Abstract
Both the natural variants of the apolipoprotein A5 (APOA5) and the glucokinase regulatory protein gene (GCKR) have been shown to associate with increased fasting triglyceride levels. Here, we investigated the possible association of the functional variants of these two genes with non-fasting triglyceride levels and their susceptibility nature in ischemic stroke. A total of 513 stroke patients and 172 healthy controls were genotyped. All the APOA5 variants (T-1131C, IVS3 + G476A, C56G, and T1259C) were associated with increased triglyceride levels in all stroke patients and controls; except for T1259C, they all conferred risk for the disease. No such association was found for the examined GCKR rs1260326 (C1337T) variant. Furthermore, we examined the effects of specific combinations of the GCKR rs1260326 and APOA5 polymorphisms. Our findings confirmed the previous results regarding the association of APOA5 variants with triglyceride-level increase and stroke susceptibility of these alleles. By contrast, we could not detect any association of the studied GCKR allele with triglyceride levels or with the susceptibility of stroke in the same cohort of patients. In addition, the effect of APOA5 did not change significantly when specific combinations of the two genes were present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the development of ischemic stroke, a common multifactorial disease which is the third leading cause of death, the role of increased triglyceride level as a risk factor is still under debate (Rothwell et al. 2005). Recently, with the help of robust genome-wide association studies, several genetic factors associated with elevated triglycerides have been identified, opening new perspectives for the research of the fine details of the triglyceride-stroke relationship (Bansal et al. 2007; Nordestgaard et al. 2007; Szolnoki et al. 2003).
The apolipoprotein A5 (APOA5) gene is located near the apolipoprotein A (APOA) cluster containing the APOAI-APOCIII-APOAIV genes at 11q23 chromosome (Havasi et al. 2006; Martinelli et al. 2007a; Pennacchio and Rubin 2003; Szalai et al. 2004), encoding 366 amino acids. The mature APOA5 protein following synthesis in the liver is secreted into the plasma and plays a central regulatory role in triglyceride metabolism (Groenendijk et al. 2001). Since the different proteins transcribed from the natural variants of the gene may have slightly different functions, the most common natural variants, the T-1131C (rs662799), T1259C (rs2266788), C56G (rs3135506), and IVS3 + G476A (rs2072560) alleles have been repeatedly reported to associate with elevated triglyceride levels; moreover, some of them were also found to be independent risk factors for cardio- and cerebrovascular diseases and metabolic syndrome (Maasz et al. 2008a, b; Willer et al. 2008).
The glucokinase regulatory protein (GCKR) gene, located on chromosome 2p23.3-p23.2, consists of 19 exons and encodes a protein of 625 amino acids (Hayward et al. 1998; Vaxillaire et al. 1994; Warner et al. 1995). The gene product functions as a regulatory protein that inhibits glucokinase in liver and pancreatic cells by binding the enzyme non-covalently to form an inactive complex (Farrelly et al. 1999; Grimsby et al. 2000; Hayward et al. 1996). Functional variants were discovered in a genome-wide association study in association with hypertriglyceridemia; later, an inverse relationship of the triglyceride and glucose levels has also been reported in relation with GCKR functional alleles. Moreover, demonstrating a central role of the gene, GCKR 446Leu (rs1260326) carriers are protected against type II diabetes (Vaxillaire et al. 2008). Recently, genome-wide association studies suggest that by combining genotype information for multiple loci, significant predictive value from genetic markers might be gained (Kathiresan et al. 2008a; Morrison et al. 2007; Perez-Martinez et al. 2009; Trichopoulou et al. 2008; Yiannakouris et al. 2006).
The aim of our work was to investigate the relationship of functional variants of APOA5 and GCKR genes with triglyceride levels and to study the allele distributions in stratified ischemic stroke patients.
Materials and Methods
Patients
The DNA samples were from the Central Biobank governed by the University of Pécs, as part of the National Biobank Network of Hungary (www.biobank.hu), which belongs also to the pan-European Biobanking and Biomolecular Resources Research Infrastructure (BBMRI) preparatory phase project (http://bbmri.eu/bbmri/). The governance, maintenance, and management principles of the Biobank had been approved by the national Scientific Research Ethics Committee, Budapest (ETT TUKEB). During the collection and use of DNA samples and the accompanying clinical and personal data, the guidelines and regulations of the Helsinki Declaration in 1975 and the currently operative National laws and regulations were also followed.
A total of 513 Caucasian patients were involved in the study (207 males, 306 females; mean age, 65.1 ± 0.62 years) with acutely developing ischemic stroke, who had never suffered a previous stroke event. All the 513 subjects underwent a detailed clinical scrutiny, including medical and familial histories, estimation of vascular risk factors, general physical and neurological explorations, urine analysis, extended laboratory examinations, electrocardiography, extracranial and transcranial Doppler sonography of the brain-supplying arteries, transthoracic and/or transesophageal echocardiography where apposite, and magnetic resonance imaging (MRI) examinations within 2 days after the outbreak of symptoms. After collecting the data of the 513 patients, we classified them into one of three stroke categories; the first two groups essentially matched with the corresponding type defined by the Trial of Org 10172 in Acute Stroke Treatment classification. The first subgroup called “small-vessel occlusion type” consists of 232 patients (91 males, 141 females; mean age, 65.1 ± 0.96 years) with small-vessel infarcts (one or more subcortical hemispheric or brainstem infarcts with a diameter <1.5 cm on MRI, with one of the features of the traditional clinical lacunar syndrome and without cerebral cortical dysfunction); the “large-vessel group” is composed of 139 patients (57 males, 82 females; mean age, 66.5 ± 1.21 years) with large-vessel infarcts (cortical or cerebellar lesions and/or brainstem infarcts or subcortical hemispheric infarcts greater than 1.5 cm in diameter on the MRI, with a cerebral cortical impairment or a brainstem or cerebellar dysfunction). The “mixed group” contains 142 patients (59 males, 83 females; mean age, 63.8 ± 1.06 years) with mixed vascular pathology (one or more lacunar and large-vessel infarcts on the MRI, and other nonspecific etiology). A total of 172 Caucasian subjects (49 males, 123 females; mean age, 56.5 ± 1.20 years) served as controls. This cohort was a neuroimaging alteration-free group, with negative brain MRI findings.
Molecular Biology Methods
Genomic DNA was extracted from peripheral EDTA-anticoagulated blood by a standard desalting method (Miller et al. 1988).For polymerase chain reaction (PCR), the following forward and reverse primers were used: for GCKR rs1260326 forward: 5′-TGC AGA CTA TAG TGG AGC CG-3′ and reverse: 5′-CAT CAC ATG GCC ACT GCT TT-3′; APOA5 gene: for the T-1131C variant forward: 5′-CCC CAG GAA CTG GAG CGA AATT-3′ and reverse: 5′-TTC AAG CAG AGG GAA GCC TGT A-3′; for the T1259C single nucleotide polymorphism (SNP) 5′-TCA GTC CTT GAA AGT GGC CT-3′ and 5′-ATG TAG TGG CAC AGG CTT CC-3′; for the IVS3 + G476A forward: 5′-CTC AAG GCT GTC TTC AG-3′ and the reverse 5′-CCT TTG ATT CTG GGG ACTG G-3′; for the C56G forward: 5′-AGA GCT AGC ACC GCT CCT TT-3′ and reverse: 5′-TAG TCC CTC TCC ACA GCG TT-3′ primers. The reactions were performed using an MJ Research PTC-200 thermal cycler (Bio-Rad, Hercules, CA, USA). The PCR conditions were the following: predenaturation at 96°C for 2 min; followed by 35 cycles of denaturation at 96°C for 20 s for the GCKR rs1260326 and all the APOA5 SNPs; annealing at 60°C for 20 s; primer extension for 30 s at 72°C; and final extension at 72°C for 5 min. For the T-1131C, PCR conditions were as follows: predenaturation at 96°C for 2 min followed by 32 cycles of 30 s at 95°C; 30 s at 55°C; 30 s at 72°C, and a final extension at 72°C. The amplicons were digested by allele-specific restriction endonucleases, HpaII for the GCKR rs1260326; TruI for the APOA5 T-1131C; BseGI for T1259C; MnlI for IVS3 + G476A; and Cfr13I for C56G. All methods were designed to include an obligate cleavage site on the amplicon thus enabling us to monitor the efficacy of the digestion.
Statistical Analysis
All clinical data are represented as mean ± standard error of the mean (SEM). The Mann–Whitney test was used to assess the differences between the clinical parameters in patients and the controls. For discreet variables, χ 2 test was applied. Odds ratios (ORs) derived from multiple logistic regression were also used to determine whether the presence of the significant genotypes of the APOA5 or GCKR gene were associated with clinical risk factors in the development of ischemic stroke. The ORs for the specific genotype combinations of the two genes (GCKR rs1260326 and APOA5 gene variants) were calculated by χ 2 test. All statistical analysis were carried out using SPSS 15.0 package for Windows (SPSS Inc, Chicago, IL, USA).
Results
The main clinical features and general laboratory data of the patients and controls are shown in Table 1. Patients were divided into groups according to our previously established stroke classification. The gender ratio, age range and body mass index (BMI) of the subgroups were as indicated in Table 1. The major risk factors for ischemic stroke, serum total cholesterol, and triglyceride levels showed significant differences between stroke patients and controls. The triglyceride levels of 1.67 ± 0.04, 1.78 ± 0.06, 1.76 ± 0.06, and 1.73 ± 0.03 mmol/l of the small vessel, large vessel, mixed, and overall groups, respectively, were significantly higher than the 1.53 ± 0.04 mmol/l triglyceride value of the controls (p < 0.05). The serum total cholesterol levels were 5.85 ± 0.08, 5.96 ± 0.11, 5.90 ± 1.12, and 5.89 ± 0.06 mmol/l of the small vessel, large vessel, mixed, and overall cohorts, respectively, and showed a significant difference between the stroke subgroups and the controls (p < 0.05).
Genotype profiles are summarized in Table 2. We analyzed the allele distribution of the different subgroups. All allele distributions were in Hardy–Weinberg equilibrium both in stroke groups and in controls. We detected a significant increase of allele frequencies of T-1131C, IVS3 + G476A, and C56G variants of the APOA5 gene in the following groups of stroke patients: −1131C (8.62%*, 8.27%*, 9.51%*, 8.77%*, respectively, in small vessel, large vessel, mixed, overall subgroups), IVS3 + G476A (6.90%*, 5.04%*, 7.75%*, 6.63%*) in all stroke groups, and the 56G (7.55%* in large-vessel group; 7.75%* in mixed group; and 7.02%* in the overall cohort) in all groups except small-vessel (6.25%) associated stroke. The T1259C polymorphism did not accumulate in any stroke groups (9.48% in small-vessel group; 9.71% in large-vessel group; 11.27% in mixed group; and 10.0% in the overall group). The rs1260326 (C1337T) variant of GCKR gene showed similar allele frequencies both in the stroke and control groups (49.4% in small vessel; 47.8% in large vessel; 48.9% in mixed group; and 48.8% in overall cohort vs. controls).
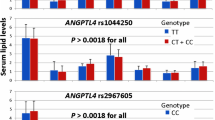
Triglyceride levels are shown in Table 2. Both in stroke patients and in controls the level of triglycerides was elevated in all APOA5 variant −1131C, 1259C, and IVS+G476A minor allele carriers compared with the non-carriers. By contrast, the GCKR gene’s minor allele 1337 T was not associated with triglyceride level changes either in stroke patients or in controls.
After adjustment for age, gender, body mass index, ischemic heart disease, hypertension, diabetes mellitus and smoking and drinking habits, the adjusted ORs for carrying −1131C, 56G, and IVS3 + 476A demonstrated an independent risk for the development of stroke (Table 2). After inclusion of triglyceride levels into the adjustment parameters, the same positive correlations were found (results are not shown). In contrast, we could not detect any association of the 1259C allele with stroke; in addition, the GCKR 1337 T allele did not exhibit a risk or protective nature for the disease.
Besides single locus genetic analysis, we also tested the effect of the specific combinations of GCKR C1337T and APOA5 polymorphisms. Individual APOA5 genotypes were studied in combination with GCKR C1337T genotypes. We generated four groups for all APOA5 variants as follows: NC C1337T –NCT-1131C; NC C1337T –CT-1131C; C C1337T –NCT-1131C and C C1337T –CT-1131C where “NC” refers to the wild genotype; “C” means carrying the minor allele of the variant indicated. Compared to individual ORs of the variants, the relative risk for ischemic stroke conferred by the presence of their combinations was significantly greater in certain groups. The relative risk for stroke was increased by CC1337T–CT-1131C in the small-vessel group, in the large-vessel group, in the mixed group, and in the overall cohort; by CC1337T–CIVS+G476A in the small and mixed groups as well as and in the whole stroke cohort; and by CC1337T–CC56G in the large-vessel group, in the mixed group, and in the whole group of stroke patients (results are shown in Table 3). For CC1337T–CT-1131C, lower ORs were seen in overall as well as in large and mixed-vessel groups. For the other combinations, we could not detect any changes affecting the risk of the disease. Although, for all the individual APOA5 variants significant association with elevated non-fasting triglyceride level was found in all stroke subgroups, for the specific genotype combinations only CC1337T–CT1259C, CC1337T–CIVS+G476A (small, overall), and CC1337T–CC56G (overall) showed the same effect (Table 3).
The C1337T variant of GCKR gene has an effect on triglyceride levels, impaired fasting glycemia, and have possible a risk of type II diabetes mellitus, as mentioned by Veiga-da Cunha (Veiga-da-Cunha et al. 2003). Carrying the 1337 T allele showed elevated triglyceride levels and higher risk of dyslipidemia but a lower fasting plasma glucose rates and a lower risk for the susceptibility of hyperglycemia (Vaxillaire et al. 2008). In our studied population, the presence of diabetes mellitus conferred increased risk for stroke in all stroke subgroups (adjusted OR in small vessel, 3.69*; in large vessel, 4.537*; in mixed, 6.157*, in overall, 4.395*; results are shown in Table 4). Subsequently, we examined the effect of GCKR C1337T variant on stroke patients with diabetes mellitus, the minor allele of GCKR (1337 T) in homozygous form showed an increased risk for stroke in two subgroups (in small-vessel group, 5.278*; in overall, 4.855*). Because of the low number of sample with diabetes mellitus, we are unable to draw a far-reaching conclusion.
Discussion
The role of triglycerides in different occlusive vascular diseases, including ischemic stroke, has been under investigation for a long while (Freiberg et al. 2008). Results are still controversial, and as the delineation of the mechanism of triglyceride elevation has already begun, there might be a chance for verification of the possible roles of APOA5 and GCKR genes (Hachinski et al. 1996; Haheim et al. 1993; Salonen et al. 1982; Simons et al. 1998).
In the postgenomic era, several new genes affecting the triglyceride metabolism had already been verified, like the APOA5 variants. These polymorphisms can influence the function of the protein transcript, which can modify secondarily the interaction of APOA5 with the lipoprotein lipase and eventually lead to increased circulating triglyceride levels (Pennacchio and Rubin 2003; Wright et al. 2006; Yang et al. 2004). Among the most frequently occurring variants of APOA5, the T-1131C, T1259C, C56G, and IVS3 + G476A have already been reported to associate with elevated triglyceride levels in several different populations, and some of them were also found to confer risk for cardio- and cerebrovascular diseases (Havasi et al. 2006; Maasz et al. 2008a; b; Martinelli et al. 2007b; Pennacchio and Rubin 2003; Vaessen et al. 2006). In the present study, we could confirm the previous findings regarding associations with triglyceride levels and stroke susceptibility.
More recently, in a genome-wide association study, variants of different genes associated with elevated triglyceride levels were identified (Saxena et al. 2007). Glucokinase regulatory protein has an inhibitory effect on glucokinase, which depends on the presence of fructose-6-phosphate, antagonized by fructose-1-phosphate (Warner et al. 1995). The mutations in GCKR gene resulting in the synthesis of proteins with increased inhibitory activity might be diabetogenic, likely reflecting elevated sensitivity to fructose-6-phosphate or reduced susceptibility to antagonism by fructose-1-phosphate. Probably, glucokinase has a role in the development of maturity-onset diabetes of the young type II. The Diabetes Genetics Initiative genome-wide association study (Saxena et al. 2007) for type II diabetes and quantitative metabolic traits described one intronic polymorphism of the GCKR gene (rs780094). This SNP showed a trend toward association with lower fasting glycemia, less insulin resistance, and lower chance for the development of type II diabetes had been verified. Previously, it was proven that the rs780094 is in strong linkage disequilibrium with the other nonsynonymous GCKR variant, the rs1260326 (Vaxillaire et al. 2008). The rs1260326 (Leu446Pro) is in connection with triglyceride levels and impaired fasting glycemia and might have a risk of type II diabetes mellitus (Veiga-da-Cunha et al. 2003). Vaxillaire et al. established that the minor allele (T) of rs1260326 variant in the GCKR gene could protect against diabetes mellitus type II (Vaxillaire et al. 2008). Although, the minor T-allele of rs1260326 was associated with increased triglyceride levels and higher risk of dyslipidemia, it showed lower fasting plasma glucose rates and decreased risk of hyperglycemia. S. Kathiresan et al. and C. J. Willer et al. investigated the effect of GCKR gene on triglyceride level elevation and have found a positive association with both the rs1260326 and the rs780094 SNPs (Kathiresan et al. 2008b; Willer et al. 2008).
In Hungarian population, in stroke patients, the GCKR gene has not been reported so far; accordingly, our goal was to scrutinize the possible gene variance and the correlation between gene effect and changes of triglyceride rates. We concluded that, except for T1259C, all APOA5 variants had a significantly increased allele frequency and were associated with significantly elevated triglyceride levels in stroke patients collated with controls, suggesting an association with the development of stroke disease. By contrast, in the case of GCKR gene (rs1260326), we could not detect any difference in allele frequencies compared with controls and also found a nonsignificant triglyceride level rise. As a result, the variant of GCKR gene (rs1260326) is not in correlation with the susceptibility for the disease in our study group. Additionally, we observed that in all stroke subgroups diabetes mellitus was significantly associated with the development of stroke disease, despite the small number of cases. Perez-Martinez et al. (2009) studied the additive and/or synergistic effects of GCKR gene rs780094 variant with APOA5 gene polymorphisms (T-1131C, C56G). We also examined the genotype combinations of the GCKR (rs1260326) and APOA5 gene variants (T-1131C, T1259C, IVS+G476A, and C56G) but could not detect relevant changes—neither in triglyceride levels nor in odds ratios for the ischemic stroke disease—compared to individual effects of these genes. In summary, in agreement with our previous results, the frequencies of APOA5 minor alleles are higher in stroke patients than in controls, and we can conclude that there is a relationship between the triglyceride increase and the variants studied. We could detect a possible risk for the development of stroke in connection with the APOA5 polymorphisms. However, we did not find any association between GCKR variant and either the triglyceride levels or the susceptibility for the disease; thus, in the Hungarian population, the GCKR gene polymorphism did not prove to be an independent risk factor. Examining the combinations of four APOA5 polymorphisms with the GCKR variant, we found that in certain subgroups, the gene combinations increased the risk for the development of stroke. In conclusion, further well-powered studies investigating larger populations are required to understand the role of GCKR mutations in ischemic stroke susceptibility and to help in developing a more effective clinical prevention.
References
Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM (2007) Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298:309–316
Farrelly D, Brown KS, Tieman A et al (1999) Mice mutant for glucokinase regulatory protein exhibit decreased liver glucokinase: a sequestration mechanism in metabolic regulation. Proc Natl Acad Sci USA 96:14511–14516
Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG (2008) Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA 300:2142–2152
Grimsby J, Coffey JW, Dvorozniak MT et al (2000) Characterization of glucokinase regulatory protein-deficient mice. J Biol Chem 275:7826–7831
Groenendijk M, Cantor RM, De Bruin TW, Dallinga-Thie GM (2001) The apoAI-CIII-AIV gene cluster. Atherosclerosis 157:1–11
Hachinski V, Graffagnino C, Beaudry M et al (1996) Lipids and stroke: a paradox resolved. Arch Neurol 53:303–308
Haheim LL, Holme I, Hjermann I, Leren P (1993) Risk factors of stroke incidence and mortality. a 12-year follow-up of the Oslo Study. Stroke 24:1484–1489
Havasi V, Szolnoki Z, Talian G et al (2006) Apolipoprotein A5 gene promoter region T-1131C polymorphism associates with elevated circulating triglyceride levels and confers susceptibility for development of ischemic stroke. J Mol Neurosci 29:177–183
Hayward BE, Fantes JA, Warner JP et al (1996) Co-localization of the ketohexokinase and glucokinase regulator genes to a 500-kb region of chromosome 2p23. Mamm Genome 7:454–458
Hayward BE, Dunlop N, Intody S et al (1998) Organization of the human glucokinase regulator gene GCKR. Genomics 49:137–142
Kathiresan S, Melander O, Anevski D et al (2008a) Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med 358:1240–1249
Kathiresan S, Melander O, Guiducci C et al (2008b) Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet 40:189–197
Maasz A, Kisfali P, Jaromi L et al (2008a) Apolipoprotein A5 gene IVS3 + G476A allelic variant confers susceptibility for development of ischemic stroke. Circ J 72:1065–1070
Maasz A, Kisfali P, Szolnoki Z, Hadarits F, Melegh B (2008b) Apolipoprotein A5 gene C56G variant confers risk for the development of large vessel associated ischemic stroke. J Neurol 255:649–654
Martinelli N, Girelli D, Ferraresi P et al (2007a) Increased factor VIII coagulant activity levels in male carriers of the factor V R2 polymorphism. Blood Coagul Fibrinolysis 18:125–129
Martinelli N, Trabetti E, Bassi A et al (2007b) The -1131 T>C and S19W APOA5 gene polymorphisms are associated with high levels of triglycerides and apolipoprotein C-III, but not with coronary artery disease: an angiographic study. Atherosclerosis 191:409–417
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Morrison AC, Bare LA, Chambless LE et al (2007) Prediction of coronary heart disease risk using a genetic risk score: the Atherosclerosis risk in communities study. Am J Epidemiol 166:28–35
Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A (2007) Non-fasting triglycerides and risk of for myocardial infarction and death among women and men. Ugeskr Laeger 169:3865–3868
Pennacchio LA, Rubin EM (2003) Apolipoprotein A5, a newly identified gene that affects plasma triglyceride levels in humans and mice. Arterioscler Thromb Vasc Biol 23:529–534
Perez-Martinez P, Corella D, Shen J et al (2009) Association between glucokinase regulatory protein (GCKR) and apolipoprotein A5 (APOA5) gene polymorphisms and triacylglycerol concentrations in fasting, postprandial, and fenofibrate-treated states. Am J Clin Nutr 89:391–399
Rothwell PM, Coull AJ, Silver LE et al (2005) Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 366:1773–1783
Salonen JT, Puska P, Tuomilehto J, Homan K (1982) Relation of blood pressure, serum lipids, and smoking to the risk of cerebral stroke. A longitudinal study in Eastern Finland. Stroke 13:327–333
Saxena R, Voight BF, Lyssenko V et al (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336
Simons LA, McCallum J, Friedlander Y, Simons J (1998) Risk factors for ischemic stroke: Dubbo study of the elderly. Stroke 29:1341–1346
Szalai C, Keszei M, Duba J et al (2004) Polymorphism in the promoter region of the apolipoprotein A5 gene is associated with an increased susceptibility for coronary artery disease. Atherosclerosis 173:109–114
Szolnoki Z, Somogyvari F, Kondacs A et al (2003) Evaluation of the modifying effects of unfavourable genotypes on classical clinical risk factors for ischaemic stroke. J Neurol Neurosurg Psychiatry 74:1615–1620
Trichopoulou A, Yiannakouris N, Bamia C, Benetou V, Trichopoulos D, Ordovas JM (2008) Genetic predisposition, nongenetic risk factors, and coronary infarct. Arch Intern Med 168:891–896
Vaessen SF, Schaap FG, Kuivenhoven JA et al (2006) Apolipoprotein A-V, triglycerides and risk of coronary artery disease: the prospective Epic-Norfolk population study. J Lipid Res 47:2064–2070
Vaxillaire M, Vionnet N, Vigouroux C et al (1994) Search for a third susceptibility gene for maturity-onset diabetes of the young. Studies with eleven candidate genes. Diabetes 43:389–395
Vaxillaire M, Cavalcanti-Proenca C, Dechaume A et al (2008) The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes 57:2253–2257
Veiga-da-Cunha M, Delplanque J, Gillain A et al (2003) Mutations in the glucokinase regulatory protein gene in 2p23 in obese French caucasians. Diabetologia 46:704–711
Warner JP, Leek JP, Intody S, Markham AF, Bonthron DT (1995) Human glucokinase regulatory protein (GCKR): cDNA and genomic cloning, complete primary structure, and chromosomal localization. Mamm Genome 6:532–536
Willer CJ, Sanna S, Jackson AU et al (2008) Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 40:161–169
Wright WT, Young IS, Nicholls DP, Patterson C, Lyttle K, Graham CA (2006) SNPs at the APOA5 gene account for the strong association with hypertriglyceridaemia at the APOA5/A4/C3/A1 locus on chromosome 11q23 in the Northern Irish population. Atherosclerosis 185:353–360
Yang Y, Ruiz-Narvaez E, Niu T, Xu X, Campos H (2004) Genetic variants of the lipoprotein lipase gene and myocardial infarction in the Central Valley of Costa Rica. J Lipid Res 45:2106–2109
Yiannakouris N, Trichopoulou A, Benetou V, Psaltopoulou T, Ordovas JM, Trichopoulos D (2006) A direct assessment of genetic contribution to the incidence of coronary infarct in the general population Greek EPIC cohort. Eur J Epidemiol 21:859–867
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Járomi, L., Csöngei, V., Polgár, N. et al. Functional Variants of Glucokinase Regulatory Protein and Apolipoprotein A5 Genes in Ischemic Stroke. J Mol Neurosci 41, 121–128 (2010). https://doi.org/10.1007/s12031-009-9301-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-009-9301-9