Abstract
Posttraumatic stress disorder (PTSD) is an anxiety disorder caused by a life-threatening traumatic experience, which affects a patient’s quality of life and social stability. The objective of this study was to investigate the change of the glucose-regulated protein (GRP) 94 and apoptosis-related caspase-12 expression in medial prefrontal cortex (mPFC) in rats to determine whether endoplasmic reticulum apoptosis pathway plays an important role in single-prolonged stress (SPS), a well-established PTSD animal model, and therefore to provide experimental evidence to reveal PTSD pathogenesis. A total of 120 healthy male Wistar rats were selected for this study, randomly divided into a normal control group and SPS groups of 1, 4, 7, 14, and 28 days. Behavioral studies of the learning and memory capabilities of rats were observed by using Morris water maze. Morphological changes were detected using transmission electron microscopy (TEM). Immunohistochemistry, Western blot, and reverse transcription polymerase chain reaction (RT-PCR) were used to detect the expressions of caspase-12 and GRP94 expressions in mPFC. Our results showed that compared with control groups, after the SPS exposure, the average escape latency was prolonged in place navigation test (P < 0.05), and swimming time in the third quadrant in spatial probe test shortened. The morphological change of mPFC in each SPS group bears typical apoptotic characteristics. The expressions of GRP94 and caspase-12 gradually increased on 1 and 4 days, peaked on 7 days after the SPS exposure, and then decreased. These results suggest that SPS exposure can induce apoptotic neurons and a change of caspase-12 and GRP94 expression in the mPFC, which may be one of the pathogenesis of mPFC abnormal function in PTSD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posttraumatic stress disorder is a severe mental and behavioral disorder that can develop after exposure to any event that results in psychological trauma. These symptoms characterized by mental disorder and anxiety syndrome may appear after a few days or months, which even last for years (Frick et al. 1995; Sherin and Nemeroff 2011). The stress-related mental disorder is caused by paroxysmal enormous natural disaster or violent personal assault or threatens and presents with characteristic symptoms including mental disorder, anxiety disorder, intrusive memories, hyperarousal, and persistence avoidance. The clinical symptoms include reexperiencing of the trauma event (Zhu et al. 2011; Pollice et al. 2012), flashback, nightmares, and avoidance of people or places that could relate to the event (Shalev 2002; Harvey and Bryant 2002; DSM-IV 1994). The pathophysiology of posttraumatic stress disorder (PTSD) has been widely studied in neuroscience (Kessler 2000). However, the mechanisms that cause such atrophy are still elusive. Single-prolonged stress (SPS) (Shin and Liberzon 2010) was shown to induce enhanced inhibition of the hypothalamic–pituitary–adrenal (HPA) axis, which is a putative neuroendocrinological hallmark of PTSD (Yehuda 2005), and SPS-exposed rats also exhibit behavioral abnormalities. Therefore, SPS paradigms have been extensively applied in the investigation of PTSD.
The pathophysiology of PTSD significantly improved our understanding of PTSD (Shin et al. 2006; Eckart et al. 2011). According to the existing studies, the hippocampus and amygdaloid nucleus are all key brain regions for morbidity of PTSD. A large number of studies have revealed that the amygdala, hippocampus, and medial prefrontal cortex (mPFC) are closely related to the occurrence of PTSD. The existing studies show that mPFC is an important brain functional area. As the executive center of brain function, it will participate in composition of emotional central loop. Traumatic injury of mPFC may cause abnormities to patients in emotional and social behaviors (Damasio et al. 1994). The mPFC has direct synaptic connections with amygdaloid nucleus, and it is also closely associated with other parts of the brain. Memory consolidation can be realized through the interaction between mPFC and amygdaloid nucleus (Roozendaal et al. 2009). As one of the important emotional central pathways, mPFC is also listed as the key brain region of PTSD together with the hippocampus and amygdaloid nucleus. Studies show that PTSD patients have a smaller mPFC than normal people in its volume (Hull 2002). Animal experiment proves that the expression changes of Bcl-2 and Bax (Li et al. 2013) of mPFC of rats after SPS stimulation exist and there also exist expression changes of caspase-3 and caspase-9 (Bing Xiao et al. 2011).
Apoptosis is also called programmed cell death. In recent years, endoplasmic reticulum stress (ERS) pathway is discovered as another important pathway of apoptosis (Nakagawa et al. 2000a). When the cell undergoes various kinds of stress distributed on endoplasmic reticulum (ER) and there are specific markers of ERS stimulus, ERS will happen. Previous work from our laboratory shows that rats exposed to SPS showed an increase in intracellular Ca2+ concentration and in signs of ERS (Wen et al. 2012). Accordingly, ERS that induced the changes of intracellular Ca2+ will activate the ER apoptotic protein caspase-12. Caspase family might play a role in adjusting and controlling apoptosis and inflammatory response. Caspase-12 is the specific medium of ERS. The activated caspase-12 will enter cytoplasm from ER and gradually activate caspase-9 and caspase-3. Glucose-regulated proteins (GRP) lingering on ER, GRP78, and GRP94 are stress proteins distributed on ER, and they are specific markers of ERS (Whitesell et al. 2003a; Chang et al. 2011).
In this study, SPS rats are extensively applied in investigation of PTSD. We aim to explore the potential mechanism of GRP94 regulating stress-induced ERS and ER-dependent apoptosis in mPFC on the SPS rats.
Materials and Methods
Animals and SPS Model Establishment
Healthy 120 male Wistar rats (Experimental Animal Center of China Medical University) aged 7 or 8 weeks were used, weighing approximately 180 g. Rats were individually housed at air-conditioned room (22 ± 1 °C and 55 ± 5 % humidity) on a 12-h light/dark schedule with free access to food and water, for at least 1 week before experiment. Animals were divided randomly into the normal control group and SPS groups (1, 4, 7, 14, and 28 days). The control group remained in their home cages with no handling; at the same time, SPS groups were exposed to an SPS event on the first day. The rats were restrained for 2 h with plastic bags (immobilization), followed immediately by forced swimming for 20 min (24 ± 1 °C), rest for 15 min, followed by drying, and ether anesthesia (until consciousness was lost). The rats were left undisturbed in their home cages until used for brain tissue sampling or behavioral study (Takahashi et al. 2006; Kohda et al. 2007; Wang et al. 2010).
Tissue Preparation
The animals were killed at the end of the experiment. The mPFC was excised immediately and fixed in 2.5 % glutaraldehyde for transmission electron microscopy (TEM); the brains were snap frozen in liquid nitrogen for RNA and protein isolation. The animals were perfused through the heart with 4 % (w/v) formaldehyde in phosphate buffer (PB, pH 7.4), fixed in the same fixative for light microscopy. The normal control group animals were processed in the same way. Then, the brains were embedded in paraffin. Sections were cut at 4-μm slices in thickness and mounted on glass slides.
Behavior in Rats Detected
As a previously described method, rats in Morris water maze, navigation test experiment, and spatial probe test are kept in water temperature (25 ± 1 °C). Surrounding environment is quiet and with constant light source. The place navigation test is carried out for 6 days: records the time from rats put into the water to climb up the platform in 60 s. A 6-day testing study was performed as previously described in detail; spatial reference and working memory deficits were assessed in the water maze fixation and section making.
Transmission Electron Microscope (TEM)
Glutaraldehyde-fixed mPFC samples were dehydrated with gradient ethanol and acetone and were embedded with EPON812 epoxy resin. The 70-nm-thick ultrathin sections were stained with uranyl acetate and lead citrate and were observed with JEOL1200EX at 100 kV.
Immunohistochemical Analysis to Detect Caspase-12 and GRP94
Immunohistochemical staining was performed using PV two-step immunohistochemical detection kit. Caspase-12- and GRP94-positive cells were detected using rabbit anti-rat caspase-12 polyclonal antibody (1:50, Beijing Biosynthesis Biotechnology Co., Ltd.) and mouse anti-GRP94 monoclonal antibody (1:200, Beyotime Institute of Biotechnology), respectively. Dewaxed sections were incubated with 3 % hydrogen peroxide for 10 min and later repaired in 10-mM citrate buffer (pH 6.0) by a microwave oven for 10 min. Sections were treated with 0.3 % Triton X-100 in PBS and 5 % bovine serum albumin (BSA) to block nonspecific staining, blocked with dripped 10 % goat serum for 30 min, and incubated with primary antibody at 4 °C overnight. Finally, 3,30′-diaminobenzidine was used as chromogen for about 10 min until the brown color appeared. Slices were then dehydrated and mounted with neutral balsam. The Image-Pro Plus image analysis system was used to analyze the average optical density (OD).
Western Blotting to Detect GRP94 and Caspase-12
Rats were decapitated; immediately, the brains were removed and placed on an ice-cold dish, and the mPFC according to the atlas of rats were dissected from the brain tissues. The protein concentration was determined using Coomassie brilliant blue method. Each sample was separated by 10 % (w/v) gradient sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to a PVDF membrane. The membrane was blocked with 5 % dried skim milk for 2 h and incubated with rabbit polyclonal antibody anti-caspase-12 (Abcam, 1:500), mouse monoclonal antibody anti-GRP94 (Abcam, 1:500), and mouse monoclonal antibody anti-GAPDH (Zhongshan Goldenbridge, China, 1:500) at 4 °C overnight. The membranes were then incubated with the secondary antibody anti-rabbit IgG-HRP and anti-mouse IgG-HRP (Zhongshan Golden bridge, 1:2,000) for 2 h at room temperature. The membranes were detected with enhanced chemiluminescence (ECL, P0018; Beyotime, China). Absorbance was measured and analyzed by the Gel Image Analysis System (Tanon 2500R, Shanghai, China). The levels were determined by calculating the OD ratio.
Semiquantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR) to Detect GRP94 mRNA
Total RNA was isolated from mPFC by Trizol (Invitrogen, USA), and 1 μg of total RNA was reverse transcribed into complementary DNA (cDNA) (AM Ver. 3.0, Takara Bio, Otsu, Japan). The specific primers were synthetized from Sangon Biotech Company (Shanghai, China). The primer sequences used for PCR amplification are shown in Table 1. The PCR products were separated on 2 % agarose gel by electrophoresis, and the density of each band was analyzed with the Gel Image Analysis System (Tanon 2500R, Shanghai, China). The levels of GRP94 messenger RNA (mRNA) were normalized by β-actin mRNA using the ratio of GRP94 mRNA/β-actin mRNA.
Statistical Analysis
The results were expressed as means ± SD. The differences between normal control group and SPS groups were analyzed by one-way analysis of variance (ANOVA) using SPSS 16.0 software. A level of P < 0.05 was considered to be statistically significant.
Results
Changes in Physiological Behavior and Morris Water Maze Test
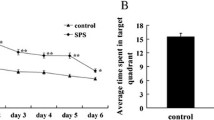
Rats in control group showed lively behavior and normal appetite dense. In contrast, SPS-stimulated rats presented multiple signs of PTSD, such as being less active, sleepiness, and loss of restlessness. Furthermore, the results of Morris water maze (MWM) test are illustrated in Fig. 1. SPS groups had significantly higher latency spent to find the underwater platform when compared with normal control group rats (P < 0.05) (Fig. 1a). When the platform was removed from the pool at day 7 for spatial memory testing, SPS rats had much less time spent in target quadrant compared with control group rats (P < 0.01) (Fig. 1b).
Morphological Change of Hippocampal Neurons by TEM
To determine SPS-caused apoptosis, as shown in Fig. 2, some neurons of mPFC in SPS group of rats showed obvious neuronal ultrastructure changes under the TEM. Neurons displayed characteristic morphological changes of early apoptosis, including cell shrinkage, nuclear pyknosis, chromatin condensation, and chromatin gathered along the inside of the nuclear. Later, stage apoptotic cells were found with liquefied vacuole, increased vacuole, and notching of the nuclear membrane (Fig. 2b). These included shrunk chromatin in the form of a set of edges in the nuclear membrane under the crescent-shaped bodies (Fig. 2c). These changes were not present in normal control rats (Fig. 2a).
Results of the TEM showed morphological changes in the medial prefrontal cortex neuron. a Normal neuron in control group. b Chromatin condensation and chromatin gathered along the inside of the nuclear membrane in SPS groups. c Crescent-shaped bodies in the nuclear membrane in SPS groups. Scale bar indicates 500 nm
Immunohistochemical Analysis Results of GRP94
GRP94-positive products were brown granular that were mainly distributed in the cytoplasm of mPFC neurons. The immunohistochemical-staining results are shown in Fig. 3a. Positive immunohistochemical cells showed a weak positive reaction in normal control group mPFC neurons with a relatively light staining. After SPS stimulation, the expression of GRP94 increased significantly in comparison with that of the normal control group (P < 0.05). The peak of the increase was in 7 days after SPS. Then, the immunoreactivity was slowly decreased in 14 and 28 days after SPS but is still higher than normal group (P < 0.05). Mean ODs of GRP94 are shown in Fig. 3b.
a Presentation of GRP94 expression in medial prefrontal cortex in each group (×400 magnification). Representative immunohistochemistry of the following groups: A indicates normal control group, B indicates SPS-1-day group, C SPS-4-day group, D SPS-7-day group, E SPS-14-day group, F SPS-28-day group. b Mean optical densities of GRP94 are shown. Data represent the means ± SD (n = 5, each). Asterisk, composed with control group, denotes P < 0.05. White triangle, composed with SPS 7 days, denotes P < 0.05
Immunohistochemical Analysis Results of Caspase-12
After SPS stimulation, caspase-12 was widely distributed throughout the mPFC, mainly in the cytoplasm, and appeared as a buffy particle, but in the normal control group, a little expression could be seen. Evaluation of caspase-12 by immunohistochemical analysis indicated a significant increase in the SPS model groups compared with the normal control group (P < 0.05). The expression reached its peak 7 days after the SPS exposure and then decreased 14 days after. The analysis results are shown in Fig. 4.
The immunohistochemistry for caspase-12 in medial prefrontal cortex of PTSD rats. a Slight caspase-12 immunoreactivity cells in normal control group. b Caspase-12 immunoreactivity on SPS-1 day. c Caspase-12 immunoreactivity on SPS-4 days. d Marked caspase-12 immunoreactivity was detected on SPS-7 days. e Caspase-12 tended to decrease on SPS-14 days. f Caspase-12 immunoreactivity on SPS-28 days (×400 magnification). g Quantitative analysis of the average optical density. Data are represented as mean ± SD. Asterisk, composed with control, denotes P < 0.05. White triangle, composed with SPS 7 days, denotes P < 0.05
Western Blotting Analysis Protein Expression Levels
Western blot was used to detect the protein expressions of GRP94 and caspase-12 in mPFC of PTSD rats. Molecular weights of GRP94, caspase-12, and GADPH were 94, 42, and 36 kD, respectively, showing clear bands. The protein expression in the SPS groups changed over time, which showed a marked increase compared with that of the control group (P < 0.05). The peak increase occurred in the SPS 7-day group (Fig. 5).
a Western blot analysis for GRP94 and caspase-12 in medial prefrontal cortex. b The quantification of Western blot analysis for GRP94/GADPH and caspase-12/GADPH is shown. Data are represented as the mean ± SD (n = 5, each). Asterisk, composed with control group, denotes P < 0.05. White triangle, composed with SPS 7 days, denotes P < 0.05
RT-PCR Results of GRP94 mRNA
There were certain expression levels in GRP94 mRNA in normal rat mPFC neurons (Fig. 6). The levels of GRP94 mRNA were normalized with β-actin mRNA. The expressions of GRP94 mRNA increased significantly after SPS stimulation and began to decline on day 14, which are consistent with the results of immunohistochemistry and Western blot.
Discussion
PTSD is a clinical syndrome with repeated recurrence of traumatic experience, high vigilance, and persistent avoidance. The patients often have experienced various catastrophic incidents such as tsunami, earthquake, water flood, and war. Memory disorder is one symptom of PTSD as well as its clinical diagnostic criterion (Hoge et al. 2004). Standard animal model is one of the foundations to carry out research on PTSD. SPS stimulation model established by the experiment is the method determined at the International PTSD Scientific Meeting held by the Japanese Ministry of Education in 2005. It is the acknowledged method of PTSD model at current stage. Experimental research verifies that rat after SPS stimulation has dysfunction in HPA axle, the animal model has a change in ethology, activity habit, and memory ability, and its environmental adaptability reduces, in conformity with clinical manifestation of human PTSD. In this experiment, after SPS stimulation, the average escape latency period of rat in Morris water maze (Morris 1981, 2008) is higher when compared with the normal control (P < 0.05). Learning and memory ability of rat in model group decreases with simulating the memory disorder symptom of PTSD rats. This hints that the model is successfully copied, which has provided guarantee for subsequent studies.
The existing studies show that mPFC is an important brain functional area. The result of MWM in this experiment shows that mPFC neuronal cell of rat in normal control has a normal shape and complete nuclear membrane, but after SPS, nuclear membrane of neuron partly disappears, pyknosis and chromatin were condensed into blocks, and cytoplasm overflows; all these indicate neuronal apoptosis. Occurrence of neuronal apoptosis in mPFC will change its structural form, and this is one of the reasons why mPFC function of rat after SPS is damaged.
Apoptosis is also called programmed cell death. As is known at present, the apoptosis process is accompanied by expression of a series of apoptosis-related genes. There are early and multiple research on death receptor pathway and mitochondrion pathway, and they play a very important role in the process of apoptosis. In recent years, ERS pathway is discovered as another important pathway of apoptosis. ER of eukaryotic cell is the major place to process protein and store calcium in the cell. It is responsible for folding, assembly, and secretion of secreted protein, and it is quite sensitive to changes of internal and external environments. When the cell undergoes various kinds of stimulus from internal and external sources, physiological function of ER will be disordered, and ERS will happen. The inducements cover calcium loss or calcium overload in ER, cell hypoxia, nutrition deficiency, pH change (Bernales et al. 2006), etc. Present research considers that dysfunction of ER may be the important cause for diseases including degenerative disease of nervous system, hepatopathy, cardiovascular disease, and inflammatory immune response (Lin et al. 2000).
Caspase family might play a role in adjusting and controlling apoptosis and inflammatory response. Caspase-12 is the specific medium of ERS (Nakagawa et al. 2000b). Increase of caspase-12 expression only happens during ERS (Bernales et al. 2006). Shibata et al. (2003) discovered in the temporary infarction model of the rat’s middle cerebral artery that TUNEL-positive cell and caspase-12-positive cell coexisted in nerve cells. All these materials proved that activation of caspase-12 is the key signal of triggering ER apoptosis. The activated caspase-12 will enter cytoplasm and act on caspase-9 and Caspase-3. Activation of caspase-12 is related to increase of free Ca2+ concentration in cytoplasm (Nieves and Moreno 2007; Sreedhar et al. 2004). Strong stress may cause high expression of glucocorticoid receptors (GR) in mPFC, and high expression of GR will promote internal flow of Ca2+ (Furay et al. 2008). Yu Wen et al. (2012) proved the abnormal expression of Ca2+-CaM-CaMKIIα pathway in mPFC. As an inducement of ERS, the change of Ca2+ concentration might be one of the reasons for the rise of early expression of caspase-12 1 day after SPS. In this experiment, results of immunological histological chemistry (IHC), Western blot, and RT-PCR of rat after SPS all show that caspase-12 starts to rise after 1 day, reaches the peak value after 7 days, and then decreases gradually. This indicates that SPS triggers ERS of mPFC neuron and apoptosis of mPFC.
After SPS stimulation, ERS, dysfunction of ER and the subsequent unfolded protein response (UPR) will affect protein assembly and secretion. With deepening of the study, more and more UPR-related proteins have been discovered. GRP lingering on ER, GRP78, and GRP94, distributed proteins on ER, are specific markers of ERS (Whitesell et al. 2003b). Under normal condition that is low expression of GRP94, its distribution and expression are both controlled by cell cycle. In normal control of this experiment, a few positive cells of GRP94 can be seen in immunohistochemical results, and the results of Western blotting and RT-PCR experiments also indicate weak expression of protein in normal control. After 1 to 28 days of SPS, both immunohistochemical and Western results show that expression of GRP94 is higher than that in normal control; it continues to rise gradually and reaches the peak value after 7 days. This shows that after ERS, the unfolded protein and misfolded protein will activate UPR pathway, induce expression of UPR target gene, increase transcription of chaperonin GRP94 (Patil and Walter 2001), and trigger endogenous protective mechanism of ER. Therefore, at the early stage of stress, GRP94 will increase. This is a reaction of the cell to try to get through the dangerous period.
At the early stage of ERS, UPR will induce redistribution of chaperonin. A large number of chaperonins and caspase-12 will form compounds to stop apoptosis. However, apoptosis will be the cause for ERS to consume a large amount of GRP94.
The variation trends of GRP94 and caspase-12 expressions are almost the same in this experiment. They all rise gradually after SPS, reach the peak on 7 days, and then decrease gradually. This phenomenon might be caused by block of protein synthesis after ERS. Synchronous peaks of these two show that a balance is reached between apoptosis and cellular tolerance controlled 7 days after SPS.
In conclusion, ER apoptosis might be one of the pathologic reasons for PTSD injury. UPR triggered by ER apoptosis pathway of mPFC in SPS-PTSD rat is the adaptation to prevent the organism from undergoing greater damages, but this might also be one of the pathologic reasons for volume decrease and cortical atrophy of mPFC in PTSD patients.
References
American psychiatric association: diagnostic and statistical manual of mental disorders (1994) DSM-IV, 4th edn. American Psychiatric Press, Washington
Bernales S, Papa FR, Walter P (2006) Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol 22:487–508
Chang YJ, Tai CJ, Kuo LJ et al (2011) Glucose–regulated protein 78 (GRP78) mediated the efficacy to curcumin treatment on hepatocellular carcinoma. Ann Surg Oncol 18:2395–2403
Damasio H, Grabowski T, Frank R et al (1994) The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 264:1102–1105
Eckart C, Stoppel C, Kaufmann J et al (2011) Structural alterations in lateral prefrontal, parietal and posterior midline regions of men with chronic posttraumatic stress disorder. J Psychiatry Neurosci 36:176–186
Frick KM, Baxter MG, Markowaska AL et al (1995) Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging 16:149–160
Furay AR, Bruestle AE, Herman JP (2008) The tole of the forebrain glucocorticoid recepror in acute and chronic srees. Endocrinology 149(11):5482–5490
Harvey AG, Bryant RA (2002) Acute stress disorder: a synthesis and critique. Psychol Bull 128(6):886–902
Hoge CW, Castro CA, Messer SC et al (2004) Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 351:13–22
Hull AM (2002) Neuroimaging findings in post-traumatic stress disorder. Systematic review. Br J Psychiatry 181:102–110
Kessler RC (2000) Post-traumatic stress disorder the burden to the individual and to society. J Clin psychiatrt 61(suppl5) 4–12
Kohda K, Harada K, Kato K et al (2007) Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single–prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience 148:22–33
Lin XY, Choi MS, Porter AG (2000) Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolys accharide and interferon–gamma. J Biol Chem 275:39920–39926
Li Y, Han F, Shi Y (2013) Increased neuronal apoptosis in medial prefrontal cortex is accompanied with changes of Bcl-2 and Bax in a rat model of post-traumatic stress disorder. J Mol Neurosci 32:625–631
Morris R (1981) Spatial localisation does not depend on the presence of local cues. Learn Motiv 12:239–260
Morris RGM (2008) Morris water maze. Scholarpedia 3:6315
Nakagawa T, Zhu H, Morishima N et al (2000) Caspase-12 mediates endoplasmic–reticulum specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Nieves D, Moreno JJ (2007) Epoxyeicosatrienoic acids induce growth inhibition and calpain/caspase-12 dependent apoptosis in PDGF cultured 3T6 fibroblast. Apoptosis 12(11):1979–1988
Patil C, Walter P (2001) Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast, and mammals. Curr Opin Cell Biol 13:349–355
Pollice R, Bianchini V, Roncone R et al (2012) Psychological distress and post-traumatic stress disorder (PTSD) in young survivors of L'Aquila earthquake. Rev Psichiatr 47:59–64
Roozendaal B, McReynolds JR, Van der Zee EA et al (2009) Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci 29:14299–308
Shalev AY (2002) Acute stress reactions in adults. Biol Psychiatry 51(7):532–543
Sherin JE, Nemeroff CB (2011) Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci 13:263–278
Shin LM, Liberzon I (2010) The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35:169–191
Shin LM, Rauch SL, Pitman RK et al (2006) Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071:67–79
Sreedhar AS, Kalmar E, Csermely P et al (2004) Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett 562:11–15
Shibata M, Hattori H, Sasaki T (2003) Activation of caspase-12 by endoplasmic reticulum stress induced by transient middle cerebral artery occlusion in mice. Neuroscience 118:491–499
Takahashi T, Morinobu S, Iwamoto Y et al (2006) Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacol (Ber) 189:165–173
Wang HT, Han F, Gao JL, Shi YX (2010) Increased phosphorylation of extracellular signal–regulated kinase in the medial prefrontal cortex of the single-prolonged stress rats. Cell Mol Neurobiol 30:437–444
Wen Y, LI B, Han F et al (2012) Dysfunction of calcium/calmodulin/CaM kinase IIα cascades in the medial prefrontal cortex in post-traumatic stress disorder. Mol Med Rep 6:1140–1144
Whitesell L, Bagatell R, Falsey R (2003) The stress response: implications for the clinical development of hsp90 inhibitors. Cancer Drug Targets 3:349–358
Xiao B, Yu B, Wang H–T et al (2011) Single-prolonged stress induces apoptosis by activating cytochrome C/caspase-9 pathway in a rat model of posttraumatic stress disorder. Cell Mol Neurobiol 31:37–43
Yehuda R (2005) Neuroendocrine aspects of PTSD. Handb Exp Pharmacol 169:371–403
Zhu CZ, Situ MJ, Zhang Y et al (2011) Influence factors of posttraumatic stress disorder (PTSD) and depression symptoms in children and adolescents after Wenchuan earthquake in China. Zhonghua Yu Fang Yi Xue Za Zhi 45:531–536
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (No. 81171282; No. 31200772) and research fund from the doctoral program of High Education of China (No. 20132104110021). The authors would like to thank the reviewers for their valuable comments on how to improve the quality of the paper.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, D., Han, F. & Shi, Y. Effect of Glucose-Regulated Protein 94 and Endoplasmic Reticulum Modulator Caspase-12 in Medial Prefrontal Cortex in a Rat Model of Posttraumatic Stress Disorder. J Mol Neurosci 54, 147–155 (2014). https://doi.org/10.1007/s12031-014-0263-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0263-1