Abstract
Induction of demyelination in the central nervous system induce the oligodendrocyte progenitors to proliferate, migrate, and differentiate for restoring new myelin sheathes around demyelinated axons. Factors which increase the response of endogenous progenitor cells could be used to improve remyelination. In the current study, the effect of bFGF on lysolecithin-induced demyelination and remyelination processes in mouse optic chiasm and nerves was investigated. Lysolecithin was injected into the optic chiasm of Balb/C mice. Two groups of animals received doses of bFGF (1 or 5 ng/kg i.p.) just before and every 3 days after lysolecithin injection. Delay and amplitude of visual evoked potential (VEP) waves were recorded as indices of axonal demyelination at 7th, 13th, and 28th days post-lesion. Myelin basic protein (MBP) and Olig2 gene expressions were studied as indices of myelination and oligodendrocyte precursors’ recruitment into the lesion. Lysolecithin elongated delay of P1 wave and declined the amplitude of P1-N1 wave. Lysolecithin decreased MBP and increased Olig2 expression in different days post-lesion. Lysolecithin-induced changes in VEPs were partially ameliorated by endogenous repair. bFGF reduced the increased delay, increased the reduced amplitude of P1-N1 wave, increased MBP gene expression, and accelerated the increasing pattern of Olig2. bFGF seems to be able to potentiate the endogenous repair mechanisms of myelin. Its effect on demyelination and remyelination processes seems to be mediated by oligodendrocyte progenitor cells and their differentiation to myelinating cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) which is characterized by the loss of oligodendrocytes and demyelination of axons is the most frequent neurological disease that causes disability in the young adults (Annapurna et al. 2002; Sun et al. 2004). MS affects the optic apparatus in about 70 % of patients (Guazzo 2005). Once axons become demyelinated, a spontaneous regenerative response named remyelination occurs (Arnett et al. 2004) which restores new myelin sheaths and saltatory conduction. Remyelination has been observed in both MS patient and in a range of experimental models of demyelination (Zhang et al. 1999), and it is dependent on proliferation, migration, and differentiation of endogenous progenitors (Franklin 2002). Although remyelination can be widespread and extensive in experimental demyelination models, it is usually incomplete in the context of chronic demyelinating diseases (Stangel and Hartung 2002). Remyelination might fail because of an inadequate response of oligodendrocyte progenitor cells (OPCs) (recruitment failure) or because of a failure in differentiation of recruited OPCs into remyelinating oligodendrocytes (differentiation failure) (Franklin 2002; Ois Lachapelle et al. 2002). Oligodendrocytes that survived in demyelinated areas remain at quiescent stage and seem unlikely to regenerate new myelin sheaths and to contribute in subsequent remyelination (Ans et al. 1998; Woodruff and Franklin 1999; Franklin 2002; Picard-Riera et al. 2002; Stangel and Hartung 2002). Therefore, probably all the remyelinating cells come from OPCs in surrounding white matter (Kuhn et al. 2001; Franklin 2002; Ois Lachapelle et al. 2002; Dubois-Dalcq et al. 2008) or from proliferative cells in subventricular zone (SVZ) of the lateral and third ventricles (Ans et al. 1998; Franklin 2002; Mozafari et al. 2010, 2011). Multipotent and self-renewable cells which are located in germinative areas of adult brain can be triggered to proliferate, migrate, and differentiate into astrocytes, neurons, and oligodendrocytes in response to environmental cues such as growth factors (Woodruff and Franklin 1999; Decker et al. 2002; Arnett et al. 2004).
There is increasing evidence that growth factors are important signaling molecules in CNS remyelination (Ans et al. 1998) with proliferative, pro-migratory, and mitogenic effects (Franklin 2002). Local overexpression of growth factors in the lesion area might be required for survival, proliferation, migration, and differentiation of OPCs. bFGF regulates multiple responses of oligodendrocyte lineage, including their proliferation and migration and then terminal differentiation to oligodendrocytes (Murtie et al. 2005).
Visual evoked potential (VEP) which is recorded from the scalp by averaging from EEG signals is a sensitive tools for studying demyelination and remyelination in optic nerves and chiasm (Mozafari et al. 2010). A highly significant correlation between changes in VEP parameters and the amount of demyelination in optic nerve is proved (You et al. 2011). Moreover, VEP is a noninvasive tool for investigating the function of visual path and provides the possibility of multiple evaluations during the course of demyelination and remyelination and subsequently provides the possibility of comparing records obtained from each subject; therefore, more precise tracking of myelination is possible following a specific intervention.
In the present study, we used a toxin-induced model of demyelination in the optic nerves and chiasm of adult mouse to test the effect of exogenous bFGF on remyelination process. We used VEP recording to investigate neural conductivity in optic nerves and chiasm as a functional index of demyelination and remyelination. The mRNA level of myelin basic protein (MBP) and Olig2 (oligodendrocyte lineage marker) were also assessed as indices of myelination and OPCs recruitment into the lesion site.
Material and Methods
Animals
All experiments were carried out on adult female Balb/C mice, weighting 20–25 g (Pastor Institute, Karaj, Iran). Room temperature of 22 ± 1°C and humidity between 40 and 60 % were maintained during a 12/12-h light–dark cycle. All animal care procedures were approved by Tarbiat Modares University ethical committee for animal research. Efforts were made to minimize the number of animals used and their suffering.
Induction of Demyelination
Lysolecithin microinjection and its conformation were done based on our previous report [18]. Concisely, after 1 week of acclimatization, animals were anesthetized using intraperitoneal (i.p.) injection of ketamine (10 mg/kg) and xylazine (2 mg/kg) and placed in a rat stereotaxic instrument (Stoelting, USA) in a skull-flat situation. After shaving the corresponding skull surface, using a bladed scalpel, a midline incision was made at the middle of shaved site. Using stereotaxic atlas of the mouse brain (Paxinos and Franklin 2004), values for appropriate coordinates to the optic chiasm (0.28 mm dorsal to the bregma, 3 mm lateral, and −5 mm deep from the dura surface) were determined. Dura was exposed using an electric high-speed drill at the appropriate previously labeled site on the skull. Injections were made with a vertical angel equal to 37 ° using a 10-μl Hamilton syringe. Mice were injected with 1 μl of 1 % lysolecithin (within 5 min) (Sigma, St. Louis, USA), and the needle was kept in place for another 5 min to avoid the possible reflux through the needle track. Control animals were injected an equal volume of sterile saline.
Treatment with bFGF
Two groups of animals received bFGF (Royan Institute, Iran) dissolved in saline (1 or 5 ng/kg i.p. in 200 μl saline) 1 h before and every 3 days after lysolecithin injection. Control animals received same volume of saline.
Visual Evoked Potential Recording
VEP is an evoked electrophysiological potential obtained by signal averaging from the electroencephalographic activity recorded at the scalp. It can provide important diagnostic information regarding the functional integrity of the visual system. The surgical process for implanting the electrodes was similar to the method described previously (Ishikawa et al. 2008; Mozafari et al. 2010). Mice were anesthetized with i.p. injection of a mixture of ketamine and xylazine (70 and 10 mg/kg, respectively) and fixed to stereotaxic apparatus. Normal body temperature was maintained in 37°C (SR-5, Narishige, Tokyo, Japan) during the anesthesia. To facilitate VEP recordings, a monopolar electrode was implanted on the occipital cortex (coordinate to lambda, −3 mm lateral contralateral to stimulated eye) and the anterior edge of the skull was implanted with the reference electrode (bregma A, 1 mm; L, 1 mm) (Paxinos and Franklin 2004). The electrodes were connected to a miniature receptacle and fixed on the skull using dental cement.
For VEP recording, animals were placed in a light-reflecting cylinder (diameter, 18 cm; height, 23 cm). The cylinder was placed in a sound-attenuating, dark, and electrically shielded box (60 × 60 × 60 cm). Mice were allowed to adapt to darkness for 5 min. Flashlight stimulation was delivered by a general evoked response stimulator (SMP-3100, Nihon Kohden, Tokyo, Japan) 300 times with a frequency of 0.5 Hz. Simultaneously with visual stimulation, voltage difference among reference and recorder electrodes was amplified for 10,000 times (with high- and low filter settings of 30 and 0.08 Hz, respectively) using a biophysical amplifier (AVB-10, Nihon Kohden) and displayed on a memory oscilloscope (VC-11, Nihon Kohden). Amplified waveforms were afterwards averaged (DAT-1100, Nihon Kohden). For each VEP recording, we studied the latency between the flashlight and the first negative peak (N1) and the amplitude of N1-P1 wave. Eight mice were used in each experimental group.
Luxol Fast Blue and Cresyl Fast Violet Counter Staining
Mice were deeply anesthetized with pentobarbital (130 mg/kg) at day 28 post-lesion and sequentially perfused (3 ml/min) with a freshly prepared 0.1 M phosphate-buffered saline and then with a solution of 4 % paraformaldehyde (pH 7.4). Brains were excised and post-fixed overnight in same fixative solution at 4°C. For paraffin embedding, brains were first dehydrated in series of alcohol with increasing concentrations, cleared by incubation in xylene (2 × 45 min) and finally embedded in paraffin for 6–12 h and blocked. Coronal serial sections (5 μm) were obtained from the chiasm using a rotary microtome (lieca, Austria) and stained with 0.1 % luxol fast blue (British drug house, UK) at 60°C for 3 h. Adequate contrast was made by rapid immersion of preparation in 0.05 % lithium carbonate and several changes of 70 % ethanol. After being washed by distilled water, the sections were counter stained with 0.1 % Cresyl fast violet (Merck, Germany) for a minute. The sections were again and dehydrated, then cleaned in xylene, coverslipped, and screened for demyelination.
Reverse Transcription-PCR
In order to determine MBP, Olig2, and GAPDH gene expression levels within optic nerve and chiasm, total cellular RNA was extracted using a modification of guanidine isothiocyonate–phenol–chloroform method, using RNX + reagent (CinnaGen, Iran). After determining the concentration of total RNA and its integrity, cDNA was prepared using 2 μg of total RNA from each sample using oligdT primer and M-MuLV reverse transcriptase (Fermentase). PCR reaction were performed using selective forward and reverse primer for GAPDH (housekeeping gene), MBP, and Olig2 and PCR master mix (Bio atlas, Germany). The sequences of primers are presented in Table 1. To amplify the cDNA, PCR reactions included incubation at 95°C for 5 min, followed by 32 cycles of thermal cycling (60 s at 95°C, 60 s at 60°C, and 60 s at 72°C). The final cycle was followed by a 5-min extension step at 72°C. The reaction parameters were adjusted to obtain a condition with linear relation between the number of PCR cycles and PCR products and with linear relation between the initial amount of cDNA template and PCR product. PCR products were subsequently analyzed on 1 % agarose gel (Roche), and bands were quantified by densitometry using lab works analyzing software (UVP) and normalizing to GAPDH for each sample.
Statistical Analysis
Data were expressed as mean ± SEM. Statistical analysis was carried out by using one-way ANOVA and Tukey post-hoc to compare the difference between experimental groups. p < 0.05 was considered as minimum significant level.
Results
Visual Evoked Potentials Recording
Experimental demyelination was induced by local injection of lysolecithin into the optic chiasm. Demyelination level was functionally assessed by recording VEPs. A sample of VEP record is presented in Fig. 1a. Seven days after lysolecithin injection, a significant increase in P1 wave delay in P1 wave latency (demyelination) was observed (p < 0.001, Fig. 1b). The elevated delay was observed also on days 13 and 28 (both p < 0.05). Maximum demyelination was observed on day 7 and the amount of endogenous remyelination was significant on day 28 (p < 0.05 vs. day 7, Fig. 1b). The amplitude of P1-N1 wave was also measured and compared in different days post-lesion. Lysolecithin injection into the chiasm reduced the amplitude on days 7, 13, and 28 (all p < 0.001), while there was a trend for restoring wave amplitude during days 7–28 (Fig. 1c).
Changes in the parameters of visual evoked potentials following lysolecithin injection into the mouse chiasm. a Shows a sample of visual evoked potential record. b Shows changes in the latency of P1 wave following lysolecithin injection. c Shows changes in P1-N1 amplitude following lysolecithin injection. *p < 0.05 and ***p < 0.001 compared to control group, +p < 0.05 compared to day 7 post-lesion. Each bar shows mean ± SEM
To study the effects of bFGF on the processes of demyelination and remyelination, two groups of animals received bFGF, 1 or 5 ng/kg of body weight, and were studied using VEPs recording from the scalp. As Fig. 2a shows, a significant increase in P1 delay on day 7 post-lesion in lysolecithin-treated group has occurred, while the application of bFGF 1 or 5 ng/kg significantly restored VEP latency (p < 0.05 and p < 0.01, respectively). On day 13 post-lesion, in animals treated with bFGF, the P1 wave delay did not change significantly (Fig. 2b). Both doses of bFGF significantly reduced P1 wave latency, compared to lysolecithin-treated animals (p < 0.01). On day 28 post-lesion, there was a significant increase in P1 wave delay in lysolecithin-treated animals (p < 0.05) while in animals treated with bFGF during days 0–28, the P1 wave latency was the same to the control group (Fig. 2c). Animals treated with a dose (5 μg/kg) of bFGF showed shorter P1 wave delay compared to lysolecithin-treated animals (p < 0.05). Changes in the amplitude of P1-N1 wave following bFGF administration are presented in Fig. 3. On day 7 post-lesion, lysolecithin induced a significant decrease in the amplitude of P1-N1 wave in bFGF-treated- and bFGF-untreated groups and there was nonsignificant difference between bFGF-treated- and bFGF-untreated groups (Fig. 3a). On day 13, the observed decrease in the amplitude of P1-N1 wave in bFGF-treated animals was not significant as compared to the control. Moreover bFGF (5 ng/kg) significantly restored the amplitude (p < 0.01, Fig. 3b). Declined amplitude was not observed in bFGF-treated animals on day 28, while the amplitude was significantly increased compared to lysolecithin-treated animals (both p < 0.01, Fig. 3c).
Effect of bFGF on the latency of P1-N1 component on days 7 (a), 13 (b), and 28 (c) post-lysolecithin injection into the optic chiasm. *p < 0.05 and ***p < 0.001 compared to control group, +p < 0.05 and ++p < 0.01 compared to lysolecithin group. LPC lysolecithin-treated group, bFGF1 lysolecithin-treated animals injected with bFGF (1 ng/kg i.p.), bFGF5 lysolecithin-treated animals injected with bFGF (5 ng/kg i.p.), each bar shows mean ± SEM, bFGF was injected just before lysolecithin injection and then repeated every 3 days
Effect of bFGF on the amplitude of P1-N1 component on days 7 (a), 13 (b), and 28 (c) post-lysolecithin injection into the optic chiasm. ***p < 0.001 compared to control group, ++p < 0.01 and +++p < 0.001 compared to lysolecithin group. LPC lysolecithin-treated group, bFGF1 lysolecithin-treated animals injected with bFGF (1 ng/kg i.p.), bFGF5 lysolecithin-treated animals injected with bFGF (5 ng/kg i.p.), each bar shows mean ± SEM, bFGF was injected just before lysolecithin injection and then repeated every 3 days
Myelin Staining
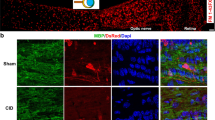
Histological evaluation of chiasm sections using luxol fast blue staining showed a normal staining for the control animals. Twenty-eight days after injection of lysolecithin, still a few amount of demyelination was detectable, while in bFGF-treated rats, myelin staining was significantly improved. Representative micrographs are shown in Fig. 4.
Gene Expression Study
Semiquantitative RT-PCR was used to study changes in the expression of marker genes for myelination (MBP) and oligodendrocytes precursor cells (olig2) in optic chiasm and nerves. Seven days after lysolecithin injection, a significant decline in MBP gene expression was observed (p < 0.001, Fig. 5a). The declined expression was also observed on days 13 and 28 (both p < 0.001). Maximum demyelination (minimum MBP expression) was observed on day 7, and the amount of endogenous remyelination was significant on days 13 and 28 (both p < 0.01 vs. day 7, Fig. 5a). The level of Olig2 expression as a marker of OPCs recruitment was not changed on day 7 post-lesion, but on days 13 and 28, its expression increased significantly (respectively, p < 0.001 and p < 0.01 vs. control and p < 0.01 and p < 0.05 vs. day 7, Fig. 5b). Figure 4c shows the representative bands for the expression of MBP, Olig2, and GAPDH as an internal control.
Changes in the expression level of marker genes for myelinating cells (MBP) and oligodendrocytes progenitors following lysolecithin injection into the rat chiasm. a Shows changes in the expression of myelin basic protein (MBP) gene following lysolecithin injection. b Shows changes in the expression of olig2 transcription factor (olig2) following lysolecithin injection. c Shows representative bands for MBP, olig2 and GAPDH (housekeeping gene). **p < 0.01 and ***p < 0.001 compared to control group, +p < 0.05 and ++p < 0.01 compared to day 7 post-lesion. Each bar shows mean ± SEM
Lysolecithin injection into the chiasm reduced the expression of MBP to 10 % of the control on day 7 post-lesion. Although in presence of bFGF (1 and 5 ng/kg), lysolecithin reduced the expression of MBP (both p < 0.001), but the expression level was significantly higher than the untreated group (respectively, p < 0.01 and p < 0.001, Fig. 6a). Higher level of myelination was observed in animals treated with bFGF (5 ng/kg) (p < 0.01) compared to bFGF (1 ng/kg). On day 13 post-lesion, in animals treated with bFGF (1 and 5 ng/kg), the MBP expression was same to control and significantly higher than lysolecithin group (p < 0.05 and p < 0.001, respectively; Fig. 6b). On day 28 post-lesion (Fig. 6c), the expression level of MBP in lysolecithin group was significantly lower than the control (p < 0.001), while bFGF (1 and 5 ng/kg) significantly reversed the MBP expression (p < 0.01 and p < 0.001, respectively). Compared to the control, higher level of MBP expression was observed in bFGF (5 μg/kg)-treated group (p < 0.05).
Effect of bFGF on the expression of myelin basic protein (MBP) gene on days 7 (a), 13 (b), and 28 (c) post-lysolecithin injection into the optic chiasm. ***p < 0.001 compared to control group, +p < 0.05, ++p < 0.01, and +++p < 0.001 compared to lysolecithin group. LPC lysolecithin-treated group, bFGF1 lysolecithin-treated animals injected with bFGF (1 ng/kg i.p.), bFGF5 lysolecithin-treated animals injected with bFGF (5 ng/kg i.p.), each bar shows mean ± SEM, bFGF was injected just before lysolecithin injection and then repeated every 3 days
Lysolecithin injection into the chiasm did not change the expression of Olig2 on day 7 post-lesion. Application of bFGF (1and 5 ng/kg) in lysolecithin-treated animals produced a trend to increase the expression level of Olig2, and the effect of bFGF (5 ng/kg) was statistically significant (p < 0.05, Fig. 7a). On day 13 post-lesion, lysolecithin-induced demyelination in chiasm and treatment of lesioned animals with both doses of bFGF increased the expression of Olig2 (respectively, p < 0.001, p < 0.001, and p < 0.01; Fig. 7b). On day 28 post-lesion (Fig. 7c), higher expression level of Olig2 was observed only in lysolecithin-treated animals (p < 0.01). Following 28 days exposure to bFGF, the expression level of Olig2 did not show significant difference compared to the control.
Effect of bFGF on the expression of olig2 transcription factor (olig2) gene on days 7 (a), 13 (b), and 28 (c) post-lysolecithin injection into the optic chiasm. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to control group, LPC lysolecithin-treated group, bFGF1 lysolecithin-treated animals injected with bFGF (1 ng/kg-i.p.), bFGF5 lysolecithin-treated animals injected with bFGF (5 ng/kg i.p.), each bar shows mean ± SEM, bFGF was injected just before lysolecithin injection and then repeated every 3 days
Discussion
VEP waves are the results of electrical activity of visual cortex in response to visual stimuli (Heckenlively and Arden 2006). These waves are very sensitive to deficits in optical apparatus and pathways and could be used for detecting demyelination and remyelination in optic nerves (Mozafari et al. 2010; You et al. 2011) Demyelinations resulting from cuprizone and/or lysolecithin give rise to the increased P1 wave latency in rat (Soto et al. 2004; Mozafari et al. 2010). Following the injection of lysolecithin which leads to the death of oligodendrocytes, a demyelinated area could be induced at the site of injection. At the present study, P1 wave latency and P1-N1 amplitude were measured as functional indices of demyelination and remyelination in optic chiasm and nerves. Under the influence of the endogenous-released effective factors, an insufficient remyelination occurred, and consequently, P1 wave latency decreased towards the control and P1-N1 amplitude increased, but this reversal was not complete and statistically differed from the control group. Although oligodendrocytes might be able to survive in the demyelinated area, based on the reports, they cannot regenerate new myelin sheaths. It is also indicated that an oligodendrocyte that has myelinated once cannot do so for a second time (Woodruff and Franklin 1999; Johansson et al. 1999; You et al. 2011). Therefore the partial endogenous repair of myelin in this report might be due to recruitment, migration, and differentiation of adjacent OPCs or neural stem cells. On the other hand, the observed deficit in remyelination process might be the result of depletion of OPCs or failure in the recruitment or differentiation phases (Franklin et al. 1997; Levine et al. 2001; You et al. 2011). Therefore, any treatment with positive effect on the mentioned processes will potentiate the repair capacity for myelin loss. Growth factors have pivotal role in efficient remyelination by providing support and incentive for myelinating cells, and one could suppose that deficiency in growth factors synthesis lead to incomplete remyelination (Murtie et al. 2005). Neural stem cells (NSCs) in SVZ are able to transform to OPCs and consequently participate in remyelination (Zhang et al. 1999; Decker et al. 2002; Mozafari et al. 2010). Moreover, myelin repair in optic chiasm is reported to be mostly mediated by neural stem cells residing in the SVZ of the third ventricle (Mozafari et al. 2011). NSCs express receptors for growth factors involved in proliferation and differentiation (Decker et al. 2002).
As the previous reports indicated, bFGF has been a mitogen factor and effective on OPCs migration and also is one of the earliest differentiation regulators of OPCs (Frost et al. 2003; You et al. 2011). bFGF receptors and their ligands have been increased following the induction of demyelination (Soto et al. 2004). In the current study, to evaluate the effects of bFGF on demyelination and remyelination processes, it was injected just before and every 3 days after lysolecithin injection and the outcome was measured using VEP recording from the scalp. In bFGF recipient animals, restoration of P1 wave latency and P1-N1 amplitude toward the control animals were significantly more than endogenous repair in control animals. The curative effect of bFGF might be the result of either decreased demyelination (oligodendrocytes protection) or increased remyelination (oligodendrogenesis). The later seems to be more plausible due to the proliferative, migrative and differentiative effects reported for growth factors. In the next step, using assessment of the expression of some marker genes, we attempted to assess the effect of bFGF on OPCs-mediated remyelination and measured the expression of olig2 and MBP (myelin basic factor) genes in the lesion site in different times post-lesion. The results of molecular studies fortified the effects of bFGF on VEP waves. MBP, a component of myelin proteins essential for myelin compaction and stability in CNS (Messersmith et al. 2000), is frequently used as an index of myelination (McDonald 1999; Messersmith et al. 2000; Baumann and Pham-Dinh 2001; Husted 2006; Aditya and Chakrabarty 2007; Goudarzvand et al. 2010). Following the injection of lysolecithin into the chiasm, the decreased MBP expression reflects the reduced number of myelinating oligodendrocytes. Lower expression level of MBP was observed on day 7 and afterward; a significant increase was seen on days 13 and 28 which support endogenous repair observed in VEP records. Applying 1 and 5 ng/kg doses of bFGF increased the level of MBP expression, and even on day 28, bFGF increased MBP expression to an amount higher than control group. Newly differentiated oligodendrocytes express higher amount of MBP compared to older ones (Goudarzvand et al. 2010); the fact that may explain why the level of MBP expression in bFGF-treated animals was higher than control.
Changes in the expression of olig2 show the relative number of activated OPCs in the site of demyelination (Fancy et al. 2004); Olig2 positive OPCs move into the lesion and differentiate to oligodendrocytes (Mason et al. 2004; Hack et al. 2004; Copray et al. 2006) and the expression of olig2 is essential for OPCs differentiation (Picard-Riera et al. 2002; Marshall et al. 2005; Vallstedt et al. 2005; Copray et al. 2006; Sun et al. 2006). Following lysolecithin injection, the expression of olig2 was increased on days 7 and 13 which show the recruitment of OPCs into the lesion. Comparing the expression of olig2 and MBP, the increase in olig2 expression has occurred prior to MBP expression and in agreement with the actual process in myelin repair, i.e., OPCs move into the lesion and differentiate to oligodendrocytes. When comparing the expression of MBP and olig2, in bFGF-treated group, the level of olig2 was higher than LPC group only on day 7 post-lesion and on days 13 and 28 even olig2 showed a trend to be reduced compared to lysolecithin-treated animals. On the other hand the expression of MBP was higher in bFGF-treated animals in all days post-lesion. This observation may be due to accelerated process of OPCs proliferation and migration towards the lesion and also due to in advanced differentiation of OPCs to myelinating oligodendrocytes. Thereupon, on days 13 and 28, higher MBP level in bFGF-treated animals were correlated with lower levels of olig2.
The present study investigated electrophysiological indices of P1 wave latency and P1-N1 amplitude and also molecular markers which indicate the activation of OPCs and their differentiation into the myelinating cells. Based on our results, it may be concluded that bFGF accelerates and potentiated the remyelination process following experimental induction of demyelination in optic nerve and chiasm. Since the limited number of endogenous OPCs and neural stem cells in thought to be the main reason for myelin repair in the context of chronic demyelinating diseases like multiple sclerosis, bFGF may be potential choice for overcoming the insufficiency.
References
Aditya GS, Chakrabarty A (2007) Multiple sclerosis and demyelination. Curr Diagn Pathol 13:193–202
Annapurna A, Krlshna KV, Murali Mohan Rao P, Someswara Rao K, Rajasekhar J (2002) Multiple sclerosis: the disease and its treatment. Ind J Pharmacol 34:3–15
Ans S, Keirstead J, Levine M, Blakemore WF (1998) Response of the oligodendrocyte progenitor cell population (defined by NG2 labeling) to demyelination of the adult spinal cord. GLIA 22:161–170
Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD (2004) bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306(5704):2111–2115
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81(2):871–927
Copray S, Balasubramaniyan V, Levenga J, De Bruijn J, Liem R, Boddekf E (2006) Olig2 Overexpression induces the in vitro differentiation of neural stem cells into mature oligodendrocytes. Stem Cells 24:1001–1010
Decker L, Picard-Riera N, Lachapelle F, Baron-Van EA (2002) Growth factor treatment promotes mobilization of young but not aged adult subventricular zone precursors in response to demyelination. J Neurosci Res 69:763–771
Dubois-Dalcq M, Williams A, Stadelmann C, Stankoff B, Zalc B, Lubetzki C (2008) From fish to man: understanding endogenous remyelination in CNS demyelinating diseases. Brain 131(Pt 7):1686–1700
Fancy SPJ, Zhao C, Franklin RJM (2004) Increased expression of Nkx2. 2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci 27(3):247–254
Franklin RJM (2002) Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci 3:705–714
Franklin RJM, Gilson JM, Blakemore WF (1997) Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res 50:337–344
Frost EE, Nielsen JA, Le TQ, Armstrong RC (2003) PDGF and FGF2 regulate oligodendrocyte progenitor responses to demyelination. J Neurobiol 54:457–472
Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T (2010) Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol 30(2):289–299
Guazzo EP (2005) A technique for producing demyelination of the rat optic nerves. J Clin Neurosci 12(1):54–58
Hack MA, Sugimori M, Lundberg C, Nakafuku M, Gotz M (2004) Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci 25:664–678
Heckenlively JR, Arden GB (2006) Principle and practice of clinical electrophysiology of vision, 2nd edn. The MIT, Cambridge
Husted C (2006) Structural insight into the role of myelin basic protein in multiple sclerosis. Proc Natl Acad Sci U S A 103(12):4339–4340
Ishikawa T, Fujiwara A, Takechi K, Matsumoto JAN, Rahman A, Kamei C (2008) Changes of visual evoked potential induced by lateral geniculate nucleus kindling in rats. Epilepsy Res 79:146–150
Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J (1999) Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96:25–34
Kuhn H, Palmer TD, Fuchs E (2001) Adult neurogenesis: a compensatory mechanism for neuronal damage. Eur Arch Psychiatry Clin Neurosci 251:152–158
Levine JM, Reynoldsand R, Fawcett JW (2001) The oligodendrocyte precursor cell in health and disease. Trends Neurosci 24:39–47
Marshall CAG, Novitch BG, Goldman JE (2005) Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci 25(32):7289–7298
Mason JL, Toews A, Hostettler JD, Morell P, Suzuki K, Goldman JE, Matsushima GK (2004) Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. Am J Pathol 164(5):1673–1682
McDonald WIMAR (1999) Multiple sclerosis: the disease and its manifestations. Phil Trans R Soc B Biol Sci 354(1390):1615–1622
Messersmith DJ, Murtie JC, Le TQ, Frost EE, Armstrong RC (2000) Fibroblast growth factor 2 (FGF2) and fgf receptor expression in an experimental demyelinating disease with extensive remyelination. J Neurosci Res 62:241–256
Mozafari S, Sherafat MA, Javan M, Mirnajafi-Zadeh J, Tiraihi T (2010) Visual evoked potentials and MBP gene expression imply endogenous myelin repair in adult rat optic nerve and chiasm following local lysolecithin induced demyelination. Brain Res 1351:50–56
Mozafari S, Javan M, Sherafat MA, Mirnajafi-Zadeh J, Heibatollahi M, Pour-Beiranvand S, Tiraihi T, Ahmadiani A (2011) Analysis of structural and molecular events associated with adult rat optic chiasm and nerves demyelination and remyelination; possible role for 3 rd ventricle proliferating cells. NeuroMol Med 13:138–150
Murtie JC, Zhou Y, Le TQ, Vana AC, Armstronga RC (2005) PDGF and FGF2 pathways regulate distinct oligodendrocyte lineage responses in experimental demyelination with spontaneous remyelination. Neurobiol Dis 19:171–182
Ois Lachapelle F, Avellana-Adalid V, Nait-Oumesmar B, Baron-Van EA (2002) Fibroblast growth factor-2 (FGF-2) and platelet-derived growth factor AB (PDGFAB) promote adult SVZ-derived oligodendrogenesis in vivo. Mol Cell Neurosci 20:390–403
Paxinos G, Franklin KBJ (2004) The mouse brain in stereotaxic coordinates, 2nd edn. Academic, San Diego
Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Baron-Van EA (2002) Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A 99(20):13211–13216
Soto A, Pérez-Samartín AL, Etxebarria E, Matute C (2004) Excitotoxic insults to the optic nerve alter visual evoked potentials. Neuroscience 123(2):441–449
Stangel M, Hartung H (2002) Remyelinating strategies for the treatment of multiple sclerosis. Prog Neurobiol 68:361–376
Sun L, Lee J, Fine HA (2004) Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest 113:1364–1374
Sun T, Hafler BP, Kaing S, Kitada M, Ligon KL, Widlund HR, Yuk DI, Stiles CD, Rowitch DH (2006) Evidence for motoneuron lineage-specific regulation of Olig2 in the vertebrate neural tube. Dev Biol 292(1):152–164
Vallstedt A, Klos JM, Ericson J (2005) Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 45:55–67
Woodruff RH, Franklin RJ (1999) Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide and complement/anti-galactocerebroside: a comparative study. Glia 25:216–228
You Y, Klistorner A, Thie J, Graham SL (2011) Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Invest Ophthalmol Vis Sci 52(9):6911–6918
Zhang S, Ge B, Duncan ID (1999) Adult brain retains the potential to generate oligodendroglial progenitors with extensive myelination capacity. Proc Natl Acad Sci U S A 96:4089–4094
Acknowledgments
This study was supported by grants from research affairs of Tarbiat Modares University and Iranian Network for Neuroscience Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dehghan, S., Javan, M., Pourabdolhossein, F. et al. Basic Fibroblast Growth Factor Potentiates Myelin Repair Following Induction of Experimental Demyelination in Adult Mouse Optic Chiasm and Nerves. J Mol Neurosci 48, 77–85 (2012). https://doi.org/10.1007/s12031-012-9777-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9777-6