Abstract
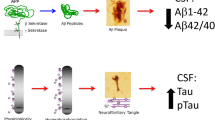
Reliable biomarkers for Alzheimer's disease (AD) are highly needed in the clinic. As a fluid surrounding the brain and reflecting the major neuropathological features characteristic to the AD brain, the cerebrospinal fluid (CSF) provides the natural source for AD biomarkers. The expected use of an ideal AD biomarker is for the following purposes: (1) diagnosis, (2) prediction, (3) monitoring of disease progression, and (4) drug discovery. Review of the literature revealed that CSF analysis, specifically amyloid-beta (Aβ42, total (T)-tau, and phosphorylated (P)-tau, are reliable markers for AD diagnosis, even at very early stages, particularly vs. healthy controls, while more limited evidence for distinguishing from other dementias. As for prediction, abnormal CSF markers are predictors of cognitive decline in healthy subjects, converting from MCI to development of AD, and of the rate of cognitive decline in mild AD. Regarding monitoring disease progression, the use of CSF biomarkers does not seem very promising since a comparison of the marker levels between baseline and following years of follow-up revealed a remarkable stability of biomarker levels in CSF. As for the use in drug discovery, it is estimated that using CSF markers for the selection of subjects for clinical trials may reduce robustly sample size and trial costs. Yet, since no effective drug is currently available, the contribution of CSF AD biomarkers in drug discovery cannot be currently fully assessed. Nevertheless, testing CSF for evidence of CNS inflammation may help safety monitoring in AD clinical trials. Factors affecting CSF biomarker levels that should be taken into account are assay variability as well as effects of age, gender, apoE and other genetic variations, education, and time of day. Much effort has been and is still being dedicated into developing and validating CSF AD biomarkers by many centers in the world. Identifying additional CSF components, reflecting not only the lesions characteristic to AD (plaques and tangles) but also more functional and structural brain parameters, may provide a wider profile of the changes taking place in AD brains, and be further used as reliable CSF biomarkers for AD monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Concomitantly with the intensive effort to develop a therapy for Alzheimer's disease (AD), there is an essential need for reliable disease-specific biomarkers in the clinic. It was well defined in the “Consensus report of the Working Group on Molecular and biochemical markers of AD” by The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group (1998) that “The ideal biomarker for AD should detect a fundamental feature of neuropathology and be validated in neuropathologically-confirmed cases; it should have a sensitivity of >80% for detecting AD and a specificity of >80% for distinguishing from other dementias; it should be reliable, non-invasive, simple to perform, and inexpensive.” Much research has been dedicated in the last decade trying to establish such disease-specific biomarkers that will fulfill the requested demands for reliable AD markers. In this review, I will focus on the relevant literature dealing with cerebrospinal fluid (CSF) biomarkers for AD.
As a fluid which is in direct contact with the brain, the CSF is believed to contain the richest pool of brain molecular components and therefore seems to be a natural source for biomarkers of AD-related brain pathology. There is accumulating evidence that the major neuropathological features characteristic of the AD brain are generally reflected in the CSF: higher amyloid plaque burden in brain is reflected as lower amyloid beta (Aβ) levels in CSF (Fagan et al. 2006; Tapiola et al. 2009; Strozyk et al. 2003; Fagan et al. 2009) and higher tangle burden as higher tau CSF levels (Tapiola et al. 1997; Buerger et al. 2006; Tapiola et al. 2009), yet with some controversies (Buerger et al. 2007; Engelborghs et al. 2007). The most studied CSF AD biomarkers (“core CSF markers”) are the Aβ42 peptide which represents the amyloid pathology, the total (T)-tau protein representing axonal injury and cell death, as well as the phosphorylated (P)-tau (mostly 181 and 231) markers for neurofibrillary tangle (NFT) pathology. These markers, and their ratio, show association with the regional distribution of NFTs according to the Braak staging (Braak and Braak 1996), and with the quantity of neuritic plaques according to the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) recommendations (Mirra et al. 1991), (Tapiola et al. 2009). Other markers, yet less studied, CSF components which may reflect additional disease pathogenic processes, are the inflammatory and oxidative stress markers as well as markers for blood–brain barrier (BBB) damage (Mattsson et al. 2009a).
As for the association of the CSF markers with the cognitive/clinical status, conflicting results have been reported. Few studies have reported an inverse correlation between CSF Aβ42 levels and clinical dementia severity (Riemenschneider et al. 2000; Csernansky et al. 2002), while others have shown no such association with CSF Aβ (Lin et al. 2009; Ivanoiu and Sindic 2005; Mehta et al. 2001); yet some studies have shown an apoE-dependent effect on an association (positive) of CSF Aβ42 levels with Mini-Mental State Examination (MMSE) (Riemenschneider et al. 2002b). There is more evidence for an inverse association of T-tau and the cognitive status (Tato et al. 1995; Kanai et al. 1998; Csernansky et al. 2002; Ivanoiu and Sindic 2005; Andersson et al. 2007; Lin et al. 2009; Samgard et al. 2010; Vemuri et al. 2010), yet conflicting results regarding P-tau181 (Buerger et al. 2002; Scheurich et al. 2010; Seppala et al. 2011). Other reports have shown that, specifically in mild cognitive impairment (MCI), but not in AD and controls, lower Aβ42 as well as higher T-tau and P-tau181 were associated with cognitive impairment (Mattsson et al. 2009b; Riepe et al. 2010). Recently, a distinct association between CSF biomarkers and memory has been suggested along the continuum from healthy subjects to AD patients: memory performance is related to Aβ42 levels in subjective memory complaints, while it is associated with tau in the prodromal stage of the disease (Antonell et al. 2010), with a similar trend also in familial AD patients [carriers of the presenilin 1 mutations]: CSF Aβ42 levels correlate with time to disease onset in asymptomatic mutation carriers to reach floor levels when symptoms appear, while CSF T-tau levels become elevated in symptomatic mutation carriers and correlate with clinical severity (Fortea et al. 2011). Recently, high-molecular-weight (HMW) Aβ oligomers showed a negative correlation with MMSE scores in the AD and MCI group (Fukumoto et al. 2010; Gao et al. 2010).
In the first part of this review, the expected use of an ideal biomarker, specifically the CSF-biomarkers, will be discussed. This will include the expected use of the markers for the following purposes: (1) diagnosis (early) of AD (discrimination from (a) non-demented subjects and (b) other dementias), (2) prediction (a—the risk of healthy subjects to develop dementia/AD; b—the risk of MCI patients to develop AD; c—the rate of cognitive decline in AD patients), (3) monitoring of disease progression, (4) drug discovery (a—selection of patients entering clinical trials; b—safety monitoring; c—theragnostics). In the second part, some confounding factors influencing the CSF markers will be presented. Finally, different approaches for the current use of CSF in clinic as well as future perspectives for CSF that would provide additional AD markers are discussed.
Part I: The Expected Use of the CSF AD Biomarkers
Diagnosis
Discrimination Between AD and Non-demented Individuals
A classical pattern of CSF markers comparing AD patients to controls (serious medical illnesses excluded) is reduced Aβ42 and increased T-tau levels in the CSF of AD patients (Motter et al. 1995; Galasko et al. 1998; Andreasen et al. 1999c; Hulstaert et al. 1999; Mehta et al. 2000; Riemenschneider et al. 2002a; Buerger et al. 2003; Sunderland et al. 2003; Zetterberg et al. 2003; Hampel et al. 2004a; Grossman et al. 2005; Herukka et al. 2005; Hansson et al. 2006; Bouwman et al. 2007a; Brys et al. 2009; Mattsson et al. 2009a; Mattsson et al. 2009b; Shaw et al. 2009), expectedly related to increase in amyloid plaque burden leaving lower levels of soluble Aβ42 to be secreted into CSF, and increase in neuronal injury/death leading to exposure of intracellular tau and its secretion into the CSF. While the vast majority of studies have shown a decrease of CSF Aβ42 levels in AD patients vs. controls, a small number of studies have shown no changes or even elevations of the protein levels (Nakamura et al. 1994; Nitsch et al. 1995; van Gool et al. 1995; Jensen et al. 1999), possibly related to the variable methods used and the sample sizes. Recently, significantly higher levels of soluble HMW Aβ oligomers have been detected in CSF samples from AD and MCI patients compared to age-matched controls (Fukumoto et al. 2010 ; Gao et al. 2010). A change in CSF levels in AD has been also detected in P-tau181, P-tau199, or P-tau231, showing an increase relative to controls (Buerger et al. 2002; Hampel et al. 2004b; Mattsson et al. 2009b), and a positive correlation has been reported in AD cases between T-tau and P-tau199 in CSF (Itoh et al. 2001), as well as a correlation between the different P-tau181, P-tau199, and P-tau231 epitopes (Hampel et al. 2004b). Recently, in a mixture modeling approach, independent of clinical AD diagnosis, an “AD signature” of CSF Aβ42/P-tau181 ratio has been suggested by De Meyer et al. (2010) and further validated in clinically diagnosed AD patients as well as in autopsy-confirmed AD cases.
Importantly, abnormalities in Aβ42, T-tau, and P-tau181 levels (but not in Aβ40) have been detected already in the early stages of AD (Galasko et al. 1998), even with clinical dementia rating (CDR) of 0.5 (Fagan et al. 2007).
Comparing CSF markers between patients with an atypical onset (non-memory) and those who are typical patients revealed a significantly higher level of CSF T-tau in the atypical patients (Koric et al. 2010).
Discrimination Between AD and Other Dementias
While CSF markers show clinical diagnostic value for the diagnosis of AD in discrimination from non-demented individuals, the use of these markers for discrimination from other dementias [such as dementia with Lewy bodies (DLB), Parkinson's disease dementia (PDD), vascular dementia (VD), and frontotemporal dementia (FTD)] seems to be more limited. Comparing the levels of Aβ42 in AD to those in non-AD dementias has shown conflicting results of either similar levels in DLB and AD (Parnetti et al. 2001; Gomez-Tortosa et al. 2003), higher in DLB and other non-AD dementias relative to AD (Kasuga et al. 2010), and even a lower level of Aβ42 in DLB (Parnetti et al. 2008). Higher Aβ42 levels have been reported in VD relative to AD and to their coexistence in the form of mixed dementia (Paraskevas et al. 2009). Higher Aβ42 levels have also been reported in FTD (Riemenschneider et al. 2002c; Grossman et al. 2005; Verbeek et al. 2005; Rosso et al. 2003; de Souza et al. 2011). Yet several reports indicate that Aβ peptide patterns reflect disease-specific pathophysiological pathways of different dementia syndromes and can help discriminate between them, such as the Aβ42/Aβ38 ratio (Mulugeta et al. 2010) or Aβ42/Aβ37 ratio (Bibl et al. 2006). In addition, the relative Aβ42 concentration [%, as compared to the sum of the peptides Aβ37-40 and oxidized Aβ40 (Aβ40ox)] as well as Aβ40ox% values have been suggested to discriminate between DLB and AD (Bibl et al. 2010).
Comparing the levels of CSF tau markers between AD and non-AD dementias revealed generally more consistent results showing a lower level in the non-AD relative to the AD dementia (yet with overlap). This includes lower T-tau and P-tau181 levels (Buerger et al. 2002; Gomez-Tortosa et al. 2003; Kasuga et al. 2010), specifically in DLB and PD (Parnetti et al. 2008) and also T-tau/P-tau181 ratio (Mollenhauer et al. 2006), as well as lower P-tau199 and P-tau231 (Hampel et al. 2004b) in non-AD dementias than in AD cases. Lower T-tau values relative to AD have also been reported specifically in FTD (Riemenschneider et al. 2002c; Grossman et al. 2005; Verbeek et al. 2005; Rosso et al. 2003; de Souza et al. 2011), yet the levels of T-tau were still higher in FTD than in healthy controls (Green et al. 1999; Fabre et al. 2001; Riemenschneider et al. 2002c; Rosso et al. 2003; Verbeek et al. 2005), while in other studies the levels were similar or even lower than in the controls (Sjogren et al. 2000; Urakami et al. 2001; Wallin et al. 2003; Grossman et al. 2005). While the vast majority of studies have shown lower T-tau levels in FTD, a report has suggested that FTD T-tau values were comparable with those in AD (Green et al. 1999). Also, lower P-tau181 (Rosso et al. 2003; Verbeek et al. 2005) and lower P-tau231 (Buerger et al. 2002) have been detected in FTD relative to AD. T-tau/P-tau ratio (and to some extent each of them separately) may contribute to the differential diagnosis of other dementias [higher T-tau/P-tau in Creutzfeldt–Jakob disease vs. AD (Hort et al. 2008); lower P-tau181, T-tau, and their ratio in normal pressure hydrocephalus (NHP) vs. AD (Kapaki et al. 2007)].
Prediction
The Risk of Healthy Subjects to Develop Dementia/AD
Individuals with high T-tau/Aβ42 and P-tau181/Aβ42 ratios were faster to display cognitive impairments (conversion from CDR=0 to CDR>0) compared with those who had lower ratios, in a longitudinal clinical follow-up of 1–8 years, pointing that CSF markers can be used as antecedent (preclinical) biomarkers that predict future dementia in cognitively normal older adults (>60 years of age) (Fagan et al. 2007). Further support for the predictive potential of these CSF markers in future deterioration stems from the finding that abnormalities in CSF Aβ42, T-tau, and mostly P-tau181 in normal individuals were associated with functional decline (Okonkwo et al. 2010). The “AD signature” of abnormal Aβ42/P-tau181 ratio in CSF, described by De Meyer et al. (2010), was unexpectedly present in more than one third of cognitively normal-aged subjects, suggesting that AD pathology is active and detectable earlier than any suspected cognitive impairment and may point that these individuals are at risk for developing AD, a prediction that is supported by the enrichment of apoE4 allele carriers among them.
MCI Conversion to AD
Comparing baseline CSF analytes of MCI patients converting to AD (MCI-AD) with those remaining stable (MCI-MCI) in studies of various time periods of follow-up (ranging from 1 to 9 years) revealed lower Aβ42 levels and higher T-tau levels, as well as their combinations in MCI-AD patients (Hampel et al. 2004a; Herukka et al. 2005; Li et al. 2007; Brys et al. 2009; Rolstad et al. 2009; Shaw et al. 2009). Higher P-tau231, P-tau231/Aβ42/40, as well as P-tau181 levels (Mattsson et al. 2009b), and P-tau181/Aβ42 at baseline were predictive of MCI-AD (Hansson et al. 2006; Ewers et al. 2007; Hertze et al. 2010 ; Landau et al. 2010). It has also been shown that actually all three P-tau epitopes associated with AD (181, 199, 231) predicted the rate of cognitive deterioration in the MCI group, yet combinations of these P-tau epitopes did not improve predictive accuracy (Buerger et al. 2005a). Combination of T-tau and Aβ42/P-tau181 ratio quintiles revealed that among MCI patients with biomarkers values in the highest quintile of T-tau, plus the lowest quintile of Aβ42/P-tau181 ratio, a high proportion were patients with incipient AD compared with patients with values in the opposite quintiles (Mattsson et al. 2009b). The “AD signature” of CSF Aβ42/P-tau181 ratio suggested by De Meyer et al. (2010) has shown to be more common among MCI-AD cases than a “healthy signature,” pointing to its predictive value. Yet in a recent study of the AD Neuroimaging Initiative (ADNI), it has been shown that Aβ abnormalities, but not tau alterations, were associated with cognitive deterioration and increased risk of conversion to AD dementia in patients with MCI (Okonkwo et al. 2011).
In addition to an association with cognitive decline, abnormalities in the CSF analytes in MCI cases showed an association with functional degradation (Okonkwo et al. 2010).
Comparing baseline CSF biomarkers in MCI-AD to those who developed other forms of dementia (MCI-other dementias) (VD, DLB and FTD) revealed higher CSF abnormalities (lower Aβ42, higher T-tau and P-tau181, and lower Aβ42/P-tau181 ratio) in the MCI-AD cases, yet the MCI-other dementias showed higher CSF marker abnormalities relative to controls (Hansson et al. 2006) (Mattsson et al. 2009b).
Cognitive Decline in AD
The rate of dementia progression in AD patients was reported to be significantly more rapid in individuals with lower baseline CSF Aβ42 levels, higher T-tau, or high T-tau/Aβ42 levels, with the P-tau-181/T-tau ratio suggested as the strongest predictor showing a dose-dependent effect (Kester et al. 2009). It has been also shown that the three CSF analytes (and their various combinations), in addition to being predictive of a faster rate of cognitive decline, are also predictive of a faster decline in the psychometric composite score (Snider et al. 2009). This was further supported by the worse clinical outcomes over time, including faster cognitive decline, as well as no response to cholinesterase inhibitor treatment, and a higher mortality reported in a subgroup of patients with AD with extreme levels of these three CSF biomarkers (Wallin et al. 2010). While the CSF analytes were predictive for functional decline in controls and MCI cases, these analytes did not predict further functional degradation in patients with AD (Okonkwo et al. 2010).
Monitoring Disease Progression
Most longitudinal studies of CSF levels of tau and Aβ have indicated a remarkable stability of the Aβ42 (Kanai et al. 1998; Andreasen et al. 1999c; Andreasen et al. 1999a; Mollenhauer et al. 2005; Bouwman et al. 2006; Blennow et al. 2007), and of the T-tau (Andreasen et al. 1999a; Andreasen et al. 1999b; Mollenhauer et al. 2005; Blennow et al. 2007) as well as of P-tau (Andreasen et al. 1999b; Mollenhauer et al. 2005; Blennow et al. 2007) in AD patients over an average interval of 6–21 months as well as in MCI-AD and those MCI cases staying stable for 24 months (Zetterberg et al. 2007). These results suggest that intra-individual levels of these biomarkers are remarkably stable over 2 years, and therefore these biomarkers do not seem to be reliable tools for monitoring disease progression. Interestingly, it has been reported that P-tau231 decreases linearly with time in AD patients, possibly reflecting the increasing sequestration of P-tau231 into the tangles, suggesting that P-tau231 becomes more insoluble rather than entering into the CSF (Hampel et al. 2001). This is further supported by a recent report suggesting that CSF P-tau181 decreases in correlation with cognitive functioning (Seppala et al. 2011).
Yet, while the vast majority of studies have reported stability, a few reports have pointed to a decrease in Aβ42 (Tapiola et al. 2000b), and an increase T-tau (Kanai et al. 1998), or increase of both Aβ42 and T-tau, but not in P-tau181 in AD, that was explained by the author to be attributed to the effect of sample storage conditions of the baseline samples (Bouwman et al. 2007b).
Drug Discovery
An important requirement for CSF biomarkers is to improve the likelihood of successful clinical trials for AD. This includes: enrichment of patient cohort entering clinical trials with pure AD patients, safety monitoring, and theragnostics.
Selection of Patients Entering Clinical Trials
CSF markers are needed to provide an accurate diagnosis in the selection of defined individuals entering clinical trials. For the treatment of AD patients, it would be desirable to enrich the patient cohort with pure AD cases and eliminate other diagnoses that might dilute treatment effects. In preventing/slowing down the progression of MCI to AD, it would also be desirable to select MCI subjects predicted to develop AD rather than including unselected MCI subjects. It has been recommended to use low Aβ42 and high T-tau:Aβ42 ratios in order to select MCI patients for clinical trials (Dubois et al. 2007; Shaw et al. 2009). It has been estimated that using CSF markers (specifically the combination of Aβ42 and T-tau) in the selection of subjects could reduce sample size by 67% and trial costs by 60% compared to a trial in which unselected subjects with MCI are enrolled (van Rossum et al. 2010). Nevertheless, in clinical trials simulations, it has been shown that the requirement of biomarker-positive patients would most likely not result in more efficient clinical trials and that trials would take longer to complete due to the scarcity of patients (Schneider et al. 2010). Therefore, in clinical trial settings, the balance between enrichment of screened in and loss of screened out patients should be critically considered (Lorenzi et al. 2010).
Safety Monitoring
Based on the encephalitis and vasogenic edema developed in AD patients participating in the amyloid-immunotherapy clinical trials, the capability of the CNS to provide evidence for inflammatory processes taking place in the CNS [(by increase in CSF mononuclear cells and intrathecal immunoglobulin production), as well as signs of BBB damage (Tibbling et al. 1977; Forsberg et al. 1984)], may be valuable in monitoring drug safety in AD clinical trials and preferable at a pre-symptomatic stage (Hampel et al. 2010).
Theragnostics
AD CSF markers are expected to provide information about the effect of tested drugs on the disease-specific processes taking place, particularly of disease-modifying therapies.
No change in levels of the CSF markers was detected under treatment with drugs which do not have a proven disease-modifying effect (specifically, cholinesterase inhibitors) (Blennow et al. 2007). As for disease-modifying candidate drugs, the metal–protein attenuating Aβ oligomerisation (PBT2) did show a decrease in CSF Aβ42 (Lannfelt et al. 2008) with no correlations with cognitive change (Faux et al. 2010). The glycosaminoglycan mimetic amyloid aggregation inhibitor (Tramiprosate, Alzhemed™) which reveals some evidence of a beneficial effect on cognition (Saumier et al. 2009) dose-dependently reduced CSF Aβ levels, with a greater reduction in mild AD subjects than in moderate AD participants (Aisen et al. 2007). Also, the amyloid precursor protein translation inhibitor (phenserine) showed a decrease in Aβ in CSF (Kadir et al. 2008). Yet, no change in CSF Aβ42 level was detected in clinical trials of the gamma-secretase inhibitor (LY450139) [(Fleisher et al. 2008), although a decrease in shorter Aβ isoforms was noticed (Portelius et al. 2010)]. Also, no change in CSF Aβ42 was detected in the interrupted phase IIa Aβ-immunotherapy trial (AN1792), despite some decrease in the CSF T-tau (Gilman et al. 2005). Testing the validity of CSF biomarkers in a meta-analysis, including observational studies, single-arm clinical trials, as well as randomized controlled trials involving AD patients, did not show an association between the CSF markers and dementia severity and did not yield conclusive results for proving that these markers serve as reliable surrogates/endpoints in early clinical trials in AD (Zhou et al. 2009). Yet, since no effective drug is currently available, the contribution of CSF AD biomarkers in drug discovery cannot be currently fully assessed. Several clinical trials with disease-modifying drugs using the CSF biomarkers as endpoints are currently ongoing.
Part II: CSF Markers—Important Considerations for Interpretation of Results (Confounding Factors)
In the context of result interpretation of the CSF marker analysis, various important factors influencing these marker levels should be taken into consideration. These are discussed in the following sections.
Assay Variability
CSF studies in AD patients and controls report different biomarker concentrations, reference ranges, and diagnostic cutoffs; in some studies, CSF marker levels in AD patients even exceeded the levels in controls in other studies (Blennow and Hampel 2003; Hort et al. 2010). The diverse cutoff values vary from 300 to 849 pg/ml for Aβ42, 195 to 450 pg/ml for T-tau, and 40 to 80 pg/ml for P-tau (Hort et al. 2010). Yet, the relative differences between the patients and controls in the different studies were generally consistent. Several multi-center studies have been conducted [such as the DESCRIPA study by Visser et al. (2009), the ADNI study by Shaw et al. (2009), the European-ADNI (E-ADNI) by Buerger et al. (2009), and the multi-center study of Mattsson et al. (2009b)], showing lower diagnostic accuracies than those of homogenous mono-center studies, which may be related to inter-center variations affecting the results, thereby blurring some effects of the AD CSF biomarkers. CSF biomarkers Aβ42, T-tau, and P-tau have very high diagnostic accuracy in a well-defined homogeneous mono-center population, demonstrating the excellent potency of CSF biomarkers to identify pathological processes in AD when a stringent analytical protocol is used (Johansson et al. 2011), but the inter-center variations make it complicated and it is even misleading to compare CSF biomarker levels between centers and studies. Possible reasons for such variations may be subject selection, CSF handling, CSF obtaining, and CSF storing (such as the type of test tubes, freeze/thaw procedures, plasma contamination, etc.), as well as analytical factors [different immunoassays (ELISA or multiplex techniques), batch-to-batch variations, and different reagents] (Buerger et al. 2009; Bjerke et al. 2010), with the variation usually being larger for Aβ42 than for T-tau and P-tau protein (Lewczuk et al. 2006; Verwey et al. 2010)]. To overcome these drawbacks, an international quality control program for CSF AD biomarkers has been started last year, run by the Alzheimer’s Association and administered from the Clinical Neurochemistry Laboratory in Molndal. Participating laboratories receive CSF samples for analysis with recommended guidelines for lumbar puncture (LP) and sample handling and storage.
Age
Various studies have pointed to an age effect on CSF markers, particularly to the effect on T-tau in physiologically normal subjects (Blomberg et al. 2001; Itoh et al. 2001; Sjogren et al. 2001; Briani et al. 2002; Glodzik-Sobanska et al. 2009) (Scheurich et al. 2010; Vemuri et al. 2010) and on P-tau181 and P-tau231 (Hampel et al. 2004b; Glodzik-Sobanska et al. 2009); subsequently, age-adjusted norms have been recommended (Sjogren et al. 2001), yet no correlation of T-tau (Lewczuk et al. 2004; Yakushev et al. 2010), P-tau181 (Lewczuk et al. 2004; Scheurich et al. 2010; Yakushev et al. 2010), or P-tau231 (Buerger et al. 2003) with age has been detected in other studies. Association with age was also detected in other non-dementia diseases (Andreasen et al. 1999b; Scheurich et al. 2010). Interestingly, no effect of age on T-tau (Andreasen et al. 1999b; Briani et al. 2002; Lewczuk et al. 2004) and of P-tau181/P-tau231 (Hampel et al. 2004b) was detected in AD patients, possibly pointing that some neuronal damage is normally taking place with age, but when prominent neuronal death is taking place like in AD, this physiological age-dependent variation is less pronounced. This may point that age adjustment is not actually needed in AD diagnosis. While CSF levels of Aβ42 seem to be unrelated to age (Kunicki et al. 1998; Maruyama et al. 2001; Sjogren et al. 2001), some effect of age has been recently demonstrated on the Aβ42 levels in apoE4 carriers, in a way that carrying the apoE4 allele was associated with a significant decrease in the Aβ42 concentrations of middle-aged and older cognitively normal individuals (Popp et al. 2010).
Gender
While many studies have shown no gender difference in tau levels (T-tau and P-tau181) (Blomberg et al. 2001; Itoh et al. 2001; Schoonenboom et al. 2004; Scheurich et al. 2010; Yakushev et al. 2010) and in Aβ levels (Galasko et al. 1998; Kunicki et al. 1998; Mehta et al. 2000; Schoonenboom et al. 2004), others reported that T-tau level was associated with male sex in AD patients (Galasko et al. 1998) and that gender was significantly associated with total Aβ levels (Kauwe et al. 2009). Some gender-specific differences were reported in a correlation of T-tau and P-tau181 levels with specific cognitive tasks [the California Verbal Learning Test score in females and Wechsler Memory Scale score in males] in MCI, but not in the controls or patients with AD (Riepe et al. 2010).
Genetic Variations
ApoE
An apoE4 allele dose effect on CSF Aβ42 levels showing a significant decrease of CSF-Aß42 levels with rising numbers of apoE4 alleles was detected within AD patients (Galasko et al. 1998; Riemenschneider et al. 2000; Tapiola et al. 2000a; Sunderland et al. 2004; Shaw et al. 2009; Vemuri et al. 2010) in a demented population (Ganzer et al. 2003), in MCI (Shaw et al. 2009; Vemuri et al. 2010), and even in healthy controls (Prince et al. 2004; Sunderland et al. 2004; Shaw et al. 2009; Vemuri et al. 2010), with the proportion of the variability in Aβ42 levels explained by apoE4 dose being significantly greater than the proportion of the variability explained by clinical parameters (Vemuri et al. 2010). In contrast, more data inconsistency exists regarding the effect of apoE on tau: some results have shown higher T-tau levels among AD patients positive for apoE4 than in those without an E4 allele (Golombowski et al. 1997; Tapiola et al. 2000a; Ganzer et al. 2003), while in other studies, no such effect was detected (Galasko et al. 1998; Lasser et al. 1998; Andreasen et al. 2001; Sunderland et al. 2004). An apoE4 effect also on P-tau231, with higher levels in apoE4 carriers compared to non-carriers, was reported in MCI, but not in AD and healthy controls (Buerger et al. 2005b). Investigating the possibility that apoE status affects the relationship between cognitive function and the CSF biomarkers revealed contradictory results (Andersson et al. 2007); (Riemenschneider et al. 2002b).
Some support for the effect of apoE genotype on CSF markers may come from the finding that the cognitively normal aged subjects who had a positive “AD signature” in CSF were enriched in apoE4 allele (De Meyer et al. 2010).
Others
Aβ-Associated
Investigating single nucleotide polymorphisms (SNPs) positive in AlzGene (http://www.alzgene.org) meta-analyses of AD genetic association studies (Bertram et al. 2007), for possible association with CSF Aβ levels, revealed that not only apoE alleles but also alleles of angiotensin-converting enzyme, brain-derived neurotrophic factor, death-associated protein kinase 1, and transferrin showed a significant association with CSF Aβ levels (Kauwe et al. 2009). Also, an association between a distinct haplotype of PSEN2 with higher CSF Aβ42 concentrations, as well as lower Aβ42 concentrations with a different haplotype in this gene, was reported (Lebedeva et al. 2010).
Tau-Associated
Alleles of several SNPs in the tau microtubule-associated protein gene that show an association with increased CSF T-tau and P-tau levels have been identified, specifically in individuals with evidence of Aβ deposition. These alleles have shown an association with an earlier age at onset but had no effect on risk for AD (Kauwe et al. 2008).
Recent studies screening SNP in genes related to the tau protein metabolism showed an association of a SNP in a tau phosphatase (PPP3R1) with P-tau181 levels in CSF, particularly with rate of progression of AD, but not risk for AD or age at onset (Cruchaga et al. 2010).
Education
It has been shown that MCI-MCI patients were better educated, performed better cognitively, and had higher Aβ42 levels and lower levels of T-tau relative to MCI-AD. Interestingly, MCI-AD patients with higher education had lower levels of Aβ42 and performed equally in neuropsychological tests compared to those with lower education (Rolstad et al. 2009). Similarly, Aβ42 was inversely associated with years of education, mainly present in mild form of the disease (CDR1), and was attenuated in more advanced forms of the disease. There was no significant relation between CSF T-tau or P-Tau181 levels and education levels. These results are consistent with the cognitive reserve theory, suggesting that cognitive reserve may be protective against brain pathology-related cognitive impairment, particularly amyloid plaques, at the onset of the clinical dementia (Dumurgier et al. 2010), as well as with the report that education modifies the association of amyloid, but not of tangles, with cognitive function (Bennett et al. 2005).
Diurnal Effect
Investigating the time course of human CSF Aβ levels over hours in non-demented participants who had CSF sampled hourly for up to 36 h revealed a significant variation in Aβ40 and Aβ42 levels of 1.5- to fourfold over 36 h, pointing to fluctuations of Aβ levels, which appear to be time of day or activity dependent (Bateman et al. 2007).
Discussion
Routine Analysis of CSF Biomarkers in Clinic—a Useful Tool but Still Not in Full Consensus
As fulfilling at least several of the requirements of a successful biomarker for AD: reflecting the AD brain pathology, providing good accurate in AD diagnosis and prediction of cognitive decline and AD development, as well as being non-invasive and non-expensive (yet less informative in discrimination from other dementias and in disease progression monitoring), the Aβ42, T-tau, and P-tau proteins in CSF seem valuable diagnostic/predictive tools in the clinic. Despite their value, and despite the large number of people affected by this disease and many others at risk, and despite the availability of user-friendly commercial assays, these CSF tests are not used routinely in clinics today. Debates yielding different views are expressed regarding the usefulness or rather the uselessness of the CSF AD tests, with an approach claiming that neurologists should “sharpen the needle” for LP (Herskovits and Growdon 2010), while a different view claiming that “for now: neurologists should keep a LP tray handy, but they needn’t stock up” (Wilner 2010). The value of CSF analysis in the diagnosis of AD is challenged as the clinical diagnosis accuracy of probable AD according to NINCDS-ADRDA criteria is quite high, exceeding 85% (confirmed by autopsy) (Geldmacher and Whitehouse 1997), speculating how much additional accuracy can be added by positive CSF analysis in patients with clinically evident AD? Yet, CSF profiling has been suggested to aid in the diagnosis of AD, according to the revised guidelines for the diagnosis of AD proposed by Dubois et al. (2007, 2010) as follows: a combination of a core diagnosis criterion (gradual, slowly progressive episodic memory impairment that is documented on formal testing), with at least one supporting feature from among four possible in vivo measurements (presence of medial temporal lobe atrophy on MRI, abnormal CSF profile, reduced glucose metabolism in bilateral temporal parietal, or a proven autosomal dominant genetic mutation), is required to diagnose AD. However, the DESCRIPA study suggested that criteria requiring MRI scanning and CSF sampling will be difficult to apply as part of a regular routine (Visser et al. 2008). Interestingly, lack of accuracy for the proposed “Dubois Criteria” in AD has been reported in a study aimed to compare the concordance in the diagnosis of AD using a broad clinical approach with access to biomarkers as proposed by Dubois et al. (2007), with clinical evaluation without access to such, using the definitions of the traditional disease classification systems ICD-10/DSM-IV for dementia and NINCDS-ANRDA for AD. These authors suggested that this discrepancy may be related to the young age of their study population and to the lack of established cutoff levels of AD CSF markers (Oksengard et al. 2010).
As for the use in early diagnosis (as well as prediction of cognitive decline and development of AD), this test can allow a person more autonomy in planning his/her future; however, it may result in negative psychological consequences in an otherwise well-functioning person. Therefore, pros and cons of early diagnosis must be carefully weighed in each individual prior to a confirmatory test (Wilner 2010). However, it is important to keep in mind that the interpretation of the CSF tests should be made in the wide context of the clinical symptoms (AD vs. other dementias) as well as of the various confounding factors (apoE4, education, etc.). Currently accepted indications for CSF testing include patients who present with diagnostic dilemmas (specifically NPH or depression—where treatment is possible), for counseling patients about lifestyle changes (work, driving), or where diagnosis will provide more relief than worry. In the future, when there are more effective drugs, the CSF analysis may be recommended as a screening test to identify healthy individuals at risk for MCI and AD.
CSF as a Source for AD Biomarkers—a Not Yet Fully Fulfilled Potential
A possible explanation for the limitations in using the CSF “core CSF markers” (Aβ42, T-tau, and P-tau), specifically in monitoring disease progression, is that these markers represent the pathological lesions of the disease, i.e., plaques and tangles, which seem to reach a plateau at a relatively early stage of the disease and stay relatively constant, not changing with disease progression. It is possible that CSF components representing more functional and structural changes in brain rather than the physical lesions may offer a superior depiction of the clinical symptoms characteristic of disease progression. A good example for functional parameters, rather than core pathological ones, being correlative with disease symptoms is the finding that educated people are less cognitively impaired than low educated people with the same amyloid burden in brain and CSF (Rolstad et al. 2009), a phenomenon explained as being attributed to brain cognitive reserve; this concept embodying a situation of increased neuronal connectivity in brain regions involved in learning and memory, which is believed to occur in response to cognitive stimulation (such as education), can compensate and withstand the detrimental symptoms of clinical dementia before they are manifested. This may suggest that functional parameters rather than the lesions per se better represent the cognitive status. While the AD core markers can be informative in diagnosing (presence of amyloid and tau pathology) or predicting who is prone to develop the disease (since pathology develops years before symptoms appear), functional and structural markers may represent the symptoms better and allow staging and monitoring of disease progression. While so far CSF has been intensively investigated mostly for amyloid and tau proteins, much effort is also needed to identify functional or structural CSF markers for the disease. Such potential CSF markers may be metabolites or proteins that represent a decrease in glucose metabolism, reduced levels of proteins expressed specifically in the hippocampus or those involved in synaptic activity, etc. Such changes in functional parameters are currently studied by imaging technologies—PET with 18-fluoro-deoxyglucose (for glucose metabolism), SPECT (for brain perfusion), and functional MRI (for neuronal connectivity)—and have been recently reported to be associated with disease progression or staging (Schöll et al. 2011; Hanyu et al. 2010; Kume et al. 2011; Tripoliti et al. 2011). Changes in structural parameters are tested by MRI (such as cortical thickness and hippocampal volume) and have been shown to progress with disease progression, while amyloid and tau in CSF did not (Vemuri et al. 2010). By virtue of being a fluid surrounding the brain and a direct source of brain proteins, the CSF has the potential to provide a wide battery of functional/structural markers which may aid in AD diagnosis and particularly in disease monitoring; moreover, CSF has the advantage of being non-expensive to analyze and easy to handle by biochemical/immunological methods in standard laboratories. Ongoing advanced proteomics of CSF samples may lead to the identification of such new markers, which with further validation may become part of routine analysis.
Summary
Aβ42, T-tau, and P-tau (“AD core markers”) in CSF provide good accuracy in AD diagnosis, including early diagnosis, as well as in predicting cognitive decline and AD development, and have the advantage of being non-invasive and non-expensive. Yet, these markers seem to be less informative in discrimination from other dementias and are particularly less effective in monitoring disease progression. To overcome inter-center variations, an international quality control program for CSF AD biomarkers has been established by the Alzheimer’s Association. Additional factors affecting these CSF AD markers are: apoE and other genetic factors, education, and time of day. As a fluid representing the brain proteins and metabolites, the CSF may provide not only markers of the AD core pathology (plaques and tangles) but also additional AD biomarkers, such as those representing functional parameters of the brain, thus affording a wider profile of the changes taking place in AD brains, and be further used as reliable CSF biomarkers for AD monitoring.
References
Aisen PS, Gauthier S, Vellas B, Briand R, Saumier D, Laurin J, Garceau D (2007) Alzhemed: a potential treatment for Alzheimer's disease. Curr Alzheimer Res 4:473–478
Andersson C, Blennow K, Johansson SE, Almkvist O, Engfeldt P, Lindau M, Eriksdotter-Jonhagen M (2007) Differential CSF biomarker levels in APOE-epsilon4-positive and -negative patients with memory impairment. Dement Geriatr Cogn Disord 23:87–95
Andreasen N, Minthon L, Vanmechelen E, Vanderstichele H, Davidsson P, Winblad B, Blennow K (1999a) Cerebrospinal fluid tau and Abeta42 as predictors of development of Alzheimer's disease in patients with mild cognitive impairment. Neurosci Lett 273:5–8
Andreasen N, Minthon L, Clarberg A, Davidsson P, Gottfries J, Vanmechelen E, Vanderstichele H, Winblad B, Blennow K (1999b) Sensitivity, specificity, and stability of CSF-tau in AD in a community-based patient sample. Neurology 53:1488–1494
Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, Vanderstichele H, Vanmechelen E, Blennow K (1999c) Cerebrospinal fluid beta-amyloid(1–42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol 56:673–680
Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, Blennow K (2001) Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 58:373–379
Antonell A, Fortea J, Rami L, Bosch B, Balasa M, Sanchez-Valle R, Iranzo A, Molinuevo JL, Llado A (2010) Different profiles of Alzheimer's disease cerebrospinal fluid biomarkers in controls and subjects with subjective memory complaints. J Neural Transm 118:259–262
Bateman RJ, Wen G, Morris JC, Holtzman DM (2007) Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology 68:666–669
Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE (2005) Education modifies the association of amyloid but not tangles with cognitive function. Neurology 65:953–955
Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE (2007) Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet 39:17–23
Bibl M, Mollenhauer B, Esselmann H, Lewczuk P, Klafki HW, Sparbier K, Smirnov A, Cepek L, Trenkwalder C, Ruther E, Kornhuber J, Otto M, Wiltfang J (2006) CSF amyloid-beta-peptides in Alzheimer's disease, dementia with Lewy bodies and Parkinson's disease dementia. Brain 129:1177–1187
Bibl M, Esselmann H, Lewczuk P, Trenkwalder C, Otto M, Kornhuber J, Wiltfang J, Mollenhauer B (2010) Combined analysis of CSF tau, Abeta42, Abeta1-42% and Abeta1-40% in Alzheimer's disease, dementia with Lewy bodies and Parkinson's disease dementia. Int J Alzheimers Dis. doi:10.4061/2010/761571
Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsater H, Anckarsater R, Andreasen N, Zetterberg H, Andreasson U, Blennow K (2010) Confounding factors influencing amyloid Beta concentration in cerebrospinal fluid. Int J Alzheimers Dis doi:10.4061/2010/986310
Blennow K, Hampel H (2003) CSF markers for incipient Alzheimer's disease. Lancet Neurol 2:605–613
Blennow K, Zetterberg H, Minthon L, Lannfelt L, Strid S, Annas P, Basun H, Andreasen N (2007) Longitudinal stability of CSF biomarkers in Alzheimer's disease. Neurosci Lett 419:18–22
Blomberg M, Jensen M, Basun H, Lannfelt L, Wahlund LO (2001) Cerebrospinal fluid tau levels increase with age in healthy individuals. Dement Geriatr Cogn Disord 12:127–132
Bouwman FH, van der Flier WM, Schoonenboom NS, van Elk EJ, Kok A, Scheltens P, Blankenstein MA (2006) Usefulness of longitudinal measurements of beta-amyloid1-42 in cerebrospinal fluid of patients with various cognitive and neurologic disorders. Clin Chem 52:1604–1606
Bouwman FH, Schoonenboom SN, van der Flier WM, van Elk EJ, Kok A, Barkhof F, Blankenstein MA, Scheltens P (2007a) CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol Aging 28:1070–1074
Bouwman FH, van der Flier WM, Schoonenboom NS, van Elk EJ, Kok A, Rijmen F, Blankenstein MA, Scheltens P (2007b) Longitudinal changes of CSF biomarkers in memory clinic patients. Neurology 69:1006–1011
Braak H, Braak E (1996) Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol 92:197–201
Briani C, Ruggero S, Naccarato M, Cagnin A, Ricchieri GL, Pasqui L, Pizzolato G, Battistin L (2002) Combined analysis of CSF betaA42 peptide and tau protein and serum antibodies to glycosaminoglycans in Alzheimer's disease: preliminary data. J Neural Transm 109:393–398
Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, Glodzik-Sobanska L, De Santi S, Zinkowski R, Mehta P, Pratico D, Saint Louis LA, Wallin A, Blennow K, de Leon MJ (2009) Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging 30:682–690
Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, Andreasen N, Hofmann-Kiefer K, DeBernardis J, Kerkman D, McCulloch C, Kohnken R, Padberg F, Pirttila T, Schapiro MB, Rapoport SI, Moller HJ, Davies P, Hampel H (2002) Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol 59:1267–1272
Buerger K, Zinkowski R, Teipel SJ, Arai H, DeBernardis J, Kerkman D, McCulloch C, Padberg F, Faltraco F, Goernitz A, Tapiola T, Rapoport SI, Pirttila T, Moller HJ, Hampel H (2003) Differentiation of geriatric major depression from Alzheimer's disease with CSF tau protein phosphorylated at threonine 231. Am J Psychiatry 160:376–379
Buerger K, Ewers M, Andreasen N, Zinkowski R, Ishiguro K, Vanmechelen E, Teipel SJ, Graz C, Blennow K, Hampel H (2005a) Phosphorylated tau predicts rate of cognitive decline in MCI subjects: a comparative CSF study. Neurology 65:1502–1503
Buerger K, Teipel SJ, Zinkowski R, Sunderland T, Andreasen N, Blennow K, Ewers M, DeBernardis J, Shen Y, Kerkman D, Du Y, Hampel H (2005b) Increased levels of CSF phosphorylated tau in apolipoprotein E epsilon4 carriers with mild cognitive impairment. Neurosci Lett 391:48–50
Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H (2006) CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain 129:3035–3041
Buerger K, Alafuzoff I, Ewers M, Pirttila T, Zinkowski R, Hampel H (2007) No correlation between CSF tau protein phosphorylated at threonine 181 with neocortical neurofibrillary pathology in Alzheimer's disease. Brain 130:e82
Buerger K, Frisoni G, Uspenskaya O, Ewers M, Zetterberg H, Geroldi C, Binetti G, Johannsen P, Rossini PM, Wahlund LO, Vellas B, Blennow K, Hampel H (2009) Validation of Alzheimer's disease CSF and plasma biological markers: the multicentre reliability study of the pilot European Alzheimer's Disease Neuroimaging Initiative (E-ADNI). Exp Gerontol 44:579–585
Cruchaga C, Kauwe JS, Mayo K, Spiegel N, Bertelsen S, Nowotny P, Shah AR, Abraham R, Hollingworth P, Harold D, Owen MM, Williams J, Lovestone S, Peskind ER, Li G, Leverenz JB, Galasko D, Morris JC, Fagan AM, Holtzman DM, Goate AM (2010) SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer's disease. PLoS Genet 6
Csernansky JG, Miller JP, McKeel D, Morris JC (2002) Relationships among cerebrospinal fluid biomarkers in dementia of the Alzheimer type. Alzheimer Dis Assoc Disord 16:144–149
De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, Blennow K, Shaw L, Trojanowski JQ (2010) Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol 67:949–956
de Souza LC, Lamari F, Belliard S, Jardel C, Houillier C, De Paz R, Dubois B, Sarazin M (2011) Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer's disease from other cortical dementias. J Neurol Neurosurg Psychiatry 82:240–246
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–746
Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O'Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P (2010) Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol 9:1118–1127
Dumurgier J, Paquet C, Benisty S, Kiffel C, Lidy C, Mouton-Liger F, Chabriat H, Laplanche JL, Hugon J (2010) Inverse association between CSF Abeta 42 levels and years of education in mild form of Alzheimer's disease: the cognitive reserve theory. Neurobiol Dis 40:456–459
Engelborghs S, Sleegers K, Cras P, Brouwers N, Serneels S, De Leenheir E, Martin JJ, Vanmechelen E, Van Broeckhoven C, De Deyn PP (2007) No association of CSF biomarkers with APOEepsilon4, plaque and tangle burden in definite Alzheimer's disease. Brain 130:2320–2326
Ewers M, Buerger K, Teipel SJ, Scheltens P, Schroder J, Zinkowski RP, Bouwman FH, Schonknecht P, Schoonenboom NS, Andreasen N, Wallin A, DeBernardis JF, Kerkman DJ, Heindl B, Blennow K, Hampel H (2007) Multicenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCI. Neurology 69:2205–2212
Fabre SF, Forsell C, Viitanen M, Sjogren M, Wallin A, Blennow K, Blomberg M, Andersen C, Wahlund LO, Lannfelt L (2001) Clinic-based cases with frontotemporal dementia show increased cerebrospinal fluid tau and high apolipoprotein E epsilon4 frequency, but no tau gene mutations. Exp Neurol 168:413–418
Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM (2006) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 59:512–519
Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM (2007) Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64:343–349
Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, Marcus D, Morris JC, Holtzman DM (2009) Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med 1:371–380
Faux NG, Ritchie CW, Gunn A, Rembach A, Tsatsanis A, Bedo J, Harrison J, Lannfelt L, Blennow K, Zetterberg H, Ingelsson M, Masters CL, Tanzi RE, Cummings JL, Herd CM, Bush AI (2010) PBT2 rapidly improves cognition in Alzheimer's disease: additional phase II analyses. J Alzheimers Dis 20:509–516
Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, Farlow MR, Galvin JE, Peskind ER, Quinn JF, Sherzai A, Sowell BB, Aisen PS, Thal LJ (2008) Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol 65:1031–1038
Forsberg P, Henriksson A, Link H, Ohman S (1984) Reference values for CSF-IgM, CSF-IgM/S-IgM ratio and IgM index, and its application to patients with multiple sclerosis and aseptic meningoencephalitis. Scand J Clin Lab Invest 44:7–12
Fortea J, Llado A, Bosch B, Antonell A, Oliva R, Molinuevo JL, Sanchez-Valle R (2011) Cerebrospinal fluid biomarkers in Alzheimer’s disease families with PSEN1 mutations. Neurodegener Dis 8:202–207
Fukumoto H, Tokuda T, Kasai T, Ishigami N, Hidaka H, Kondo M, Allsop D, Nakagawa M (2010) High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J 24:2716–2726
Galasko D, Chang L, Motter R, Clark CM, Kaye J, Knopman D, Thomas R, Kholodenko D, Schenk D, Lieberburg I, Miller B, Green R, Basherad R, Kertiles L, Boss MA, Seubert P (1998) High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol 55:937–945
Ganzer S, Arlt S, Schoder V, Buhmann C, Mandelkow EM, Finckh U, Beisiegel U, Naber D, Muller-Thomsen T (2003) CSF-tau, CSF-Abeta1-42, ApoE-genotype and clinical parameters in the diagnosis of Alzheimer's disease: combination of CSF-tau and MMSE yields highest sensitivity and specificity. J Neural Transm 110:1149–1160
Gao CM, Yam AY, Wang X, Magdangal E, Salisbury C, Peretz D, Zuckermann RN, Connolly MD, Hansson O, Minthon L, Zetterberg H, Blennow K, Fedynyshyn JP, Allauzen S (2010) Abeta40 oligomers identified as a potential biomarker for the diagnosis of Alzheimer's disease. PLoS One 5:e15725
Geldmacher DS, Whitehouse PJ Jr (1997) Differential diagnosis of Alzheimer's disease. Neurology 48:S2–S9
Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM (2005) Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64:1553–1562
Glodzik-Sobanska L, Pirraglia E, Brys M, de Santi S, Mosconi L, Rich KE, Switalski R, Saint Louis L, Sadowski MJ, Martiniuk F, Mehta P, Pratico D, Zinkowski RP, Blennow K, de Leon MJ (2009) The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer's disease. Neurobiol Aging 30:672–681
Golombowski S, Muller-Spahn F, Romig H, Mendla K, Hock C (1997) Dependence of cerebrospinal fluid Tau protein levels on apolipoprotein E4 allele frequency in patients with Alzheimer's disease. Neurosci Lett 225:213–215
Gomez-Tortosa E, Gonzalo I, Fanjul S, Sainz MJ, Cantarero S, Cemillan C, Yebenes JG, del Ser T (2003) Cerebrospinal fluid markers in dementia with Lewy bodies compared with Alzheimer disease. Arch Neurol 60:1218–1222
Green AJ, Harvey RJ, Thompson EJ, Rossor MN (1999) Increased tau in the cerebrospinal fluid of patients with frontotemporal dementia and Alzheimer's disease. Neurosci Lett 259:133–135
Grossman M, Farmer J, Leight S, Work M, Moore P, Van Deerlin V, Pratico D, Clark CM, Coslett HB, Chatterjee A, Gee J, Trojanowski JQ, Lee VM (2005) Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer's disease. Ann Neurol 57:721–729
Group TRaNRRIotAsAatNIoAW (1998) Consensus report of the Working Group on: "Molecular and Biochemical Markers of Alzheimer's Disease". Neurobiol Aging 19:109–116
Hampel H, Buerger K, Kohnken R, Teipel SJ, Zinkowski R, Moeller HJ, Rapoport SI, Davies P (2001) Tracking of Alzheimer's disease progression with cerebrospinal fluid tau protein phosphorylated at threonine 231. Ann Neurol 49:545–546
Hampel H, Teipel SJ, Fuchsberger T, Andreasen N, Wiltfang J, Otto M, Shen Y, Dodel R, Du Y, Farlow M, Moller HJ, Blennow K, Buerger K (2004a) Value of CSF beta-amyloid1-42 and tau as predictors of Alzheimer's disease in patients with mild cognitive impairment. Mol Psychiatry 9:705–710
Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, Sjoegren M, DeBernardis J, Kerkman D, Ishiguro K, Ohno H, Vanmechelen E, Vanderstichele H, McCulloch C, Moller HJ, Davies P, Blennow K (2004b) Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry 61:95–102
Hampel H, Shen Y, Walsh DM, Aisen P, Shaw LM, Zetterberg H, Trojanowski JQ, Blennow K (2010) Biological markers of amyloid beta-related mechanisms in Alzheimer's disease. Exp Neurol 223:334–346
Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L (2006) Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5:228–234
Hanyu H, Sato T, Hirao K, Kanetaka H, Iwamoto T, Koizumi K (2010) The progression of cognitive deterioration and regional cerebral blood flow patterns in Alzheimer’s disease: a longitudinal SPECT study. J Neurol Sci 290:96–101
Herskovits AZ, Growdon JH (2010) Sharpen that needle. Arch Neurol 67:918–920
Hertze J, Minthon L, Zetterberg H, Vanmechelen E, Blennow K, Hansson O (2010) Evaluation of CSF biomarkers as predictors of Alzheimer's disease: a clinical follow-up study of 4.7 years. J Alzheimers Dis 21:1119–1128
Herukka SK, Hallikainen M, Soininen H, Pirttila T (2005) CSF Abeta42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Neurology 64:1294–1297
Hort J, Valis M, Waberzinek G, Talab R, Glossova L, Bojar M, Vyhnalek M, Skoda D, Masopust J, Stourac P (2008) Proportion of tau protein to phosphorylated tau protein CSF levels in differential diagnosis of dementia. Nervenarzt 79:891–892, 894–896, 898
Hort J, Bartos A, Pirttila T, Scheltens P (2010) Use of cerebrospinal fluid biomarkers in diagnosis of dementia across Europe. Eur J Neurol 17:90–96
Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, Bancher C, Cras P, Wiltfang J, Mehta PD, Iqbal K, Pottel H, Vanmechelen E, Vanderstichele H (1999) Improved discrimination of AD patients using beta-amyloid(1–42) and tau levels in CSF. Neurology 52:1555–1562
Itoh N, Arai H, Urakami K, Ishiguro K, Ohno H, Hampel H, Buerger K, Wiltfang J, Otto M, Kretzschmar H, Moeller HJ, Imagawa M, Kohno H, Nakashima K, Kuzuhara S, Sasaki H, Imahori K (2001) Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer's disease. Ann Neurol 50:150–156
Ivanoiu A, Sindic CJ (2005) Cerebrospinal fluid TAU protein and amyloid beta42 in mild cognitive impairment: prediction of progression to Alzheimer's disease and correlation with the neuropsychological examination. Neurocase 11:32–39
Jensen M, Schroder J, Blomberg M, Engvall B, Pantel J, Ida N, Basun H, Wahlund LO, Werle E, Jauss M, Beyreuther K, Lannfelt L, Hartmann T (1999) Cerebrospinal fluid A beta42 is increased early in sporadic Alzheimer's disease and declines with disease progression. Ann Neurol 45:504–511
Johansson P, Mattsson N, Hansson O, Wallin A, Johansson JO, Andreasson U, Zetterberg H, Blennow K, Svensson J (2011) Cerebrospinal fluid biomarkers for Alzheimer’s disease: diagnostic performance in a homogeneous mono-center population. J Alzheimers Dis 2:537–546
Kadir A, Andreasen N, Almkvist O, Wall A, Forsberg A, Engler H, Hagman G, Larksater M, Winblad B, Zetterberg H, Blennow K, Langstrom B, Nordberg A (2008) Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer's disease. Ann Neurol 63:621–631
Kanai M, Matsubara E, Isoe K, Urakami K, Nakashima K, Arai H, Sasaki H, Abe K, Iwatsubo T, Kosaka T, Watanabe M, Tomidokoro Y, Shizuka M, Mizushima K, Nakamura T, Igeta Y, Ikeda Y, Amari M, Kawarabayashi T, Ishiguro K, Harigaya Y, Wakabayashi K, Okamoto K, Hirai S, Shoji M (1998) Longitudinal study of cerebrospinal fluid levels of tau, A beta1-40, and A beta1-42(43) in Alzheimer's disease: a study in Japan. Ann Neurol 44:17–26
Kapaki EN, Paraskevas GP, Tzerakis NG, Sfagos C, Seretis A, Kararizou E, Vassilopoulos D (2007) Cerebrospinal fluid tau, phospho-tau181 and beta-amyloid1-42 in idiopathic normal pressure hydrocephalus: a discrimination from Alzheimer's disease. Eur J Neurol 14:168–173
Kasuga K, Tokutake T, Ishikawa A, Uchiyama T, Tokuda T, Onodera O, Nishizawa M, Ikeuchi T (2010) Differential levels of alpha-synuclein, beta-amyloid42 and tau in CSF between patients with dementia with Lewy bodies and Alzheimer's disease. J Neurol Neurosurg Psychiatry 81:608–610
Kauwe JS, Cruchaga C, Mayo K, Fenoglio C, Bertelsen S, Nowotny P, Galimberti D, Scarpini E, Morris JC, Fagan AM, Holtzman DM, Goate AM (2008) Variation in MAPT is associated with cerebrospinal fluid tau levels in the presence of amyloid-beta deposition. Proc Natl Acad Sci USA 105:8050–8054
Kauwe JS, Wang J, Mayo K, Morris JC, Fagan AM, Holtzman DM, Goate AM (2009) Alzheimer's disease risk variants show association with cerebrospinal fluid amyloid beta. Neurogenetics 10:13–17
Kester MI, van der Vlies AE, Blankenstein MA, Pijnenburg YA, van Elk EJ, Scheltens P, van der Flier WM (2009) CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology 73:1353–1358
Koric L, Felician O, Guedj E, Hubert AM, Mancini J, Boucraut J, Ceccaldi M (2010) Could clinical profile influence CSF biomarkers in early-onset Alzheimer disease? Alzheimer Dis Assoc Disord 24:278–283
Kume K, Hanyu H, Sato T, Hirao K, Shimizu S, Kanetaka H, Sakurai H, Iwamoto T (2011) Vascular risk factors are associated with faster decline of Alzheimer disease: a longitudinal SPECT study. J Neurol 258:1295–1303
Kunicki S, Richardson J, Mehta PD, Kim KS, Zorychta E (1998) The effects of age, apolipoprotein E phenotype and gender on the concentration of amyloid-beta (A beta) 40, A beta 4242, apolipoprotein E and transthyretin in human cerebrospinal fluid. Clin Biochem 31:409–415
Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, Petersen RC, Shaw LM, Trojanowski JQ, Jack CR Jr, Weiner MW, Jagust WJ (2010) Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75:230–238
Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW (2008) Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol 7:779–786
Lasser RA, Dukoff R, Levy J, Levin R, Lehtimaki T, Seubert P, Sunderland T (1998) Apolipoprotein E epsilon 4 allele in association with global cognitive performance and CSF markers in Alzheimer's disease. Int J Geriatr Psychiatry 13:767–774
Lebedeva E, Stingl JC, Thal DR, Ghebremedhin E, Strauss J, Ozer E, Bertram L, von Einem B, Tumani H, Otto M, Riepe MW, Hogel J, Ludolph AC, von Arnim CA (2010) Genetic variants in PSEN2 and correlation to CSF beta-amyloid42 levels in AD. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2010.07.017
Lewczuk P, Esselmann H, Bibl M, Beck G, Maler JM, Otto M, Kornhuber J, Wiltfang J (2004) Tau protein phosphorylated at threonine 181 in CSF as a neurochemical biomarker in Alzheimer's disease: original data and review of the literature. J Mol Neurosci 23:115–122
Lewczuk P, Beck G, Ganslandt O, Esselmann H, Deisenhammer F, Regeniter A, Petereit HF, Tumani H, Gerritzen A, Oschmann P, Schroder J, Schonknecht P, Zimmermann K, Hampel H, Burger K, Otto M, Haustein S, Herzog K, Dannenberg R, Wurster U, Bibl M, Maler JM, Reubach U, Kornhuber J, Wiltfang J (2006) International quality control survey of neurochemical dementia diagnostics. Neurosci Lett 409:1–4
Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ (2007) CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology 69:631–639
Lin YT, Cheng JT, Yao YC, Juo Lo YK, Lin CH, Ger LP, Lu PJ (2009) Increased total TAU but not amyloid-beta(42) in cerebrospinal fluid correlates with short-term memory impairment in Alzheimer's disease. J Alzheimers Dis 18:907–918
Lorenzi M, Donohue M, Paternico D, Scarpazza C, Ostrowitzki S, Blin O, Irving E, Frisoni GB (2010) Enrichment through biomarkers in clinical trials of Alzheimer's drugs in patients with mild cognitive impairment. Neurobiol Aging 31:1443–1451, 1451 e1441
Maruyama M, Arai H, Sugita M, Tanji H, Higuchi M, Okamura N, Matsui T, Higuchi S, Matsushita S, Yoshida H, Sasaki H (2001) Cerebrospinal fluid amyloid beta(1–42) levels in the mild cognitive impairment stage of Alzheimer's disease. Exp Neurol 172:433–436
Mattsson N, Blennow K, Zetterberg H (2009a) CSF biomarkers: pinpointing Alzheimer pathogenesis. Ann N Y Acad Sci 1180:28–35
Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosen E, Aarsland D, Visser PJ, Schroder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Pirttila T, Wallin A, Jonhagen ME, Minthon L, Winblad B, Blennow K (2009b) CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302:385–393
Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM (2000) Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol 57:100–105
Mehta PD, Pirttila T, Patrick BA, Barshatzky M, Mehta SP (2001) Amyloid beta protein 1-40 and 1-42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease. Neurosci Lett 30:102–106
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 41:479–486
Mollenhauer B, Bibl M, Trenkwalder C, Stiens G, Cepek L, Steinacker P, Ciesielczyk B, Neubert K, Wiltfang J, Kretzschmar HA, Poser S, Otto M (2005) Follow-up investigations in cerebrospinal fluid of patients with dementia with Lewy bodies and Alzheimer's disease. J Neural Transm 112:933–948
Mollenhauer B, Bibl M, Wiltfang J, Steinacker P, Ciesielczyk B, Neubert K, Trenkwalder C, Otto M (2006) Total tau protein, phosphorylated tau (181p) protein, beta-amyloid(1–42), and beta-amyloid(1–40) in cerebrospinal fluid of patients with dementia with Lewy bodies. Clin Chem Lab Med 44:192–195
Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, Chang L, Miller B, Clark C, Green R et al (1995) Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol 38:643–648
Mulugeta E, Londos E, Ballard C, Alves G, Zetterberg H, Blennow K, Skogseth R, Minthon L, Aarsland D (2010) CSF amyloid beta38 as a novel diagnostic marker for dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 82:160–164
Nakamura T, Shoji M, Harigaya Y, Watanabe M, Hosoda K, Cheung TT, Shaffer LM, Golde TE, Younkin LH, Younkin SG et al (1994) Amyloid beta protein levels in cerebrospinal fluid are elevated in early-onset Alzheimer's disease. Ann Neurol 36:903–911
Nitsch RM, Rebeck GW, Deng M, Richardson UI, Tennis M, Schenk DB, Vigo-Pelfrey C, Lieberburg I, Wurtman RJ, Hyman BT et al (1995) Cerebrospinal fluid levels of amyloid beta-protein in Alzheimer's disease: inverse correlation with severity of dementia and effect of apolipoprotein E genotype. Ann Neurol 37:512–518
Okonkwo OC, Alosco ML, Griffith HR, Mielke MM, Shaw LM, Trojanowski JQ, Tremont G (2010) Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol 67:688–696
Okonkwo OC, Mielke MM, Griffith HR, Moghekar AR, O'Brien RJ, Shaw LM, Trojanowski JQ, Albert MS (2011) Cerebrospinal fluid profiles and prospective course and outcome in patients with amnestic mild cognitive impairment. Arch Neurol 68:113–119
Oksengard AR, Cavallin L, Axelsson R, Andersson C, Nagga K, Winblad B, Eriksdotter-Jonhagen M, Wahlund LO (2010) Lack of accuracy for the proposed 'Dubois criteria' in Alzheimer's disease: a validation study from the Swedish brain power initiative. Dement Geriatr Cogn Disord 30:374–380
Paraskevas GP, Kapaki E, Papageorgiou SG, Kalfakis N, Andreadou E, Zalonis I, Vassilopoulos D (2009) CSF biomarker profile and diagnostic value in vascular dementia. Eur J Neurol 16:205–211
Parnetti L, Lanari A, Amici S, Gallai V, Vanmechelen E, Hulstaert F (2001) CSF phosphorylated tau is a possible marker for discriminating Alzheimer's disease from dementia with Lewy bodies. Phospho-Tau International Study Group. Neurol Sci 22:77–78
Parnetti L, Tiraboschi P, Lanari A, Peducci M, Padiglioni C, D'Amore C, Pierguidi L, Tambasco N, Rossi A, Calabresi P (2008) Cerebrospinal fluid biomarkers in Parkinson's disease with dementia and dementia with Lewy bodies. Biol Psychiatry 64:850–855
Popp J, Lewczuk P, Frommann I, Kolsch H, Kornhuber J, Maier W, Jessen F (2010) Cerebrospinal fluid markers for Alzheimer's disease over the lifespan: effects of age and the APOEepsilon4 genotype. J Alzheimers Dis 22:459–468
Portelius E, Dean RA, Gustavsson MK, Andreasson U, Zetterberg H, Siemers E, Blennow K (2010) A novel Abeta isoform pattern in CSF reflects gamma-secretase inhibition in Alzheimer disease. Alzheimers Res Ther 2:7
Prince JA, Zetterberg H, Andreasen N, Marcusson J, Blennow K (2004) APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurology 62:2116–2118
Riemenschneider M, Schmolke M, Lautenschlager N, Guder WG, Vanderstichele H, Vanmechelen E, Kurz A (2000) Cerebrospinal beta-amyloid ((1–42)) in early Alzheimer's disease: association with apolipoprotein E genotype and cognitive decline. Neurosci Lett 284:85–88
Riemenschneider M, Lautenschlager N, Wagenpfeil S, Diehl J, Drzezga A, Kurz A (2002a) Cerebrospinal fluid tau and beta-amyloid 42 proteins identify Alzheimer disease in subjects with mild cognitive impairment. Arch Neurol 59:1729–1734
Riemenschneider M, Schmolke M, Lautenschlager N, Vanderstichele H, Vanmechelen E, Guder WG, Kurz A (2002b) Association of CSF apolipoprotein E, Abeta42 and cognition in Alzheimer's disease. Neurobiol Aging 23:205–211
Riemenschneider M, Wagenpfeil S, Diehl J, Lautenschlager N, Theml T, Heldmann B, Drzezga A, Jahn T, Forstl H, Kurz A (2002c) Tau and Abeta42 protein in CSF of patients with frontotemporal degeneration. Neurology 58:1622–1628
Riepe MW, Karl J, Tumani H, von Arnim CA (2010) Tau-proteins as gender-specific state markers in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord 30:93–100
Rolstad S, Nordlund A, Eckerstrom C, Gustavsson MH, Zetterberg H, Wallin A (2009) Biomarkers in relation to cognitive reserve in patients with mild cognitive impairment—proof of concept. Dement Geriatr Cogn Disord 27:194–200
Rosso SM, van Herpen E, Pijnenburg YA, Schoonenboom NS, Scheltens P, Heutink P, van Swieten JC (2003) Total tau and phosphorylated tau 181 levels in the cerebrospinal fluid of patients with frontotemporal dementia due to P301L and G272V tau mutations. Arch Neurol 60:1209–1213
Samgard K, Zetterberg H, Blennow K, Hansson O, Minthon L, Londos E (2010) Cerebrospinal fluid total tau as a marker of Alzheimer's disease intensity. Int J Geriatr Psychiatry 25:403–410
Saumier D, Duong A, Haine D, Garceau D, Sampalis J (2009) Domain-specific cognitive effects of tramiprosate in patients with mild to moderate Alzheimer's disease: ADAS-cog subscale results from the Alphase Study. J Nutr Health Aging 13:808–812
Scheurich A, Urban PP, Koch-Khoury N, Fellgiebel A (2010) CSF phospho-tau is independent of age, cognitive status and gender of neurological patients. J Neurol 257:609–614
Schneider LS, Kennedy RE, Cutter GR (2010) Requiring an amyloid-beta1-42 biomarker for prodromal Alzheimer's disease or mild cognitive impairment does not lead to more efficient clinical trials. Alzheimers Dement 6:367–377
Schöll M, Almkvist O, Bogdanovic N, Wall A, Långström B, Viitanen M, Nordberg A (2011) Time course of glucose metabolism in relation to cognitive performance and postmortem neuropathology in Met146Val PSEN1 mutation carriers. J Alzheimers Dis 24:495–506
Schoonenboom NS, Pijnenburg YA, Mulder C, Rosso SM, Van Elk EJ, Van Kamp GJ, Van Swieten JC, Scheltens P (2004) Amyloid beta(1–42) and phosphorylated tau in CSF as markers for early-onset Alzheimer disease. Neurology 62:1580–1584
Seppala TT, Koivisto AM, Hartikainen P, Helisalmi S, Soininen H, Herukka SK (2011) Longitudinal changes of CSF biomarkers in Alzheimer’s disease. J Alzheimers Dis 25:583–594
Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ (2009) Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 65:403–413
Sjogren M, Minthon L, Davidsson P, Granerus AK, Clarberg A, Vanderstichele H, Vanmechelen E, Wallin A, Blennow K (2000) CSF levels of tau, beta-amyloid(1–42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J Neural Transm 107:563–579
Sjogren M, Vanderstichele H, Agren H, Zachrisson O, Edsbagge M, Wikkelso C, Skoog I, Wallin A, Wahlund LO, Marcusson J, Nagga K, Andreasen N, Davidsson P, Vanmechelen E, Blennow K (2001) Tau and Abeta42 in cerebrospinal fluid from healthy adults 21–93 years of age: establishment of reference values. Clin Chem 47:1776–1781
Snider BJ, Fagan AM, Roe C, Shah AR, Grant EA, Xiong C, Morris JC, Holtzman DM (2009) Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol 66:638–645
Strozyk D, Blennow K, White LR, Launer LJ (2003) CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 60:652–656
Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM (2003) Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 289:2094–2103
Sunderland T, Mirza N, Putnam KT, Linker G, Bhupali D, Durham R, Soares H, Kimmel L, Friedman D, Bergeson J, Csako G, Levy JA, Bartko JJ, Cohen RM (2004) Cerebrospinal fluid beta-amyloid1-42 and tau in control subjects at risk for Alzheimer's disease: the effect of APOE epsilon4 allele. Biol Psychiatry 56:670–676
Tapiola T, Overmyer M, Lehtovirta M, Helisalmi S, Ramberg J, Alafuzoff I, Riekkinen P Sr, Soininen H (1997) The level of cerebrospinal fluid tau correlates with neurofibrillary tangles in Alzheimer's disease. Neuroreport 8:3961–3963
Tapiola T, Pirttila T, Mehta PD, Alafuzofff I, Lehtovirta M, Soininen H (2000a) Relationship between apoE genotype and CSF beta-amyloid (1–42) and tau in patients with probable and definite Alzheimer's disease. Neurobiol Aging 21:735–740
Tapiola T, Pirttila T, Mikkonen M, Mehta PD, Alafuzoff I, Koivisto K, Soininen H (2000b) Three-year follow-up of cerebrospinal fluid tau, beta-amyloid 42 and 40 concentrations in Alzheimer's disease. Neurosci Lett 280:119–122
Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, Pirttila T (2009) Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66:382–389
Tato RE, Frank A, Hernanz A (1995) Tau protein concentrations in cerebrospinal fluid of patients with dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry 59:280–283
Tibbling G, Link H, Ohman S (1977) Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest 37:385–390
Tripoliti EE, Fotiadis DI, Argyropoulou M (2011) A supervised method to assist the diagnosis and monitor progression of Alzheimer’s disease using data from an fMRI experiment. Artif Intell Med 53:35–45. doi:10.1016/j.artmed.2011.05.005
Urakami K, Wada K, Arai H, Sasaki H, Kanai M, Shoji M, Ishizu H, Kashihara K, Yamamoto M, Tsuchiya-Ikemoto K, Morimatsu M, Takashima H, Nakagawa M, Kurokawa K, Maruyama H, Kaseda Y, Nakamura S, Hasegawa K, Oono H, Hikasa C, Ikeda K, Yamagata K, Wakutani Y, Takeshima T, Nakashima K (2001) Diagnostic significance of tau protein in cerebrospinal fluid from patients with corticobasal degeneration or progressive supranuclear palsy. J Neurol Sci 183:95–98
van Gool WA, Kuiper MA, Walstra GJ, Wolters EC, Bolhuis PA (1995) Concentrations of amyloid beta protein in cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol 37:277–279
van Rossum IA, Vos S, Handels R, Visser PJ (2010) Biomarkers as predictors for conversion from mild cognitive impairment to Alzheimer-type dementia: implications for trial design. J Alzheimers Dis 20:881–891
Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Shaw LM, Trojanowski JQ, Aisen PS, Weiner M, Petersen RC, Jack CR Jr (2010) Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol 67:308–316
Verbeek MM, Pijnenburg YA, Schoonenboom NS, Kremer BP, Scheltens P (2005) Cerebrospinal fluid tau levels in frontotemporal dementia. Ann Neurol 58:656–657, author reply 657
Verwey NA, Kester MI, van der Flier WM, Veerhuis R, Berkhof H, Twaalfhoven H, Blankenstein MA, Scheltens And P, Pijnenburg YA (2010) Additional value of CSF amyloid-beta 40 levels in the differentiation between FTLD and control subjects. J Alzheimers Dis 20:445–452
Visser PJ, Verhey FR, Boada M, Bullock R, De Deyn PP, Frisoni GB, Frolich L, Hampel H, Jolles J, Jones R, Minthon L, Nobili F, Olde Rikkert M, Ousset PJ, Rigaud AS, Scheltens P, Soininen H, Spiru L, Touchon J, Tsolaki M, Vellas B, Wahlund LO, Wilcock G, Winblad B (2008) Development of screening guidelines and clinical criteria for predementia Alzheimer's disease. The DESCRIPA Study. Neuroepidemiology 30:254–265
Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, Tsolaki M, Minthon L, Wallin AK, Hampel H, Burger K, Pirttila T, Soininen H, Rikkert MO, Verbeek MM, Spiru L, Blennow K (2009) Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol 8:619–627
Wallin A, Sjogren M, Blennow K, Davidsson P (2003) Decreased cerebrospinal fluid acetylcholinesterase in patients with subcortical ischemic vascular dementia. Dement Geriatr Cogn Disord 16:200–207
Wallin AK, Blennow K, Zetterberg H, Londos E, Minthon L, Hansson O (2010) CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology 74:1531–1537
Wilner A (2010) Alzheimer's CSF test: useful or useless? Medscape Neurology & Neurosurgery (Ask the Experts) 10/15/2010
Yakushev I, Bartenstein P, Siessmeier T, Hiemke C, Scheurich A, Lotz J, Fellgiebel A, Muller MJ (2010) Cerebrospinal fluid tau protein levels and 18 F-fluorodeoxyglucose positron emission tomography in the differential diagnosis of Alzheimer's disease. Dement Geriatr Cogn Disord 30:245–253
Zetterberg H, Wahlund LO, Blennow K (2003) Cerebrospinal fluid markers for prediction of Alzheimer's disease. Neurosci Lett 352:67–69
Zetterberg H, Pedersen M, Lind K, Svensson M, Rolstad S, Eckerstrom C, Syversen S, Mattsson UB, Ysander C, Mattsson N, Nordlund A, Vanderstichele H, Vanmechelen E, Jonsson M, Edman A, Blennow K, Wallin A (2007) Intra-individual stability of CSF biomarkers for Alzheimer's disease over two years. J Alzheimers Dis 12:255–260
Zhou B, Teramukai S, Yoshimura K, Fukushima M (2009) Validity of cerebrospinal fluid biomarkers as endpoints in early-phase clinical trials for Alzheimer's disease. J Alzheimers Dis 18:89–102
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenmann, H. CSF Biomarkers for Amyloid and Tau Pathology in Alzheimer's Disease. J Mol Neurosci 47, 1–14 (2012). https://doi.org/10.1007/s12031-011-9665-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9665-5