Abstract
Mesenchymal stem cells (MSCs) are considered as promising candidates for new clinical trials of cell therapies. Bone marrow (BM) was the first source reported to contain MSCs; however, using it may be detrimental due to the highly invasive aspiration procedures. More recently, adipose tissue, attainable by a less invasive method, has been introduced as an alternative source of MSCs. So far, MSCs derived from these two sources have been compared in different characters; however, one of the main properties, i.e., the expression of chemokine receptors, has been ignored in these comparisons. In the present study, human MSCs were derived from bone marrow and adipose tissues and characterized by their expression of some cell surface antigens and also differentiation capacity. The expression of five selected chemokine receptors, which seems to be important in cell homing, was also compared. Semiquantitative reverse transcription-polymerase chain reaction method was used to assess gene expression levels of these chemokine receptors. Our results indicate that expression of these receptors in human MSCs, derived from adipose tissue, was higher than MSCs from bone marrow. Chemokine receptors and their ligands and adhesion molecules play an important role in tissue-specific homing of leukocytes and have also been implicated in trafficking of hematopoietic precursors into and through tissues. Therefore, MSCs from adipose tissue may show a better migration and homing capacity and they might be a better candidate for therapeutic purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In spite of various prevention programs, many kinds of devastating, incurable central nervous system (CNS) injuries and diseases continue to occur, which result in physical and emotional problems and also increasing health care costs. So tremendous efforts have been made to develop therapeutic strategies for eliminating these offensive consequences. In recent years, a very promising approach using adult stem cells has been introduced to treat CNS disorders (Slavin et al. 2008; Hou and Hong 2008). Many groups have been working independently to introduce the better cell sources for cell therapies in neurodegenerative diseases (reviewed by Zietlow et al. 2008).
The optimal characters for candidate cells to be used in therapeutic practices include their accessibility and success rate of isolation, proliferative capacity, ability to differentiate to various tissues, low immunogenicity, and high potentials for targeted homing. Homing is the ability of cells in migration and engraftment to affected tissues. The mechanism of cell homing is still unclear, but some evidences indicate that chemokine receptors might be important in this process (Chamberlain et al. 2007; Kitaori et al. 2009).
Currently, among the most studied adult stem cells are mesenchymal stem cells (MSCs) derived from bone marrow (BM), but a major obstacle for their application is the highly invasive procedure of their harvesting. Therefore, alternative sources for isolation of MSCs are subject to intensive investigations. Recently, adipose tissue (AT) is being represented as an alternative source of MSCs. Subcutaneous adipose depots are accessible, abundant, and replenishable, thereby providing a potential adult stem cell reservoir for each individual (Madonna and De Caterina 2010; Vieira et al. 2010). However, there are controversies in whether AT can be substituted for BM. Some studies have been carried out to compare MSCs of these two sources, and the results show that they have similar differentiation and immunogenicity potentials and even in some cases, more proliferation capacity has been reported for AT-MSCs (De Ugarte et al. 2003; Vidal et al. 2007; Yoo et al. 2009).
As mentioned before, another important characteristic of stem cells, used in therapies, is their efficient homing, which based on recent studies is closely related to the expression of chemokine receptors (Chamberlain et al. 2007; Kitaori et al. 2009).
Despite the valuable studies on chemokine receptors on BM-MSCs, there is no significant report covering this character of the AT-MSCs. Therefore, we aimed to study the chemokine receptors in AT-MSCs and compare them with those from BM. The chemokine receptors examined in this study are those which seem to be more functional in migration and homing of cells in various diseases (Sordi et al. 2005; Bhakta et al. 2006; Gavina et al. 2006; Croitoru-Lamoury et al. 2007; Perez et al. 2009). Considering the probable role of chemokine receptors in cell homing, comparison of the expression level of chemokine receptors would help scientists to candidate and introduce better cell sources for therapies. According to recent studies, modulation of the expression of these receptors could affect the engraftment efficiency to the damaged sites (Gul et al. 2009; Kalwitz et al. 2009; Tondreau et al. 2009; Ringe et al. 2007; Ponte et al. 2007). The basic information provided in this study may help to decide what extent of gene manipulation should be developed to improve cell homing in some diseases including neurodegenerative diseases.
Materials and Methods
Collection of BM
BM aspirates were obtained from the sternum of three patients undergoing heart surgery. The BM aspirates were received in accordance with the ethical standards of the local ethical committee.
Cell Isolation and Culture of Mononuclear Cells from BM
The mononuclear cells (MNCs) were isolated from BM by using Lympholyte-H (Cedarlane, Canada) and seeded into T25 cell culture flasks containing Dulbecco's Modified Eagle's Medium (DMEM, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS, Gibco). The first change of medium was accomplished within 3 days after isolation and cells were harvested at subconfluency using 0.25% trypsin/EDTA (Gibco) (passage 0). Cell viability and numbers at the time of passage were determined by trypan blue exclusion and hemocytometer cell counts, respectively. Six mesenchymal stem cell lines were established successfully from BM of three individual donors. MSCs in passage 4–6 were used for further analysis.
Collection of AT
Liposuction aspirates from subcutaneous AT sites were obtained from three individuals undergoing elective procedures in local plastic surgical offices. Lipoaspirates were obtained in accordance with the regulations of ethical committee.
Cell Isolation and Culture of MNCs from AT
Adipose tissues were washed three to four times with phosphate-buffered saline (PBS) supplemented with penicillin–streptomycin (Biosera) and then incubated with 0.1% collagenase type I (Sigma) at 37°C for 1 h. They were manually shaken vigorously for 5 to 10 s every 15 min. On completion of digestion period (1 h), FBS was added to final concentration of 10% to stop collagenase activity. The digested AT was centrifuged at room temperature at 800g for 10 min. The supernatant, containing mature adipocytes, was then aspirated, and the pellet was resuspended and plated immediately in T25 flasks containing DMEM, 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. This initial passage of the primary cell culture was referred to as passage 0 (P0). After the first 72 h of incubation at 37°C and 5% CO2, the cultures were washed with PBS and maintained in media until they achieved 75–90% confluency (approximately 6 days in culture). Cells were passaged repeatedly after achieving a density of 75–90% until passage 4–6. Six cell lines were derived from fresh adipose tissue samples of three healthy individuals.
Osteogenesis
Differentiation along osteogenic lineage was assessed qualitatively based on cytochemical analysis (alizarin red). For this purpose, the culture media were replaced with osteogenic induction medium composed of DMEM with 10% FBS, 50 μg/ml ascorbate-2-phosphate, 100 nM dexamethasone, 10 mM B-glycerol phosphate (Sigma), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cultures were fed with fresh osteogenic induction medium every 7 days for a period of up to 3 weeks. At the end of the cultivation period, the cells were fixed with methanol for 10 min and stained with alizarin red (Sigma) for 15 min at room temperature so that the mineralized matrix of the bone could be examined.
Adipogenesis
Differentiation along adipogenic lineage was cytochemically (Oil Red O) assessed (Oil Red O). For this purpose, the culture media were replaced with adipocyte induction medium composed of DMEM with 10% FBS, 50 μg/ml ascorbate-2-phosphate (Sigma), 100 nM dexamethasone (Sigma), 50 μg/ml indomethacin (Sigma), and 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained in culture for up to 3–4 weeks, with adipogenic media replaced every 7 days. Cultures were rinsed with PBS and fixed in formalin solution. At the end of this period, the cells were stained with 0.5% Oil Red O (Sigma) in methanol for 15 min for adipocyte detection.
Flow Cytometry
For flow cytometry analysis, about 200,000 cells at passage 4 were removed from flasks using trypsin–EDTA and precipitated by centrifugation. The cells were then resuspended in 1 ml PBS and stained with PE-conjugated CD105, CD44, CD34, and FITC-conjugated CD90 and CD45 (all purchased from Becton Dickenson) by adding antibodies at a concentration of 2 μg/ml at 4°C for 20 min. Finally, the cells were washed and analyzed by flow cytometry (FACScalibur Cytometer equipped with 488-nm argon laser) and WinMDI software.
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction
Cells in passage 4–6 were used for reverse transcription-polymerase chain reaction (RT-PCR) analysis. Briefly, total RNA was prepared using a modification of guanidine isothiocyanate–phenol–chloroform method. cDNA was synthesized using oligo(dT) primer and M-MuLV reverse transcriptase (Fermentas, Germany) according to the manufacturer's instructions. PCR reactions were carried out using selective forward and reverse primers for GAPDH (internal control), CCR7, CXCR4, CCR1, CXCR6, and CX3CR. The sequences of primers are presented in Table 1.
The DNA amplification program was as below: Incubation at 94°C for 5 min, followed by 30 cycles of (60 s at 94°C, 60 s at 60°C, and 60 s at 72°C). The final cycle was followed by a 5-min extension step at 72°C. The reaction parameters were adjusted to obtain a linear relation between the number of PCR cycles and PCR products and also a linear relation between the initial amount of cDNA templates and PCR products. PCR products were subsequently analyzed on 1% agarose gel (Roche), and bands were quantified by densitometry using Image J software.
Data Analysis
In gene expression studies, the band densities were normalized to GAPDH for each sample. Data are presented as mean ± SD for statistical comparison; a two-sided, nonpaired t test was used to analyze the RT-PCR results. Differences were considered significant at P < 0.05.
Results
Isolation of the Stem Cells and their Expansion
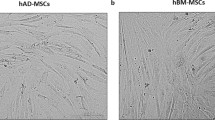
MSCs from both BM and AT were isolated successfully and formed a monolayer about 1 week after initial plating. No obvious differences concerning the morphology of MSCs derived from these two tissues were detected (Fig. 1).
Multilineage Differentiation Capacity
To investigate the differentiation potential of MSCs from the two different they sources were directed towards the osteogenic and adipogenic lineages. The osteogenic differentiation was confirmed by deposition of alizarin red staining in a mineralized matrix (Fig. 2a). Adipogenic differentiation was demonstrated by accumulation of lipid vacuoles in cytoplasm of the cells indicated by Oil Red O staining (Fig. 2b). All BM and AT samples (100%) showed both osteogenic and adipogenic differentiation capacities.
Differentiation capacity of MSCs, 20 days after induction with osteogenic medium. Differentiated human BM-MSCs were stained with alizarin red (a). BM-MSCs induced with adipogenic medium were stained with Oil Red O, 20 days after induction (b). These micrographs are representative examples of the experiments
Immunophenotypic Characterization of the Cells
For further characterization of the MSCs derived from BM or AT, expression of their cell surface antigens was examined at passages 2–4 by flow cytometry (Fig. 3). MSCs derived from BM and AT displayed no expression of hematopoietic markers of CD34 and CD45. They expressed the typical MSC markers CD44, CD90, and CD105.
Gene Expression Study
Expression of five genes related to the chemokine receptors and one housekeeping gene was determined by RT-PCR as presented in Fig. 4. Results indicated low or no detectable mRNA expression of CCR1, CXCR4, and CXCR6 in BM-MSCs. The expression of CX3CR was very low or not detectable in samples derived from both sources. There was a significant difference for expression of CCR1 between BM-MSCs and AT-MSCs. Expressions of all studied genes were lower in BM-MSCs in comparison to that of AT-MSCs (Fig. 5).
Quantitative analysis of MSCs representing relative expression of selected chemokine receptors in BM- and AT-derived MSCs. As seen in the figure, expression of all studied receptors was lower in BM-MSCs compared with AT-MSCs. The statistical significance (P < 0.05) of the difference is represented by star. BM bone marrow, AT adipose tissue
Discussion
To date, many investigations have been carried out to answer the question of which cell source is most proper for clinical use. The main characters considered in these studies include differentiation capacities and expansion potentials of the cells (De Ugarte et al. 2003; Vidal et al. 2007; Yoo et al. 2009).
On the other hand, one of the biggest obstacles in cell transplantation especially in systemic disorders is improper migration and homing of systemic administrated cells. So, study of other characters, which may influence the migration capacity of cells, should also be considered in such investigations. In this study, the chemokine receptors of CCR1, CXCR4, CCR7, CXCR6, and CXCR3, which may play an important role in cell migration (Sordi et al. 2005; Bhakta et al. 2006; Gavina et al. 2006; Croitoru-Lamoury et al. 2007; Perez et al. 2009), were considered in human MSCs, derived from BM and AT. Several groups have recently been studying the expression of chemokine receptors in human MSCs, derived from BM, but the results have been variable and also different from our results (Chamberlain et al. 2008; Croitoru-Lamoury et al. 2007; Kortesidis et al. 2005; Wynn et al. 2004). These differences may be because of different cell preparation methods in various laboratories, different culture conditions, and the heterogeneity of cultured MSCs. So introducing a standard cell preparation method and culture conditions may be essential in this area.
In this study, for the first time, analysis of these chemokine receptors was carried out in human AT-MSCs and also a comprehensive gene expression profile of them was created and compared between BM and AT-MSCs.
Our results indicated that expression of CCR1, CXCR4, CCR7, CXCR6, and CXCR3 in mRNA level was relatively low in MSCs derived from AT and very low or not detectable in most samples derived from BM. Previous reports have shown that these chemokine receptors are important in regulating the migration and engraftment of different types of stem/progenitor cells (Sordi et al. 2005; Bhakta et al. 2006; Gavina et al. 2006; Croitoru-Lamoury et al. 2007; Perez et al. 2009). Some reports also indicated that they are essential for transendothelial cancer cell migration as well as vascular permeability (Krohn et al. 2009; Lin et al. 2009; Pradelli et al. 2009; Wang et al. 2009; Deutsch et al. 2008; Varney et al. 2006). Overexpression of these chemokine receptors in cancer specimens predicts a worse outcome in patients with cancer (Xiang et al. 2009; Chu et al. 2010).
Considering the role of these receptors in stem cell migration in normal and pathologic conditions and their low expression in MSCs derived from BM and AT, it seems that modulation of their expression might affect the engraftment efficiency of these cells to the damaged sites.
Our results demonstrates that expression of these chemokine receptors is higher in MSCs derived from AT in comparison with BM-MSCs. Considering the probable role of chemokine receptors in cell migration and homing, it seems that MSCs derived from AT might be better candidates for transplantation, although further investigations are required both in vitro and in vivo to confirm it.
References
Bhakta S, Hong P, Koc O (2006) The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med 7(1):19–24
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25(11):2739–2749
Chamberlain G, Wright K, Rot A, Ashton B, Middleton J (2008) Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS One 3(8):e2934
Chu QD, Panu L, Holm NT, Li BD, Johnson LW, Zhang S (2010) High chemokine receptor CXCR4 level in triple negative breast cancer specimens predicts poor clinical outcome. J Surg Res 159(2):689–695
Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA, Brew BJ (2007) Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and Copaxone. J Interferon Cytokine Res 27(1):53–64
De Ugarte DA, Morizono K, Elbarbary A et al (2003) Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174(3):101–109
Deutsch AJ, Aigelsreiter A, Steinbauer E et al (2008) Distinct signatures of B-cell homeostatic and activation-dependent chemokine receptors in the development and progression of extragastric MALT lymphomas. J Pathol 215(4):431–444
Gavina M, Belicchi M, Rossi B et al (2006) VCAM-1 expression on dystrophic muscle vessels has a critical role in the recruitment of human blood-derived CD133+ stem cells after intra-arterial transplantation. Blood 108(8):2857–2866
Gul H, Marquez-Curtis LA, Jahroudi N, Lo J, Turner AR, Janowska-Wieczorek A (2009) Valproic acid increases CXCR4 expression in hematopoietic stem/progenitor cells by chromatin remodeling. Stem Cells Dev 18(6):831–838
Hou L, Hong T (2008) Stem cells and neurodegenerative diseases. Sci China C Life Sci 51(4):287–294
Kalwitz G, Endres M, Neumann K et al (2009) Gene expression profile of adult human bone marrow-derived mesenchymal stem cells stimulated by the chemokine CXCL7. Int J Biochem Cell Biol 41(3):649–658
Kitaori T, Ito H, Schwarz EM et al (2009) Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 60(3):813–823
Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S (2005) Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 105(10):3793–3801
Krohn A, Song YH, Muehlberg F, Droll L, Beckmann C, Alt E (2009) CXCR4 receptor positive spheroid forming cells are responsible for tumor invasion in vitro. Cancer Lett 280(1):65–71
Lin S, Sun L, Hu J, Wan S, Zhao R, Yuan S, Zhang L (2009) Chemokine C–X–C motif receptor 6 contributes to cell migration during hypoxia. Cancer Lett 279(1):108–117
Madonna R, De Caterina RJ (2010) Adipose tissue: a new source for cardiovascular repair. J Cardiovasc Med (Hagerstown) 11(2):71–80
Perez AL, Bachrach E, Illigens BM et al (2009) CXCR4 enhances engraftment of muscle progenitor cells. Muscle Nerve 40(4):562–572
Ponte AL, Marais E, Gallay N et al (2007) The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25:1737–1745
Pradelli E, Karimdjee-Soilihi B, Michiels JF et al (2009) Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. Int J Cancer 125(11):2586–2594
Ringe J, Strassburg S, Neumann K et al (2007) Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem 101:135–146
Slavin S, Kurkalli BG, Karussis D (2008) The potential use of adult stem cells for the treatment of multiple sclerosis and other neurodegenerative disorders. Clin Neurol Neurosurg 110(9):943–946
Sordi V, Malosio ML, Marchesi F et al (2005) Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 106(2):419–427
Tondreau T, Meuleman N, Stamatopoulos B et al (2009) In vitro study of matrix metalloproteinase/tissue inhibitor of metalloproteinase production by mesenchymal stromal cells in response to inflammatory cytokines: the role of their migration in injured tissues. Cytotherapy 11(5):559–569
Varney ML, Johansson SL, Singh RK (2006) Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol 125(2):209–216
Vidal MA, Kilroy GE, Lopez MJ, Johnson JR, Moore RM, Gimble JM (2007) Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg 36(7):613–622
Vieira N, Brandalise V, Zucconi E, Secco M, Strauss B, Zatz M (2010) Isolation, characterization and differentiation potential of canine adipose-derived stem cells. Cell Transplant 19(3):279–289
Wang CL, Sun BS, Tang Y, Zhuang HQ, Cao WZ (2009) CCR1 knockdown suppresses human non-small cell lung cancer cell invasion. J Cancer Res Clin Oncol 135(5):695–701
Wynn RF, Hart CA, Corradi-Perini C et al (2004) A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104(9):2643–2645
Xiang ZL, Zeng ZC, Tang ZY, Fan J, Zhuang PY, Liang Y, Tan YS, He J (2009) Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer 9:176
Yoo KH, Jang IK, Lee MW et al (2009) Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 259(2):150–156
Zietlow R, Lane EL, Dunnett SB, Rosser AE (2008) Human stem cells for CNS repair. Cell Tissue Res 331(1):301–322
Acknowledgment
We gratefully acknowledge the collaboration of Dr. Nasser Sanjar Moosavi in supplying liposuction material and also Dr. Ali Azari for providing bone marrow samples. We thank Mr. Majid Moameni-Moghaddam for his expert technical assistance. In addition, the authors gratefully acknowledge the assistance of Raheleh Ahmadian Kia and Fahimeh Najib Bagherzadeh in the preparation of this manuscript. This work was supported by grants from Iranian Academic Center for Education, Culture and Research (ACECR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmadian kia, N., Bahrami, A.R., Ebrahimi, M. et al. Comparative Analysis of Chemokine Receptor's Expression in Mesenchymal Stem Cells Derived from Human Bone Marrow and Adipose Tissue. J Mol Neurosci 44, 178–185 (2011). https://doi.org/10.1007/s12031-010-9446-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-010-9446-6