. Male C57/BL6 mice were given either an artificially modified NP-free diet (NF) or NF supplied with 1.2 % NP for 1 week. Then, mice were injected intraperitoneally four times with 20 mg/kg MPTP. Seven days later, locomotor activity was examined, and the brains were immunostained with tyrosine hydroxylase (TH) and Iba1 antibodies. Moreover, in situ detection of superoxide anion (O2 −) and gene expression of mitochondrial electron transfer chain gene, Cox8b was evaluated in midbrains. NP-fed animals showed significantly reduced locomotor impairment and an increased number of TH-positive cells in the SNc compared with NF animals. The NP-fed animals also showed reduced lower levels of O2 − and up-regulation of Cox8b levels and Iba1 immunoreactivity, suggesting that inflammation and oxidative stress were suppressed and mitochondrial impairment was relieved in these animals. Supplementation of the diet with NP may serve as a useful preventive measure to slow the onset of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease which is characterized by loss of somata and processes of dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNc) and in the striatum and by an impairment of the motor functions (Dauer and Przedborski 2003). Although effective symptomatic therapies such as dopamine analogues, dopamine receptor agonists, and monoamine oxidase-B inhibitors exist to be delayed the progression of the PD, the PD patients are increasing with the advancement of an aging society, and the medical costs in the disease compress the financial burden in countries. Therefore, in addition to the development of the novel pharmacotherapy, it is required to develop the complementary and/or alternative medicines for prevention of the onset.
Neuroinflammatory responses including oxidative damages have been thought to be involved in the PD onset (Hirsch and Hunot 2009; Taylor et al. 2013). We have reported that mice which were injected lipopolysaccharide (LPS) into substantia nigra increased microglial activation and induced PD-like pathogenesis and symptoms. However, same stimuli in a proinflammatory cytokine, interleukin-1α/β gene-deficient (IL-1 KO) mice decreased the symptoms (Tanaka et al. 2013). It implies that neuroinflammation exceedingly relates to PD. Because the blockade of microglial activation by selective inhibitors prevented 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity (Wu et al. 2003; Chung et al. 2011), it has been also thought to play an important role in a chemical-induced PD model (Turski et al. 1991; Tipton and Singer 1993). Injected MPTP is converted into MPP+ in astroglia, and MPP+ is transported into cytoplasm of DAergic neurons via dopamine transporter. Then, the MPP+ presumably reacts and impairs mitochondrial function to increase oxidative stress by reactive oxygen species (ROS) and activates glia including microglia (Singer et al. 1987; Langston 1996; Speciale 2002; Mosley et al. 2012; Khan et al. 2013). In this way, MPTP could induce the DAergic cell death and could result in impairment of the motor function.
Nucleoprotein (NP) is extracted from salmon soft roe and consists mainly of a mixture of DNA nucleotide (38 %) and protamine (56 %). The NP is used as a nutritious supplement in humans and was first reported for prevention in animal model of an atopic disease by immunomodulation (Sudo et al. 2000). We have reported that NP-fed mice decreased hippocampal neural death after forebrain ischemia (Matsunaga et al. 2003), clinical symptom and joint swelling on rheumatoid arthritis (Ohtaki et al. 2010), and hepatitis by LPS injection (Yofu et al. 2010). The NP also directly scavenged ROS, singlet oxygen (1O2), and nitric oxide (NO) in vitro (Ohtaki et al. 2010; Yofu et al. 2010). Moreover, the long-term dietary intake of nucleoside-nucleotide mixture decreased an aging lipopigment, lipofuscin (Chen et al. 2000). Accumulation of the lipofuscin-like substances in the brain has been suggested the participation of memory deterioration, and then, accumulation of them is often recognized with a parkinsonian inclusion (Lewy bodies) (Chen et al. 2000; Meredith et al. 2002; Seehafer and Pearce 2006). While our and other studies implicate in a possibility of prevention of PD by NP diet, nothing has been shown so far.
We examined a preventive effect of oral supplementary NP on MPTP-induced PD mice and demonstrated that the NP-fed mice exhibited less locomotor deficit and DAergic neuronal loss of SNc. We showed that the mice fed NP decreased in situ detection of superoxide anion (O2 −) and mitochondrial gene expression in the SNc. These might decrease an inflammatory response as represented by Iba1-positive microglial immunoreactions. These results suggested a possibility that consumption of NP from food or nutritious supplement could redress the PD symptoms.
Methods
Materials
MPTP-HCl was obtained from Sigma (St. Louis, MO). Normal horse serum (NHS), avidin-biotin complex solution (ABC; Vectastain ABC Kit), and the diaminobenzidine (DAB) kit were obtained from Vector Laboratories (Burlingame, CA). Rabbit polyclonal anti-tyrosine hydroxylase (TH) antibody was obtained from Millipore (Billerica, MA), and rabbit polyclonal anti-Iba1 antibody was obtained from Wako (Osaka, Japan). Biotinylated goat anti-rabbit IgG antibody was obtained from Santa Cruz (Santa Cruz, CA), and Alexa 488-labeled anti-rabbit IgG and hydroethidine (HEt) were from Invitrogen (Carlsbad, CA).
Animals
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of Showa University (#00178 and 01118). Male C57BL/6 mice aged 7 weeks were purchased from Sankyo Labo Service (Tokyo, Japan). All mice were maintained with a commercial chow (Labo MR Stock, Nosan, Yokohama, Japan) on a 12-h light/dark cycle at 23 ± 2 °C with constant humidity (40 ± 15 %).
The experimental chows were produced by Crea Japan (Tokyo, Japan) and based on an artificially formulated nucleoprotein-free (NF) chow with/without added 1.2 % nucleoprotein (1.2 % NP) that was extracted from salmon soft roe via an industrial procedure. Following the previous studies (Matsunaga et al. 2003; Ohtaki et al. 2010; Yofu et al. 2010), we choose 1.2 % NP in the experimental chows in the present study. Compositions of the experimental diets are shown in Table 1.
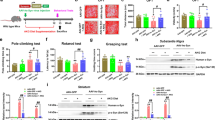
A diagram to describe the protocol followed for animal experiments is shown in Fig. 1a. After acclimation for 1 week on standard chow, the animals were divided into two groups. One group was given NF chow, and the other was given 1.2 % NP chow for the remainder of the experimental period. After 1 week on this diet, mice were administered intraperitoneally (i.p.) with MPTP (20 mg/kg dissolved in sterilized saline) on four occasions at 2-h intervals. Non-MPTP-injected animals were administered saline alone. The animals were maintained in standard cages, and motor function, measured using the foot-fault test (see below), was examined 7 days later. One day after the foot-fault test, animals were sacrificed and the brains removed for histological assessment as described below.
Schematic diagram of the experimental protocol and midbrain dissection. a Animals were given either NF or 1.2 % NP experimental chow for 1 week and then administered intraperitoneally 20 mg/kg of MPTP four times at 2-h intervals. One week later, motor function was assessed by the foot-fault test. The brains of animals were collected on day 8 after the MPTP treatment and prepared for histological assessment. b Midbrain containing SNc was dissected as follows: Coronal section between bregma −2.5 to −4.5 mm was dissected using the brain matrix. The midbrain containing SNc (arrow) was collected by removing the upper half brain and neocortical region as shown in the right image
Locomotor Assessment
Locomotor function in mice (n = 5–9) was examined using the foot-fault test (Hernandez and Schallert 1988; Goldberg et al. 2011) 7 days after MPTP injection. In brief, a wire net of dimensions 47 × 30.5 cm consisting of 1.6 × 1.6-cm squares was placed on a transparent table of height 30 cm. Mice were placed on the wire net, and the total number of steps and false steps made by the hind limbs (dropping of the hind limb through the grid) was counted for 5 min. Trials on the same mouse were repeated three times per mouse on the same day over a period of several hours. Foot-fault rates were expressed as a ratio of false steps to the total number of steps taken. The average of the three trials was expressed as the foot-fault rate. The examination was performed with two investigators who were unaware of the dietary group.
Immunohistochemistry
Mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) 8 days after the MPTP treatment and perfused with saline followed by 10 % neutralized buffer formalin into the left cardiac ventricle for fixation. The brains were removed and post-fixed in formalin for 3 days and then embedded in paraffin following dehydration with a series of alcohol and xylene solutions. The embedded brains were cut into six coronal sections (4 μm thick) at 200-μm intervals from −2.5 to −3.6 mm with respect to bregma for subsequent histological examination.
DAergic neurons and microglia were detected by TH and Iba1 immunostaining, respectively. The sections were de-paraffinized in a series of xylene and alcohol and washed in phosphate-buffered saline (PBS, pH 7.2). After treatment with 0.3 % H2O2 in PBS to quench endogenous peroxidase, the sections were placed in 2.5 % NHS in PBS to block non-specific binding and incubated with either rabbit polyclonal anti-TH antibody (1:2000) or rabbit polyclonal anti-Iba1 antibody (1:500) overnight at 4 °C. The sections were rinsed several times with PBS and further incubated with biotinylated goat anti-rabbit IgG (1:200) for 2 h. The sections were developed using the ABC method with DAB as chromogen, the nuclei were counterstained with hematoxylin, and slices mounted with malinol (Muto Chemical, Tokyo, Japan) on glass slides following dehydration.
The number of DAergic neurons was determined from cells that co-stained for both anti-TH antibody and hematoxylin in the SNc region. This was done for six coronal sections spaced 200 μm apart as described above and expressed as the total number of such cells summed from all sections. Eight and nine animals fed NF and 1.2 % NP diets, respectively, were used for this analysis.
The area of brown DAB-stained Iba1-immunoreactive cells in the SNc was calculated with ImageJ software, and mean of the area from five animals was used as an indicator of the microglial content.
An investigator (N.K.) who was unaware of the dietary group to which each animal belonged performed the cell counting and the analysis.
In Situ Detection of Superoxide Anion (O2 −) Levels
The production of O2 − in MPTP-treated PD animals was determined by in situ detection of oxidized hydroethidine (HEt) (Ohtaki et al. 2007). HEt rapidly penetrates the brain parenchyma, reacts with O2 −, and generates ethidium (Et) which is detected at an emission wavelength of 510–550 nm.
After being fed the experimental chows for 1 week, animals were administered MPTP in four doses as described above. Six hours, 3 days, and 7 days after the first MPTP injection, the animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and injected with HEt solution (1 mg/ml 0.9 % NaCl with 1 % DMSO) into the jugular vein (n = 3 per condition). Ten minutes after the HEt administration, the animals were perfused with 2 % paraformaldehyde (PFA), and the brains were then removed and post-fixed in 2 % PFA overnight. Control animals in which MPTP was not administered were also injected with HEt solution and the brains collected in a similar manner.
The brains were cryoprotected in 20 % sucrose for 2 days under dark conditions and then freeze-embedded with OCT compound in liquid nitrogen-cooled 2-methylbutane. Frozen blocks were cryosectioned in the coronal plane from bregma −2.5 to −3.9 mm at a thickness of 10 μm. To demonstrate the distribution of Et signals in the SNc, the sections were co-stained with anti-TH antibody. After blocking with 2.5 % NHS, the sections were incubated with rabbit anti-TH antibody (1:2000) and visualized by using Alexa 488-labeled anti-rabbit IgG (1:500). Three animals in each experimental group were analyzed.
Reverse Transcription Polymerase Chain Reaction
After fourth MPTP or saline injection, the animals fed either NF or 1.2 % NP diet were sacrificed. The midbrains containing SNc were immediately dissected as follow: Bregma −2.5 to −4.5 mm was extracted, and then, superior half of the brains and the temporal lobes were cut off from the extracted brain sections (see Fig. 1b) with razors and brain matrix. The midbrain tissues were snap-frozen in liquid nitrogen and stored at −80 °C until assayed. The RNA extraction and complementary DNA (cDNA) synthesis were performed as described previously (Hori et al. 2012). In briefly, the deep-frozen midbrains were transferred to a pre-chilled (in liquid nitrogen) mortar and ground with a pestle to a very fine powder with liquid nitrogen. Total RNA was extracted from ~60-mg sample powder using the QIAGEN RNeasy Mini Kit (QIAGEN, Germantown, MD). Total RNA samples were first DNase-treated with RNase-free DNase (Stratagene, Agilent Technologies, La Jolla, CA, USA). First-strand cDNA was then synthesized in a 20-μl reaction mixture with an AffinityScript QPCR cDNA Synthesis Kit (Stratagene) according to the protocol provided by the manufacturer, using 1 μg total RNA. The synthesized cDNA was made up to a volume of 50 μl with sterile water supplied in the kit. The reaction mixture contained 0.6 μl of the first-strand cDNA, 7 pmol of each primer set, and 6.0 μl of the Emerald Amp PCR Master Mix (2× premix) (TaKaRa Shuzo, Shiga, Japan) in a total volume of 12 μl. Primers were as follows: for cytochrome c oxidase subunit VIIIb (Cox8b), ggctacctaagtggtcagagga (forward) and aatctctcagggatgtgcaact (reverse); for β-actin, acgttgacatccgtaaagacct (forward) and ggtgtaaaacgcagctcagtaa (reverse). Thermal cycling (Applied Biosystems, Tokyo, Japan) parameters were as follows: After an initial denaturation at 97 °C for 5 min, the samples were subjected to a cycling regime of 20 to 40 cycles at 95 °C for 45 s, 55 °C for 45 s, and 72 °C for 1 min. The cDNA was amplified as follows: 97 °C for 5 min, then cycles of 95 °C for 45 s, 55 °C for 45 s, and 72 °C for 1 min, and, at last, 72 °C for 10 min. PCR products were analyzed by electrophoresis on 1.6 % agarose gel containing Et bromide. All data were calculated using the density with the ChemiDoc XRS+ imaging system (Bio-Rad, 6000 Alfred Nobel Drive, Hercules, CA 94547, USA) and corrected by β-actin.
Statistical Analysis
All data are expressed as the mean ± SD. Statistical comparisons were made using the two-tailed Student’s t test or Mann-Whitney U test. A value of p < 0.05 was considered to indicate statistical significance.
Results
Preventive Effect of Nucleoprotein on Locomotor Deficit in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Animals
PD patients are characterized in part according to the severity of their locomotor impairment. Here, using MPTP-treated mice, we performed firstly the foot-fault test to evaluate the degree of impairment in the mice fed normal commercial chow. In saline-injected control animals (n = 6), the mean foot-fault rate was 0.035 ± 0.009 (Fig. 2a). In contrast, the mean foot-fault rate in MPTP-injected animals was 0.088 ± 0.025 (n = 8), accounting for a significant impairment of locomotor activity compared with control (p < 0.05).
Effect of NP on locomotor activity 7 days after MPTP injection. Locomotor activity was determined by the foot-fault test. a MPTP-treated mice (n = 8) showed a significantly increased foot-fault rate compared with non-MPTP-treated control mice (n = 6). b Differences in foot-fault rates between NF- (n = 5) and 1.2 % NP- (n = 9) fed mice showing a significantly improved value for the latter. Mean foot-fault rate is shown by a line. *p < 0.05 vs control group (a) or NF group (b) by Student’s t test
We next examined the locomotor activity of animals fed the NF and 1.2 % NP diets. Seven days after MPTP injection, the mean foot-fault rate in NF fed animals was 0.066 ± 0.017 (n = 5), which was similar to that of MPTP-injected control animals (Fig. 2b). The foot-fault rate of animals fed the 1.2 % NP diet was 0.038 ± 0.015 (n = 9), suggesting a significantly reduced locomotor impairment compared with the NF diet group (p < 0.05).
Effect of Nucleoprotein Diet on Dopaminergic Neuronal Cell Loss
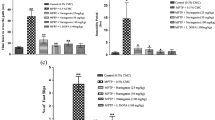
DAergic neuronal cell loss in the SNc due to MPTP treatment was examined histochemically in the brains of animals fed the NP and NF experimental diets. Red-brown TH-positive immunoreactivity was clearly observed in DA neuronal somata and fibers, but not in nuclei (Fig. 3a). Compared with saline-treated control mice, the number of TH-positive cells was clearly decreased after MPTP treatment (Fig. 3b). The mean number of TH-positive cells in six sections from the brains of control animals fed a normal chow diet was 1,262.0 ± 361.3 (n = 8), whereas for MPTP-treated animals maintained under the same conditions, it was 698.4 ± 144.5 (n = 10, p < 0.05 vs control, Fig. 3c). We examined the TH-positive cell number in the SNc of both NF- and 1.2 % NP-diet-fed animals (Fig. 3d–f), with mean values of 452 ± 122 (n = 9) and 704 ± 214 (n = 8, p < 0.05), respectively, obtained for animals in each group (Fig. 3f). The number of TH-positive cells was thus significantly improved in animals fed 1.2 % NP chow.
Effect of NP on DAergic neuronal cell loss in the SNc on day 8 after MPTP treatment. DAergic neurons were detected by TH immunostaining. a–c Representative images of DAergic neurons in the SNc of saline- (non-MPTP-treated control) (a) and MPTP-treated mice (b), and the cell counting comparison (c). The MPTP-treated animals (n = 10) showed a decreased staining intensity and a reduced number of DAergic neurons compared with control animals (n = 8). d–f Comparison of the DAergic neuronal cell loss in the SNc of the two experimental groups. Representative images of the SNc in NF- (d) and 1.2 % NP- (e) diet-fed groups 8 days after MPTP injection. f The 1.2 % NP-diet-fed animals (n = 8) had a significantly reduced DAergic neuron loss compared with the NF diet group (n = 9). The mean number of DAergic neurons is shown by a line. Scale bar, 100 μm. *p < 0.05 vs control group by Mann-Whitney U test (c) or vs NF group by Student’s t test (f)
Effect of Nucleoprotein Diet on Superoxide Anion (O2 −) Production in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Animals
We previously reported that NP has 1O2 and NO scavenging potential as determined by in vitro electron spin resonance analysis (Ohtaki et al. 2010; Yofu et al. 2010) and that NP decreases the level of oxidative metabolites in the serum of a rheumatoid arthritis animal model (Ohtaki et al. 2010). To determine if NP had similar effects in MPTP-treated mice, we next used the in situ detection of O2 − (Bindokas et al. 1996; Murakami et al. 1998; Ohtaki et al. 2007).
Time dependence of the O2 − in the MPTP-induced PD model is determined in Fig. 4a–d. A weak Et signal in the SNc was observed in animals on a standard chow diet that had not been treated with MPTP; the signal intensity increased immediately after the fourth MPTP injection and merged with TH-positive cells (Fig. 4e–g). The signals could not mostly detect at 3 and 7 days after MPTP injection.
Temporal profile of O2 − detection by the HEt method. Oxidized HEt (Et, red), which is an indicator of O2 −, was evaluated before MPTP injection (a) and 6 h (b), 3 days (c), and 7 days (d) after the first MPTP injection. Scale bar, 50 μm. e–g TH immunoreactivity (green) merged with Et signals (arrowhead). Scale bar, 50 μm. h, i Effect of NP on the generation of O2 − in the SNc 6 h after the first MPTP injection. In situ detection of O2 − was compared for NF- (h) and 1.2 % NP- (i) diet-fed animals. The red Et signals for 1.2 % NP-fed animals were clearly less intense than those for NF-fed animals. Scale bar, 200 μm
Then, O2 − levels in animals fed the 1.2 % NP and NF diets 6 h after the first MPTP injection were examined (Fig. 4h, i). The Et signals were observed in both experimental groups. However, the intensity of the Et signal in 1.2 % NP-fed animals was clearly less than that of NF-fed animals (n = 3).
Variation of Cox8b Messenger RNA Expression in Midbrain After 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Treatment
We have shown that the oxidative stress increased in the mitochondria in the neural damage animals (Ohtaki et al. 2007). Cox8b messenger RNA (mRNA) expression of midbrain in the MPTP-injected mice was analyzed by reverse transcription polymerase chain reaction (RT-PCR). Cox8b expression in NF group was significantly decreased by MPTP injection to compare with non-MPTP-injected control one (Fig. 5c, p < 0.05). However, the expression of MPTP-treated 1.2 % NP animal is not different from that of non-MPTP control one.
The alteration in Cox8b gene expression after MPTP injection. Individual gene expressions of Cox8b (a) and β-actin (b) were determined by RT-PCR in mice midbrains at 6 h after the first injection of saline (top) or MPTP (bottom). c Cox8b expression level normalized to β-actin. Cox8b expression level in NF group was decreased by MPTP injection (light bars). In contrast, Cox8b expression level in 1.2 % NP group showed no difference after MPTP injection (dark bars). Mean ± SD of n = 5 mouse midbrains; *p < 0.05 vs saline-injected mice in NF group, Student’s t test
Decrease in the Number of Iba1-Positive Microglia in 1.2 % Nucleoprotein Diet-Fed Animals
Neuroinflammation is a putative mechanism underlying the development of PD in human patients and in animal models of the disease. We next examined the level of inflammatory reaction as indicated by microglial activation in MPTP-treated animals. Staining of the SNc region with anti-Iba1 antibody 8 days after MPTP injection highlighted a 30 % higher number of Iba1-immunoreactive cells in NF-fed animals (Fig. 6a) compared with 1.2 % NP-fed animals (Fig. 6b), although this result was not significant. However, semiquantification with image analysis software of the Iba1-stained area in the SNc (Fig. 6c) showed that the area for 1.2 % NP-fed mice was significantly less than that of NF-fed mice (n = 5, p < 0.05).
Effect of NP on microglia in the SNc 8 days after MPTP injection. Microglia were detected by Iba1 immunostaining. Representative images of microglia in the SNc of NF (a) and 1.2 % NP (b) groups 8 days after MPTP injection. c The Iba1-positive area in the SNc was calculated with ImageJ software. Scale bar, 100 μm. n = 5; *p < 0.05 vs NF group, Mann-Whitney U test
Discussion
PD is a neurodegenerative disease characterized by a loss of DAergic neurons in the SNc and by impaired motor function (Dauer and Przedborski 2003). Although effective symptomatic therapies have been developed to delay progression of the disease, the overall number of PD patients continues to increase in line with aging populations in developed countries. In the present study, we investigated the manner in which NP obtained from salmon soft roe can hinder the progression of PD-like symptoms in MPTP-treated mice with a view to the possible use of NP as an alternative medicine.
Salmon is one of the most highly consumed fish species in the world. According to the Japanese government, the catch of salmon in 2011 was 147,000 t, for which the amount of soft roe associated with this catch is thought to be around 6,000 t. In spite of this large quantity, soft roe from this fish is used only sparingly in feeds produced for other animals and fish, and most of the roe is discarded as industrial waste. Our previous studies on mice demonstrated that oral supplementation of the diet with NP reduced ischemic neural cell death (Matsunaga et al. 2003), clinical symptoms of rheumatoid arthritis (Ohtaki et al. 2010), and hepatitis induced by LPS (Yofu et al. 2010). We also demonstrated that NP in the diet increased cerebral blood flow during ischemia (Matsunaga et al. 2003) and decreased oxidative stress and macrophage activation as putative mechanisms in inflammation (Ohtaki et al. 2010; Yofu et al. 2010). It has been also reported that the long-term diet of nucleoside-nucleotide mixture decreased an aging lipopigment, lipofuscin (Chen et al. 2000).
In line with those previous results, we examined the preventive effect of NP on MPTP-induced PD model of mice and determined that the NP-based diet prevented locomotor impairment and DAergic neuronal degeneration in the SNc. These results suggest that NP in the diet may suppress the PD symptoms.
We next determined the mitochondrial damage with in situ detection of the O2 − and the potential of the mitochondrial turnover with Cox8b mRNA expression. The animals with NP diet decreased oxidative stress in SNc neurons and kept Cox8b gene expression. These results suggest that NP might maintain the DAergic neurons by suppression of the oxidative stress. The NP diet also decreased microglial activation, which was examined by Iba1 immunostaining. The suppression of ROS and glial activation by NP might result in reduction following neuroinflammation.
We used MPTP to generate PD-like symptoms in mice. It is considered that MPTP is converted into MPP+ and the MPP+ produces ROS directly in the mitochondria of DAergic neurons. NP might influence MPTP metabolism in the present study because the NPs were given to the mice prior to MPTP injection and decreased MPTP toxicity directly. Moreover, protamine, which is a main component of NP, might decrease the PD symptom by improvement of cerebral blood flow (Matsunaga et al. 2003). Because we performed a short-period study, we did not observe insoluble substances such as α-synuclein. NP might be able to decrease those kinds of insoluble substances. Further studies are needed to clarify and to determine the real effect and mechanism of NP on PD and the other diseases.
In conclusion, supplementation of NP prevented PD-like symptoms such as DAergic neuronal loss and locomotor impairment in MPTP-treated mice. Moreover, NP diet suppressed the mitochondrial impairment and the inflammatory reactions like microglial activities and ROS production. These findings suggest that NP might be an alternative medicine on PD.
Abbreviations
- DAergic:
-
Dopaminergic
- HEt:
-
Hydroethidine
- MPP+ :
-
1-Methyl-4-phenylpyridinium
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NF:
-
Nucleoprotein-free
- NP:
-
Nucleoprotein
- O2 − :
-
Superoxide anion
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive oxygen species
- SNc:
-
Substantia nigra pars compacta
- TH:
-
Tyrosine hydroxylase
References
Bindokas VP, Jordán J, Lee CC, Miller RJ (1996) Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci 16(4):1324–1336
Chen TH, Wang MF, Liang YF, Komatsu T, Chan YC, Chung SY, Yamamoto S (2000) A nucleoside-nucleotide mixture may reduce memory deterioration in old senescence-accelerated mice. J Nutr 130:3085–3089
Chung YC, Kim SR, Park JY, Chung ES, Park KW, Won SY, Bok E, Jin M, Park ES, Yoon SH, Ko HW, Kim YS, Jin BK (2011) Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology 60(6):963–974
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39(6):889–909
Goldberg NR, Hampton T, McCue S, Kale A, Meshul CK (2011) Profiling changes in gait dynamics resulting from progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nigrostriatal lesioning. J Neurosci Res 89(10):1698–1706
Hernandez TD, Schallert T (1988) Seizures and recovery from experimental brain damage. Exp Neurol 102(3):318–324
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8(4):382–397
Hori M, Nakamachi T, Rakwal R, Shibato J, Nakamura K, Wada Y, Tsuchikawa D, Yoshikawa A, Tamaki K, Shioda S (2012) Unraveling the ischemic brain transcriptome in a permanent middle cerebral artery occlusion mouse model by DNA microarray analysis. Dis Model Mech 5(2):270–283
Khan MM, Kempuraj D, Thangavel R, Zaheer A (2013) Protection of MPTP-induced neuroinflammation and neurodegeneration by Pycnogenol. Neurochem Int 62(4):379–388
Langston JW (1996) The etiology of Parkinson’s disease with emphasis on the MPTP story. Neurology 47(6 Suppl 3):S153–S160
Matsunaga M, Ohtaki H, Takaki A, Iwai Y, Yin L, Mizuguchi H, Miyake T, Usumi K, Shioda S (2003) Nucleoprotamine diet derived from salmon soft roe protects mouse hippocampal neurons from delayed cell death after transient forebrain ischemia. Neurosci Res 47(3):269–276
Meredith GE, Totterdell S, Petroske E, Santa CK, Callison RC, Lau YS (2002) Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson’s disease. Brain Res 956(1):156–165
Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE (2012) Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med 2(1):a009381
Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH (1998) Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci 18(1):205–213
Ohtaki H, Takeda T, Dohi K, Yofu S, Nakamachi T, Satoh K, Hiraizumi Y, Miyaoka H, Matsunaga M, Shioda S (2007) Increased mitochondrial DNA oxidative damage after transient middle cerebral artery occlusion in mice. Neurosci Res 58(4):349–355
Ohtaki H, Yofu S, Nakamachi T, Satoh K, Shimizu A, Mori H, Sato A, Iwakura Y, Matsunaga M, Shioda S (2010) Nucleoprotein diet ameliorates arthritis symptoms in mice transgenic for human T-cell leukemia virus type I (HTLV-1). J Clin Biochem Nutr 46(2):93–104
Seehafer SS, Pearce DA (2006) You say lipofuscin, we say ceroid: defining autofluorescent storage material. Neurobiol Aging 27(4):576–588
Singer TP, Castagnoli N, Ramsay RR, Trevor AJ (1987) Biochemical events in the development of parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem 49(1):1–8
Speciale SG (2002) MPTP: insights into Parkinsonian neurodegeneration. Neurotoxicol Teratol 24(5):607–620
Sudo N, Aiba Y, Takaki A, Tanaka K, Yu XN, Oyama N, Koga Y, Kubo C (2000) Dietary nucleic acids promote a shift in Th1/Th2 balance toward Th1-dominant immunity. Clin Exp Allergy 30(7):979–987
Tanaka S, Ishii A, Ohtaki H, Shioda S, Yoshida T, Numazawa S (2013) Activation of microglia induces symptoms of Parkinson’s disease in wild-type, but not in IL-1 knockout mice. J Neuroinflammation 10:143
Taylor JM, Main BS, Crack PL (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62(5):803–819
Tipton KF, Singer TP (1993) Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem 61(4):1191–1206
Turski L, Bressler K, Rettig KJ, Löschmann PA, Wachtel H (1991) Protection of substantia nigra from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Nature 349(6308):414–418
Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S (2003) NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci U S A 100(10):6145–6150
Yofu S, Ogawa T, Nakamachi T, Satoh K, Shimizu A, Matsunaga M, Shioda S (2010) Nucleoprotein diet prevents LPS-induced liver injury in mice. Jpn J Appl Physiol 40(3):137–143
Acknowledgments
This work was supported in part by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2008–2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiriyama, K., Ohtaki, H., Kobayashi, N. et al. A Nucleoprotein-Enriched Diet Suppresses Dopaminergic Neuronal Cell Loss and Motor Deficit in Mice with MPTP-Induced Parkinson’s Disease. J Mol Neurosci 55, 803–811 (2015). https://doi.org/10.1007/s12031-014-0432-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0432-2