Abstract
Current knowledge concerning the molecular mechanisms of the cellular response to excitotoxic insults in neurodegenerative diseases is insufficient. Although glutamate (Glu) has been widely studied as the main excitatory neurotransmitter and principal excitotoxic agent, the neuroprotective response enacted by neurons is not yet completely understood. Some of the molecular participants have been revealed, but the signaling pathways involved in this protective response are just beginning to be identified. Here, we demonstrate in vivo that, in response to the cell damage and death induced by Glu excitotoxicity, neurons orchestrate a survival response through the extracellular signal-regulated kinase (ERK) signaling pathway by increasing ERK expression in the rat hippocampal (CA1) region, allowing increased neuronal survival. In addition, this protective response is specifically reversed by U0126, an ERK inhibitor, which promotes cell death only when it is administered together with Glu. Our findings demonstrate that the ERK signaling pathway has a neuroprotective role in the response to Glu-induced excitotoxicity in hippocampal neurons. Therefore, the ERK signaling pathway may be activated as a cellular response to excitotoxic injury to prevent damage and neural loss, representing a novel therapeutic target in the treatment of neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, multiple sclerosis, and stroke continue to represent major challenges for devising therapies that reverse or prevent neuronal loss. Our knowledge regarding the fundamental regulation of cellular processes that lead to cellular demise is incomplete. In addition, there is insufficient information regarding the mechanisms utilized by cells to protect themselves after the excitotoxic injuries that accompany degenerative processes.
Glutamate (Glu) is the main excitatory neurotransmitter in the mammalian brain. It functions through two main types of Glu receptors (GluR), ionotropic and metabotropic (Kew and Kemp 2005, and it has been shown to play important roles in many brain processes, including neurodevelopment (e.g., differentiation, migration, and survival) (Maiese et al. 2005) and learning (e.g., long-term potentiation and depression) (Gladding et al. 2009; Kullmann and Lamsa 2008). In contrast, Glu has also been implicated in acute neurodegeneration (e.g., cerebral ischemia, traumatic brain injury) (Grewer et al. 2008; Paschen 1996), chronic neurodegeneration (e.g., Huntington’s disease, Alzheimer disease) (André et al. 2010; Lee et al. 2004), and stress response and anxiety disorders (Cortese and Phan 2005). Therefore, Glu is also considered the major cause of excitotoxic damage. This excitotoxic effect has been widely studied (reviewed in Schousboe and Waagepetersen 2005; Lau and Tymianski 2010), but the molecular mechanisms underlying endogenous neuroprotection are not completely understood.
Understanding the molecular mechanisms involved in protecting neurons from excitotoxic cell death has been a significant focus of neuroscience research. Recently, we demonstrated that both in vitro and in vivo Glu-induced excitotoxicity increases the astrocytic expression of NCX3/Slc8a3, an Na+/Ca2+ membrane exchanger, which partially explains the activation of a molecular response to cell damage (Ortuño-Sahagún et al. 2010). In addition, we described the hippocampal gene expression profile after neonatal monosodium glutamate (MSG)-induced excitotoxicity, which is consistent with the activation of mechanisms that modulate the N-methyl-d-aspartic acid (NMDA) receptor function and intracellular calcium homeostasis (Ortuño-Sahagún et al. 2010); however, the specific signaling pathways involved in the brain cells remain unknown.
Mitogen-activated protein kinases (MAPKs) are signal transducers that have been implicated in cellular events that result in both cell death (Yoon et al. 2009) and survival (Park et al. 2008). Of the three major mammalian MAPK pathways, the MAPK/extracellular signal-regulated kinase (ERK) pathway is commonly associated with survival (Anderson and Tolkovsky 1999), whereas the p38/MAPK (Asomugha et al. 2010; O’Sullivan et al. 2009) and stress-activated protein kinase/Jun N-terminal kinase (JNK) pathways are often implicated in cell death (Asomugha et al. 2010; Verheij et al. 1998; Lei and Davis 2003).
Interestingly, some evidence indicates the relevance of MAPK for regulating physiological responses to Glu in the central nervous system in vivo. For instance, following the systemic administration of either of the Glu analogs quinolinic acid (Ferrer et al. 2001) or kainic acid (KA) at convulsant doses (Ferrer et al. 2002) in rats, the expression levels of MAPK, JNK, and p38 vary, particularly in the hippocampus, which strongly suggests that co-regulation could exist among these pathways in association with either cell death or cell survival triggered by overactivation of the glutamatergic system. Therefore, these pathways might be involved in the response to excitotoxic injury.
Recently, Karmarkar et al. (2011) demonstrated that MAPK/ERK is essential for endogenous neuroprotection in suprachiasmatic nuclei 2.2 (SCN2.2) cells in vitro. This finding is noteworthy because the SCN is resistant to Glu excitotoxicity (Barnes et al. 2003; Tischkau et al. 2000). The neuroprotective effect of ERK1/2 activation usually results in the subsequent activation of several key regulators. Among these, B cell lymphoma-2 (Bcl-2) is a key regulator of apoptosis and one of the main targets of ERK proteins (Wang et al. 2006) that regulates the transcriptional activities of multiple transcription factors related to apoptosis/survival (Feng et al. 2004).
However, the role of ERK in the regulation of neuronal survival during an excitotoxic process induced in vivo during the early stages of life is not clear. The goal of this work was to delineate the contribution of the MAPK/ERK signaling pathway to neuronal survival under neonatal excitotoxic conditions by using an in vivo model of neonatal excitotoxicity induced by MSG. We measured the expression levels of the phosphorylated ERK1/2 protein under excitotoxic conditions with or without an ERK inhibitor. In addition, we performed a histological analysis in the different areas of the rat hippocampus. Our results indicate that the MAPK/ERK signaling pathway is induced in response to in vivo excitotoxic neuronal damage.
Materials and Methods
Animal Treatment
Neonatal Wistar rats were utilized in all of the studies. The rats were maintained under normal conditions (free access to water and food, 12/12-h light–dark cycles, and 23–25 °C) during and after treatment. Animal care and handling was performed in agreement with the Mexican General Laws of Health and its corresponding chapters (1987) and in accordance with procedures approved by the University of Barcelona animal experimentation ethics committee, which conform to governmental regulations, and with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH publication no. 80-23, revised 1996). The experimental scheme was designed to minimize animal suffering and the number of animals used.
All litters were adjusted to eight pups per mother on the day of birth. The experimental animals received subcutaneous (s.c.) administration of MSG (4 mg/g body weight [BW]) according to a procedure described previously (Ortuño-Sahagún et al. 1997; Beas-Zárate et al. 1994). A total of 30 animals were treated with MSG on postnatal days (PND) 1, 3, 5, and 7, and 18 animals did not receive any treatment (intact control groups). The animals were killed by decapitation at 3, 6, 12, 24, 72, or 120 h after the last MSG dose, and the hippocampus was dissected out at 4 °C for further molecular analysis. Brains from different groups of animals were used for the histological analysis.
In a parallel series of experiments, 15 animals divided into three groups were used to determine the optimal concentration of U0126, an efficient inhibitor of MAPK/ERK signaling (Satoh et al. 2000; Duncia et al. 1998). We tested different doses (0.15, 0.30, and 0.50 μg/g BW) of U0126 (U-120; Sigma-Aldrich, St. Louis, MO, USA) diluted in 10 % dimethyl sulfoxide (DMSO, D-8418; Sigma-Aldrich, St. Louis, MO, USA) and administered s.c. 30 min prior to each MSG treatment following the previously described experimental scheme. A 0.1-M sodium phosphate-buffered solution (PBS) was used as a vehicle to obtain a maximum volume of 25 μL. The maximum amount of DMSO applied on the last treatment day and with the highest dose of U0126 was approximately 1 μL per animal. In addition, two control groups of three animals each were included as follows: one was treated with 10 % DMSO + vehicle (0.1 M PBS) and the other was treated with the higher experimental dose of U0126 (0.500 μg/g of BW) under the same experimental conditions.
Western Blot Analysis
After treatment, the animals were killed, and their hippocampi were rapidly removed and frozen at −95 °C. The tissues were homogenized in 1 mL cold lysis solution containing 920 μL of lysis buffer (1 M Tris base pH 7.5, 5 M NaCl, 0.2 M NaF, and 10 mM Na3VO4) and 80 μL of a protease inhibitor cocktail (complete tablets, 1842196; Hoffmann-La Roche, Basel, Switzerland). Then, the homogenates were incubated on ice for 30 min and centrifuged at 13,000×g for 30 min at 4 °C. The supernatants were collected, and the protein content was determined using the bicinchoninic acid method (Pierce Company, Rockford, MI, USA). Samples containing 50 μg of protein were placed in sample buffer, denatured by boiling, and separated by electrophoresis on 10 % acrylamide gels.
Next, the proteins were transferred onto polyvinylidene fluoride sheets (0.45 μm; Millipore, Bedford, MA, USA). The membranes were blocked for 24 h with 100 g/L nonfat milk in Tris-buffered saline with 0.1 % Tween (TBST) and then incubated with anti-phospho-ERK1 and ERK2 antibody (cat. E 7028 anti-MAPK, 1:1,000; Sigma, St. Louis, MO, USA) for 24 h at 4 °C. Subsequently, the blots were thoroughly washed in TBST buffer and incubated for 24 h with horseradish peroxidase-conjugated anti-mouse IgG (1:1,000; Vector Laboratories, Burlingame, CA, USA). The immunoreactive proteins were revealed with an ABC Standard Kit (Vectastain; Vector Laboratories, Burlingame, CA, USA) and detected by diaminobenzidine (DAB) (Sigma-Aldrich, St. Louis, MO, USA). Using the same blot, we also detected actin (1:10,000; Sigma-Aldrich, St. Louis, MO, USA) using a method that has been described previously. Protein expression levels were measured using Quantity One (Bio-Rad Laboratories, Hercules, CA, USA). The optical density (OD) of ERK1/2 bands was normalized to the actin band.
Histological Procedures
For histological analysis, the control and experimental animals were intracardially perfused with 4 % paraformaldehyde in 0.1 M phosphate buffer after MSG treatment. The brains were extracted from the animals on PND 12 and fixed in fixing solution for 1 week. Next, the whole brains were processed and embedded in paraffin, and 8-μm-thick coronal sections were obtained every 50 μm using an American Optical 820 Microtome. Finally, the sections were stained with hematoxylin and eosin (H&E) and mounted for observation.
For all morphological studies, the data correspond to three animals for each group studied. For the immunohistochemistry experiments, five animals were studied from each group and four to six sections from each animal were examined. The images were captured using a Media Cybernetics Photometric CoolSNAP-Pro CF color digital camera mounted on a binocular Olympus IX50 inverted microscope. The immunolabeling intensity was measured using Image-Pro Plus ver.4.5 analysis software. The OD of the immunopositive cells/counting area was plotted for each hippocampal region (CA1, CA2, and CA3) of both cerebral hemispheres and expressed as the mean ± the standard error of the mean (SEM). For the neurodegenerative index (H&E method), four to six sections were examined for each animal, and cell counting was performed for each hippocampal region of both cerebral hemispheres using a Leica DME light microscope and a 1-mm2 square reticule. The mean percentage of dead cells was plotted for each hippocampal region and expressed as the mean ± SEM. For the neurodegenerative index (H&E method), cell counting was performed to determine the number of neurons with normal characteristics in the dorsal hippocampus at that level. The cells in the CA1, CA2, and CA3 regions were included in the counting. In each region, the amount of neurons with normal characteristic against those with neuronal damage was determined using a Leica DME light microscope and a 1-mm2 square reticule. The data were obtained by analyzing eight fields per section in four sections from each of the five animals from each group studied. The results were expressed as the number of cells with morphology indicating neuronal damage.
Immunohistochemistry
For the immunohistochemistry studies, the animals were anesthetized by pentobarbital injection (50 mg/kg BW, intraperitoneally) at the previously determined time point of maximum ERK1/2 expression. Perfusion was performed using 4 % paraformaldehyde in 0.1 M phosphate buffer. The brains were removed and postfixed for 1 week at room temperature (RT). Then, the brains were sliced with a vibratome (Leica VT1000 S, Wetzlar, Germany) to obtain 40-μm-thick coronal sections.
Free-floating sections were rinsed in sodium borohydrate for 10 min and with 0.5 % Triton X-100 for 1 h. These sections were then preincubated in blocking solution (normal serum with PBS–sodium azide, 1:100) for 30 min. Later, the sections were incubated for 72 h at 4 °C with an anti-phospho-ERK1 and ERK2 antibody (cat. no. E 7028 anti-MAPK, 1:1,000; Sigma-Aldrich, St. Louis, MO, USA). Subsequently, the sections were sequentially incubated for 24 h with an anti-mouse IgG (1:1,000; Vector Laboratories, Burlingame, CA, USA) and an avidin–biotin complex (ABC Vectastain; Vector Laboratories, Burlingame, CA, USA) for 2 h. Finally, the tissues were visualized with DAB (Vector Laboratories, Burlingame, CA, USA). Each step of the technique was performed at RT and preceded by a minimum of three 15-min washes in PBS. The preparations were dehydrated and mounted for microscopic observation.
Hippocampal Cell Culture
Primary hippocampal neurons were isolated from the frontal/parietal hippocampus of embryonic Wistar rats (E16–E18) as previously described (Petegnief et al. 2001). The tissue sections were pooled in ice-cold PBS/glucose and dissociated with trypsin (trypsin 0.05 %–EDTA 0.53 mM for 15 min at 37 °C). After centrifugation (5 min at 800×g), the supernatant was transferred onto Neurobasal™ A complete medium (Gibco, Carlsbad, CA, USA) containing penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA), 45 % glucose solution (Sigma-Aldrich, St. Louis, MO, USA), sodium bicarbonate 7.5 % (Sigma-Aldrich, St. Louis, MO, USA), glutamine (Sigma-Aldrich, St. Louis, MO, USA), and B27 supplement (Gibco, Carlsbad, CA, USA). The dissociated cells were plated onto poly-l-lysine-coated (25 mg/mL) culture dishes at a density of 0.25 × 106 cells/mL. The cells were maintained in culture for up to 12–14 days at 37 °C in an atmosphere of 5 % CO2/95 % air. The culture medium was replaced at 7 and 10 days in vitro (DIV). To prevent non-neuronal cell proliferation, 6 μM cytosine arabinoside (Sigma, Madrid, Spain) was added at 4 DIV and 3 μM cytosine arabinoside was added at 7 and 10 DIV.
Treatment of Hippocampal Neurons
The hippocampal cells were treated at 12–14 DIV. A 24-h dose–response curve was generated for Glu (0–50 μM). The ERK inhibitor U0126 (Calbiochem, Darmstadt, Germany) was added to the cell culture at a concentration of 25 μM for 30 min prior to Glu treatment. The cell viability was measured 24 h after treatment.
Cell Viability
Cell death was determined by two different methods: lactate dehydrogenase (LDH) release and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA). We evaluated the toxicity of Glu by assaying cell death, which was based on the measurement of the LDH released from the cytosol of damaged cells. Culture medium was collected for each well after a 24-h Glu incubation period and placed on ice until use.
LDH was assayed with a kit (Roche Diagnostics GmbH, Mannheim, Germany). Photometric measurements were performed at 490 nm in a Benchmark plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA). The results were expressed as percentages of LDH released vs. non-treated cells. Cell cytotoxicity was measured utilizing the MTT assay. The MTT stock solution (5 mg MTT/mL distilled water) was added to cell cultures at a final concentration of 0.5 mg/mL for 1 h at 37 °C. MTT was reduced to non-water-soluble violet formazan crystals within the cell. The medium was removed, and the cells were dissolved in DMSO. Formazan formation was determined spectrophotometrically, employing a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). Viability was expressed as the percentage of absorbance measured in non-treated cells.
PCR of Identified Genes and Densitometric Scanning
The oligonucleotide primers used for polymerase chain reaction (PCR) were the following: Bcl-2 sense 5′-TAAAAA GCTGAAAGGAATTTGAATA-3′, antisense 5′-AAGTCGCGACGGTAGCGACGAGAGA-3′ (24 cycles) and β-actin sense 5′-CACCACAGCTGAGAGGGAAATCGTGCGTGA-3′, antisense 5′-ATTTGCGGTGCACGATGGAGGGGCCGGAACT-3′ (18 cycles) as a control gene. All PCRs were performed after a single denaturation step at 94 °C for 5 min, and they involved 24 or 18 denaturation cycles, respectively, at 94 °C for 1 min, annealing at 60 °C for 1 min, and extension at 72 °C for 1 min, followed by a final extension for 5 min at 72 °C. The samples were electrophoresed and photographed with a Kodak Digital Science Scanner, and the analysis was carried out with the same system. The amplified PCR products were calculated automatically and normalized against the area representing the expression of the constitutive gene. The results are expressed as the relative intensity in arbitrary units (AU) compared to the control value. Statistical comparisons were conducted using analysis of variance (ANOVA) for comparison between groups; a p < 0.05 was considered significant.
Statistical Analysis
For the analysis of neuronal viability and LDH release experiments, the data were analyzed using one-way ANOVA followed by Bonferroni post hoc tests; p < 0.05 was considered significant. Statistical comparisons of the morphological findings and Western blot analyses were performed by ANOVA, and the Fisher test was utilized to perform comparisons between groups; p < 0.05 was considered significant. The data represent the values obtained from four to five independent experiments, each of which was performed in duplicate.
Results
MSG Treatment Induces an Early Increase in ERK1/2 Expression in the Hippocampus
To explore the contribution of ERK signaling to the neuroprotective response, its expression level was determined by Western blotting. The results are presented in reference to the control levels. The data indicate that, from 3 to 120 h after MSG treatment, phosphorylated ERK1/2 expression levels increased, reaching a maximum at 24 h after treatment and decreasing thereafter (Fig. 1). The treatment of cells with the mitogen-activated protein kinase kinase enzyme (MEK)-specific inhibitor U0126 has been demonstrated to block the enhanced human kidney-type glutaminase activity with parallel inhibition of ERK phosphorylation (Thangavelu et al. 2012). When 150 ng/g U0126 was administered before MSG, the results clearly indicated that the levels of phosphorylated ERK1/2 were reduced (Fig. 2). This finding indicates that ERK phosphorylation is involved in the cellular response to excitotoxicity induced by neonatally administered MSG.
Upper panel representative image of phosphorylated extracellular signal-regulated kinase (ERK1/2) protein expression in the hippocampus. The protein expression levels were measured by Western blotting at different times (3, 6, 12, 24, 72, and 120 h) after the animals received the last MSG dose on PND 7. Lower panel a semiquantitative analysis expressed in AU corresponding to phosphorylated ERK1 and ERK2 expression. The data represent the mean ± SEM of five independent experiments (n = 5) with duplicate samples; F value = 7.1, *p < 0.05 was considered significant when the experimental group was compared to the control group
Upper panel representative image of hippocampal β-actin expression in all groups studied and phosphorylated extracellular signal-regulated kinase (ERK1/2) protein expression in the presence of different U0126 concentrations. Representative image of hippocampal phosphorylated ERK1/2 protein expression in the presence of different U0126 concentrations. ERK activity was evaluated 24 h (PND 8) after the last MSG dose. Lower panel a semiquantitative analysis expressed in AU corresponding to the phosphorylated ERK1 and ERK2 activities. The data represent the mean ± SEM of five independent experiments (n = 5) with duplicate samples. F value = 2.4, *p < 0.05 was considered significant when the experimental group was compared to the control group
In addition, the neurodegenerative index under excitotoxic conditions was evaluated and quantified (as the percentage of dead cells) in the control, MSG, and MSG + U0126 groups. The severity of neuronal damage in the evaluated regions was estimated based on the mean number of dead neurons and the observation of degenerative signs, including the following types of cells with neuronal necrosis patterns: fusiform neurons with cytoplasmic retraction or high basophilic intensity (live—blue); cells with pyknotic nuclei; condensed chromatin and without apparent nucleoli; and dark and edematous neurons.
Histological analysis confirmed that U0126 inhibited ERK, which increased the percentage of cell death (Fig. 3) and the number of neurons exhibiting degenerative changes. This phenomenon was observed throughout the CA region of the hippocampus, from CA1 to CA3 (Fig. 3), indicating that the protective response orchestrated in this region involves the ERK signaling pathway.
Left panel representative photomicrograph showing the effect of MSG on neuronal survival in the presence and absence of U0126 in different hippocampal areas (CA1, CA2, and CA3). The magnifications are ×4 in the upper photo and ×10 in the lower photo. The hippocampal slices were stained with the Nissl technique. Right panel respective quantitative analysis expressed as the percentage of neuronal death. The data represent the mean ± SEM of five independent experiments (n = 5) with duplicate samples. F value = 3.5, *p < 0.05 was considered significant (bar = 25 μm)
Glutamate Induces LDH Release to Mediate In Vitro Hippocampal Neuron Viability Through ERK Signaling
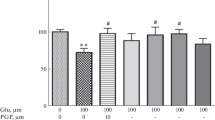
We confirmed previously published results that Glu clearly compromised hippocampal neuronal viability in culture. Our results show that Glu treatment caused a significant reduction in cell viability (a decrease between 25 and 50 %) (Fig. 4a). This effect markedly correlated with the increase in LDH release (Fig. 4b). In addition, the ERK inhibitor U0126 further affected cell viability, but only when it was administered together with Glu (Fig. 4c). U0126 increased LDH release in treated cells, but not in controls (Fig. 4d), indicating that the ERK signaling pathway is involved in the cellular response to excitotoxic damage in hippocampal neurons and that blocking this pathway increases cell survival.
Extracellular signal-regulated protein kinase (ERK) inhibition decreases hippocampal neuronal viability after 24 h of Glu treatment. (a) Glu decreases neuronal viability at concentrations ranging from 10 to 50 μM, measured by the MTT assay. (b) The LDH released increases after 24 h of Glu treatment. (c) Pretreatment with U0126 decreases cell viability and (d) increases LDH release. The data are expressed as the mean ± SEM of three independent experiments (n = 3). F value = 23.4, p < 0.05 was considered significant. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control (CTL); &&p < 0.01 vs. Glu
Hippocampal CA Regions Respond to Excitotoxicity by Increasing the Levels of Phosphorylated ERK Protein
We evaluated hippocampal phosphorylated ERK immunoreactivity to determine the precise area (CA1, CA2, or CA3) in which ERK activity was highest under excitotoxic conditions. The ERK-immunopositive cells were counted, and the results obtained confirm a high increase in phosphorylated ERK immunoreactivity in the hippocampi of the treated animals (Figs. 5, 6, and 7). When selected CA regions were evaluated, we found a higher increase of immunoreactivity in CA2 (Fig. 6) and CA3 (Fig. 7). The increase was also significant in CA1, but to a lesser extent (Fig. 5). Therefore, although the excitotoxic response appears to increase ERK protein phosphorylation throughout the hippocampus, a partial regionalization can be observed (Figs. 5, 6, and 7).
Left panel representative image showing phosphorylated extracellular signal-regulated protein kinase (ERK)-immunopositive neurons in the control (CTL) group (a) and the MSG group (b) in the CA1 hippocampal area (magnification, ×4). The square indicates ERK-immunopositive cells (arrows) at a greater magnification in the CTL (c) and the MSG-treated (d) groups. Right panel quantitative analysis of the number of ERK-immunoreactive cells (upper right graphic) and their intensity expressed as the OD (lower right graphic). The data represent the mean ± SEM of five independent experiments (n = 5), each performed in duplicate; *p < 0.05 was considered significant
Left panel images showing phosphorylated extracellular signal-regulated protein kinase (ERK)-immunopositive neurons in the control (CTL) group (a) and the MSG-treated group (b) in the CA2 hippocampal area (magnification, ×4). The square indicates ERK-immunopositive cells (arrows) at the greatest magnification in the CTL (c) and the MSG-treated (d) groups. Right panel quantitative analysis of the number of ERK-immunoreactive cells (upper right graphic) and their respective intensities expressed as OD (lower right graphic). The data represent the mean ± SEM of five independent experiments (n = 5). *p < 0.05 was considered significant
Left panel images showing phosphorylated extracellular signal-regulated protein kinase (ERK)-immunopositive neurons in the control (CTL) group (a) and the MSG-treated group (b) in the CA3 hippocampal area (magnification, ×4). The square indicates the ERK-immunopositive cells (arrows at greatest magnification) in the CTL (c) and the MSG-treated (d) groups. Right panel quantitative analysis of the number of ERK-immunoreactive cells (upper right graphic) and their respective intensities expressed as OD (lower right graphic). The data represent the mean ± SEM of five independent experiments (n = 5). *p < 0.05 was considered significant
Finally, as a measure of a neuroprotective mechanism that reinforces the role of ERK1/2 on hippocampal neurons, we assessed Bcl-2 mRNA expression and found a significant increase in the hippocampi of animals treated neonatally with MSG (Fig. 8). This result strongly suggests that an anti-apoptotic response through ERK1/2 activation appears to mediate this neuroprotective mechanism, although further experiments are required to test whether the effect can be blocked by inhibitors of ERK activation.
Upper panel representative RT-PCR products demonstrating the Bcl-2 expression levels after MSG-induced excitotoxicity in the presence or absence of the extracellular signal-regulated protein kinase (ERK1/2) inhibitor U0126. Lower panel semiquantitative analysis. The results represent the mean ± SEM of five experiments (n = 5), each performed in duplicate. *p < 0.05 was considered significant
Discussion
There is abundant evidence that excitotoxicity has a key role in neurodegenerative processes (reviewed in Dong et al. 2009; Wang and Qin 2010) and that neurons can trigger protective mechanisms that lead to neuronal survival (reviewed in Naoi and Maruyama 2010; Esposito and Cuzzocrea 2010; Sahota and Savitz 2011). Importantly, neurons attempt to control Ca2+ disruption, inactivate excitatory inputs, and reestablish homeostasis. All of these responses could be coordinated by either one or a few intracellular signaling pathways.
The link between ERK signaling and survival has been widely studied in different neuronal models (Hetman and Gozdz 2004), but its specific participation after excitotoxic damage during early postnatal stages in vivo was previously undetermined.
The ERK signaling pathway is known to be involved in the neuroprotective role of some factors, such as brain-derived neurotrophic factor (BDNF) (Melo et al. 2013; Almeida et al. 2005; Hetman et al. 1999), vascular endothelial growth factor (Ma et al. 2011; Zachary 2005; Matsuzaki et al. 2001), stem cell factor (Dhandapani et al. 2005), and 17β-estradiol (Wu et al. 2005). Additionally, ERK activation has a protective role during pilocarpine-induced seizures (Berkeley et al. 2002) and after KA-induced damage (Ferrer et al. 2002). In these cases, upregulation of the senescence marker protein-30 (SMP30) in astrocytes (Son et al. 2009) is observed, indicating an important supportive role for SMP30 after brain damage.
More recent evidence directly relates ERK signaling to the response to neural cell damage induced by Glu excitotoxicity in vitro, especially in cerebellar granule neurons (Ortega et al. 2011), rat adrenal pheochromocytoma 12 cells (PC12) (Wang et al. 2011), SCN neurons (Karmarkar et al. 2011), cultured rat hippocampal neurons (Melo et al. 2013), CA1/CA3 hippocampal neurons from organotypic hippocampal slices exposed to NMDA insult (Boscia et al. 2009), and cortical neurons affected by mechanical traumatic injury in vitro, which undergo a response mediated by the MEK/ERK pathway that is modulated by metabotropic glutamate receptor 5 (mGluR5) (Chen et al. 2012). In addition, the neuroprotective effect mediated via ERK1/2 and Bcl-2 has also been demonstrated in cultured primary cortical neurons against oxidant injury (Sánchez et al. 2012). However, until now, in vivo evidence has been absent. Combined with the previous evidence obtained in vitro, the present in vivo results suggest that ERK may be a common signaling pathway that is activated in response to excitotoxic injury and that promotes cell survival.
Neonatally administered MSG has been broadly described by our group and others as a useful in vivo model of excitotoxicity (Babai et al. 2006; Ortuño-Sahagún et al. 1997; Beas-Zárate et al. 1994). Some of the molecular mechanisms involved have been partially elucidated (Ortuño-Sahagún et al. 2010). Here, we reveal another piece of the puzzle by demonstrating the involvement of the ERK1/2 signaling pathway, which could coordinate the protective cellular response to excitotoxic damage. Previously, we showed that the p38 pathway could be involved in the response to excitotoxicity induced by MSG in the brain cortex (Segura Torres et al. 2006). Given that, in the hippocampus, the three main MAPK pathways can be differentially activated (Giovannini et al. 2008), it is possible to interpret the results presented here, in which the ERK pathway is involved in the excitotoxicity process in the hippocampus. Our in vitro and in vivo results support the key role of MAPK and ERK1/2 signaling in cellular mechanisms that are induced by excitotoxic damage. These results are reinforced by synergistic responses at the genomic and proteomic levels. It is noteworthy that there is an apparently higher protection in vivo than in that obtained in vitro, in the pure cultures of hippocampal neurons; therefore, it could be feasible to speculate about the relevant participation of not only glial cells (mainly astrocytes) but also others that provide some additional protection or at least somehow participate in the protective response. Thus, additional experiments, such as coculturing of hippocampal neurons with astrocytes, would be necessary to determine whether the non-neuronal cells are also modified in their ERK expression and/or its phosphorylation.
The active role of ERK in neuroprotection is also confirmed by the increase in Bcl-2 mRNA levels in our model. Bcl-2 is a member of the Bcl-xL protein family that functions as an anti-apoptotic factor in several cell types, including neurons (Akhtar et al. 2004). Overexpression or upregulation of Bcl-2 mRNA indicates that ERK1/2 activation possesses an active role in the neuroprotection of hippocampal neurons by inducing downstream molecular mechanisms that augment the neuroprotective response induced by MSG excitotoxicity. Taken together, the results presented here indicate that ERK1/2 involvement is a pivotal piece of the neural response to excitotoxicity, which is ongoing and successfully described by others and our group.
On the basis of previously reported in vitro studies, one attractive hypothesis of the molecular mechanism involved in neuroprotection against Glu excitotoxicity is that it could be mediated by c-fos expression (Rogers et al. 2004; Zhang et al. 2002), which in turn induces BDNF expression (Dong et al. 2006). BDNF is involved in the low-level stimulation of NMDA receptors, which protect hippocampal neurons in culture against Glu excitotoxicity by activation of the ERK pathway (Melo et al. 2013; Almeida et al. 2005) through TrkB receptor activation (Numakawa et al. 2010; Jiang et al. 2003). However, given that nearly all of the knowledge about the molecular pathways that may be involved in Glu excitotoxicity in neurons is derived from in vitro studies using isolated neurons or explants, it will be relevant to translate these results into an in vivo model, such as the one we describe here (this work and previously in 13 other studies; Malik et al. 2011), to establish the relative participation of each component in the neurotrophin-mediated neuroprotection against Glu excitotoxicity.
Deviation from the strict control of MAPK signaling pathways (ERK, p38, and JNK) has been implicated in the development of neurodegenerative diseases, including AD, PD, and amyotrophic lateral sclerosis (reviewed in Zhu et al. 2003; Kim and Choi 2010). Additionally, these three MAPK pathways are differentially activated in the hippocampus of the CRND8 transgenic mouse, a model of AD (Giovannini et al. 2008), which suggests a final equilibrium under these pathological neurodegenerative conditions.
Collectively, the results presented here further suggest that the ERK signaling pathway is involved in the in vivo cellular response to extracellular Glu injury. This evidence implies that it would be beneficial to explore ways to manipulate the ERK pathway, such as treatment with imipramine, a tricyclic antidepressant drug, which has recently been shown to promote neurogenesis and improve the survival rate of neurons in the hippocampus by activation of the MAPK pathway (Peng et al. 2008), as well as other therapeutic avenues to protect neurons from neurodegenerative disease pathophysiology.
References
Akhtar RS, Ness JM, Roth KA (2004) Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta 1644:189–203
Almeida RD, Manadas BJ, Melo CV et al (2005) Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12:1329–1343
Anderson CN, Tolkovsky AM (1999) A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci 19:664–673
André VM, Cepeda C, Levine MS (2010) Dopamine and glutamate in Huntington’s disease: a balancing act. CNS Neurosci Ther 16:163–178
Asomugha CO, Linn DM, Linn CL (2010) ACh receptors link two signaling pathways to neuroprotection against glutamate-induced excitotoxicity in isolated RGCs. J Neurochem 112:214–226
Babai N, Atlasz T, Tamás A et al (2006) Search for the optimal monosodium glutamate treatment schedule to study the neuroprotective effects of PACAP in the retina. Ann NY Acad Sci 1070:149–155
Barnes JW, Tischkau SA, Barnes JA et al (2003) Requirement of mammalian timeless for circadian rhythmicity. Science 302:439–442
Beas-Zárate C, Schliebs R, Ortuño-Sahagún D, Morales-Villagrán A, Feria-Velasco A (1994) Effect of systemic monosodium l-glutamate on muscarinic cholinergic receptors in selected rat brain regions during development. Arch Med Res 25:341–346
Berkeley JL, Decker MJ, Levey AI (2002) The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem 82:192–201
Boscia F, Esposito CL, Di Crisci A, de Franciscis V, Annunziato L, Cerchia L (2009) GDNF selectively induces microglial activation and neuronal survival in CA1/CA3 hippocampal regions exposed to NMDA insult through Ret/ERK signalling. PLoS One 4:e6486
Chen T, Cao L, Dong W et al (2012) Protective effects of mGluR5 positive modulators against traumatic neuronal injury through PKC-dependent activation of MEK/ERK pathway. Neurochem Res 37:983–990
Cortese BM, Phan KL (2005) The role of glutamate in anxiety and related disorders. CNS Spectr 10:820–830
Dhandapani KM, Wade FM, Wakade C, Mahesh VB, Brann DW (2005) Neuroprotection by stem cell factor in rat cortical neurons involves AKT and NFkappaB. J Neurochem 95:9–19
Dong M, Wu Y, Fan Y, Xu M, Zhang J (2006) c-fos modulates brain-derived neurotrophic factor mRNA expression in mouse hippocampal CA3 and dentate gyrus neurons. Neurosci Lett 400:177–180
Dong X-X, Wang Y, Qin Z-H (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sinica 30:379–387
Duncia JV, Santella JB 3rd, Higley CA et al (1998) MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett 8:2839–2844
Esposito E, Cuzzocrea S (2010) New therapeutic strategy for Parkinson’s and Alzheimer’s disease. Curr Med Chem 17:2764–2774
Feng H, Xiang H, Mao YW et al (2004) Human Bcl-2 activates ERK signaling pathway to regulate activating protein-1, lens epithelium-derived growth factor and downstream genes. Oncogene 23:7310–7321
Ferrer I, Blanco R, Carmona M (2001) Differential expression of active, phosphorylation-dependent MAP kinases, MAPK/ERK, SAPK/JNK and p38, and specific transcription factor substrates following quinolinic acid excitotoxicity in the rat. Brain Res Mol Brain Res 94:48–58
Ferrer I, Blanco R, Carmona M, Puig B, Domínguez I, Viñals F (2002) Active, phosphorylation-dependent MAP kinases, MAPK/ERK, SAPK/JNK and p38, and specific transcription factor substrates are differentially expressed following systemic administration of kainic acid to the adult rat. Acta Neuropathol 103:391–407
Giovannini MG, Cerbai F, Bellucci A et al (2008) Differential activation of mitogen-activated protein kinase signalling pathways in the hippocampus of CRND8 transgenic mouse, a model of Alzheimer’s disease. Neuroscience 153:618–633
Gladding CM, Fitzjohn SM, Molnár E (2009) Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol Rev 61:395–412
Grewer C, Gameiro A, Zhang Z, Tao Z, Braams S, Rauen T (2008) Glutamate forward and reverse transport: from molecular mechanism to transporter-mediated release after ischemia. IUBMB Life 60:609–619
Hetman M, Kanning K, Cavanaugh JE, Xia Z (1999) Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem 274:22569–22580
Hetman M, Gozdz A (2004) Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem 271:2050–2055
Jiang X, Zhu D, Okagaki P et al (2003) N-methyl-d-aspartate and TrkB receptor activation in cerebellar granule cells: an in vitro model of preconditioning to stimulate intrinsic survival pathways in neurons. Ann N Y Acad Sci 993:134–145
Karmarkar SW, Bottum KM, Krager SL, Tischkau SA (2011) ERK/MAPK is essential for endogenous neuroprotection in SCN2.2 cells. PLoS One 6:e23493
Kew JN, Kemp JA (2005) Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacol (Berl) 179:4–29
Kim EK, Choi E-J (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802:396–405
Kullmann DM, Lamsa K (2008) Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation. J Physiol 586:1481–1486
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542. doi:10.1007/s00424-010-0809-1
Lee HG, Zhu X, O’Neill MJ et al (2004) The role of metabotropic glutamate receptors in Alzheimer’s disease. Acta Neurobiol Exp (Wars) 64:89–98
Lei K, Davis RJ (2003) JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A 100:2432–2437. doi:10.1073/pnas.0438011100
Ma Y, Liu W, Wang Y et al (2011) VEGF protects rat cortical neurons from mechanical trauma injury induced apoptosis via the MEK/ERK pathway. Brain Res Bull 86:441–446
Maiese K, Chong ZZ, Li F (2005) Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res 2:425–446
Malik SZ, Motamedi S, Royo NC, LeBold D, Watson DJ (2011) Identification of potentially neuroprotective genes upregulated by neurotrophin treatment of CA3 neurons in the injured brain. J Neurotrauma 28:415–430
Matsuzaki H, Tamatani M, Yamaguchi A et al (2001) Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J 15:1218–1220
Melo CV, Okumoto S, Gomes JR et al (2013) Spatiotemporal resolution of BDNF neuroprotection against glutamate excitotoxicity in cultured hippocampal neurons. Neuroscience 237:66–86
Naoi M, Maruyama W (2010) Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr Pharm Des 16:2799–2817
Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H (2010) BDNF function and intracellular signaling in neurons. Histol Histopathol 25:237–258
O’Sullivan AW, Wang JH, Redmond HP (2009) p38 MAP kinase inhibition promotes primary tumour growth via VEGF independent mechanism. World J Surg Oncol 7:89
Ortega F, Pérez-Sen R, Delicado EG, Teresa M-PM (2011) ERK1/2 activation is involved in the neuroprotective action of P2Y13 and P2X7 receptors against glutamate excitotoxicity in cerebellar granule neurons. Neuropharmacol 61:1210–1221
Ortuño-Sahagún D, Beas-Zárate C, Adame-Gonzalez G, Feria-Velasco A (1997) Effect of l-glutamate on cholinergic neurotransmission in various brain regions and during the development of rats, when administered perinatally. Neurochem Int 31:683–692
Ortuño-Sahagún D, Rivera-Cervantes MC, Gudino-Cabrera G et al (2010) Microarray analysis of rat hippocampus exposed to excitotoxicity: reversal Na(+)/Ca(2+) exchanger NCX3 is overexpressed in glial cells. Hippocampus 22:128–140
Park MA, Zhang G, Mitchell C et al (2008) Mitogen-activated protein kinase kinase 1/2 inhibitors and 17-allylamino-17-demethoxygeldanamycin synergize to kill human gastrointestinal tumor cells in vitro via suppression of c-FLIP-s levels and activation of CD95. Mol Cancer Ther 7:2633–2648
Paschen W (1996) Glutamate excitotoxicity in transient global cerebral ischemia. Acta Neurobiol Exp (Wars) 56:313–322
Peng CH, Chiou SH, Chen SJ et al (2008) Neuroprotection by imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol 18:128–140
Petegnief V, Saura J, de Gregorio-Rocasolano N, Paul SM (2001) Neuronal injury-induced expression and release of apolipoprotein E in mixed neuron/glia co-cultures: nuclear factor kappaB inhibitors reduce basal and lesion-induced secretion of apolipoprotein E. Neuroscience 104:223–234
Rogers A, Schmuck G, Scholz G, Williams DC (2004) c-fos mRNA expression in rat cortical neurons during glutamate-mediated excitotoxicity. Toxicol Sci 82:562–569
Sahota P, Savitz SI (2011) Investigational therapies for ischemic stroke: neuroprotection and neurorecovery. Neurotherapeutics 8:434–451
Sánchez A, Tripathy D, Yin X, Luo J, Martínez J, Grammas P (2012) Pigment epithelium-derived factor (PEDF) protects cortical neurons in vitro from oxidant injury by activation of extracellular signal-regulated kinase (ERK) 1/2 and induction of Bcl-2. Neurosci Res 72:1–8
Satoh T, Nakatsuka D, Watanabe Y, Nagata I, Kikuchi H, Namura S (2000) Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neurosci Lett 288:163–166
Schousboe A, Waagepetersen HS (2005) Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotox Res 8:221–225
Segura Torres JE, Chaparro-Huerta V, Rivera Cervantes MC, Montes-González R, Flores Soto ME, Beas-Zárate C (2006) Neuronal cell death due to glutamate excitotoxicity is mediated by p38 activation in the rat cerebral cortex. Neurosci Lett 403:233–238
Son TG, Park HR, Kim SJ et al (2009) Senescence marker protein 30 is up-regulated in kainate-induced hippocampal damage through ERK-mediated astrocytosis. J Neurosci Res 87:2890–2897
Thangavelu K, Pan CQ, Karlberg T et al (2012) Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf–Mek–Erk signaling in cancer cell metabolism. Proc Natl Acad Sci U S A 109:7705–7710
Tischkau SA, Gallman EA, Buchanan GF, Gillette MU (2000) Differential cAMP gating of glutamatergic signaling regulates long-term state changes in the suprachiasmatic circadian clock. J Neurosci 20:7830–7837
Verheij M, Ruiter GA, Zerp SF et al (1998) The role of the stress-activated protein kinase (SAPK/JNK) signaling pathway in radiation-induced apoptosis. Radiother Oncol 47:225–232
Wang CX, Song JH, Song DK, Yong VW, Shuaib A, Hao C (2006) Cyclin-dependent kinase-5 prevents neuronal apoptosis through ERK-mediated upregulation of Bcl-2. Cell Death Differ 13:1203–1212
Wang X, Zhu G, Yang S et al (2011) Paeonol prevents excitotoxicity in rat pheochromocytoma PC12 cells via downregulation of ERK activation and inhibition of apoptosis. Planta Med 77:1695–1701
Wang Y, Qin ZH (2010) Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 15:1382–1402
Wu TW, Wang JM, Chen S, Brinton RD (2005) 17Beta-estradiol induced Ca2+ influx via l-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and Bcl-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience 135:59–72
Yoon CH, Kim MJ, Park MT et al (2009) Activation of p38 mitogen-activated protein kinase is required for death receptor-independent caspase-8 activation and cell death in response to sphingosine. Mol Cancer Res 7:361–370
Zachary I (2005) Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals 14:207–221
Zhang J, Zhang D, McQuade JS, Behbehani M, Tsien JZ, Xu M (2002) c-fos regulates neuronal excitability and survival. Nat Genet 30:416–420
Zhu X, Lee H-G, Raina AK, Perry G, Smith MA (2003) The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals 11:270–281
Acknowledgments
This work was partially supported by the Network GENIAR from CYTED (the Program for Science and Technology for Development), by Universidad de Guadalajara P3E/2012/137505 to DO-S, and by CONACyT Grant No. 0177594 to CB-Z.
Author information
Authors and Affiliations
Corresponding author
Additional information
Raúl Montes González and Ester Verdaguer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ortuño-Sahagún, D., González, R.M., Verdaguer, E. et al. Glutamate Excitotoxicity Activates the MAPK/ERK Signaling Pathway and Induces the Survival of Rat Hippocampal Neurons In Vivo. J Mol Neurosci 52, 366–377 (2014). https://doi.org/10.1007/s12031-013-0157-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-013-0157-7