Abstract
Acetylcholinesterase (AChE) is well known to process different molecular forms via the distinct interacting partners. Proline-rich membrane anchor (PRiMA)-linked tetrameric globular AChE (G4 AChE) is mainly found in the vertebrate brain; however, recent studies from our laboratory have suggested its existence at neuromuscular junctions (nmjs). Both muscle and motor neuron express AChE at the nmjs. In muscle, the expression of PRiMA-linked AChE is down-regulated during myogenic differentiation and by motor neuron innervation. As compared with muscle, spinal cord possessed higher total AChE activity and contained PRiMA-linked AChE forms. The spinal cord expression of this form increased during development. More importantly, PRiMA-linked G4 AChE identified as aggregates localized at nmjs. These findings suggest that the restricted localization of PRiMA-linked G4 AChE at the nmjs could be contributed by the pre-synaptic motor neuron and/or the post-synaptic muscle fiber.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a cholinergic synapse, acetylcholinesterase (AChE; EC 3.1.1.7) is responsible for hydrolyzing the neurotransmitter acetylcholine in order to effectively and efficiently control the neurotransmission process. Different molecular forms of AChE are found in various tissues of vertebrates, where they are controlled by the C terminus of AChE catalytic subunit (AChET) via alternative splicing, as well as the molecular interaction of the associating partners, namely collagen-tailed subunit (ColQ) and proline-rich membrane anchor (PRiMA) (Massoulié et al. 2005). In brain and neurons, AChET subunit interacts with PRiMA to form a tetrameric globular form (PRiMA-linked G4 AChE) (Falasca et al. 2005; Perrier et al. 2002), while in muscle, it assembles with another partner ColQ to produce asymmetric forms (A-form AChE) at vertebrate neuromuscular junctions (nmjs; Massoulié 1993). For PRiMA, two different isoforms, namely PRiMA I and PRiMA II, are generated from the PRiMA gene by alternative splicing, which PRiMA I contains a long C-terminal cytoplasmic tail, while PRiMA II contains a short motif after the transmembrane region (Perrier et al. 2003).
At the nmjs, the ColQ-associated A-form AChE is the predominant species and its existence in muscle coincides with the establishment of neuromuscular contacts during development and regeneration (Lyles et al. 1979; Massoulié 1993). However, G4 AChE indeed also exists in muscles. Previous studies found that the amount of G4 enzyme is regulated by the dynamic activity of skeletal muscles. The transcriptional regulation of PRIMA gene is suppressed during myogenic differentiation process (Xie et al. 2007). In accordance to the change of PRiMA expression, the proportion of PRiMA-linked G4 AChE is also decreased during the myogenic differentiation process in C2C12 muscle (Xie et al. 2007). In addition, the level of G4 enzyme is different in muscle fiber types; fast-twitch muscles contain a high amount of G4 AChE, whereas slow-twitch muscles contain a much smaller amount (Bacou 1982; Leung et al. 2009). Although PRiMA and G4 enzyme are shown to exist in muscle, the presence of PRiMA expression in spinal cord and/or motor neuron has not been revealed yet. In our present work, we examined the existence of PRiMA mRNA and G4 AChE in spinal cord, and we revealed the synaptic localization of PRiMA at the nmjs, which support the notion that PRiMA-linked G4 AChE might be important for synaptic transmission.
Materials and Methods
Tissue Collection
Adult rats were sacrificed by decapitation under the regulation of the Animal Care and Plant Facility of The Hong Kong University of Science and Technology. Total brain (include cerebrum and cerebellum), spinal cord, and skeletal muscle (tibialis) were collected and immediately frozen in liquid nitrogen. Tissues were stored at −80°C for subsequent analysis. For developmental studies, rats at different postnatal stages were sacrificed to collect tibialis and spinal cord.
PCR Analysis
Total RNAs from the tissues were isolated by TRIzol reagent (Invitrogen, Carlsbad, CA), and reverse-transcribed by Moloney Murine Leukemia Virus Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. The presence of transcripts encoding AChET, PRiMA I and II isoforms were analyzed by standard polymerase chain reaction (PCR). The primers were: 5’-TCT GAC TGT CCT GGT CAT CAT TTG CTA C-3’ and 5’-TCA CAC CAC CGC AGC GTT CAC-3’ for PRiMA isoform I and II [Genbank number NM 133364 and NM 178023], 5’-CTG GGG TGC GGA TCG GTG TAC CCC-3’ and 5’-TCA CAG GTC TGA GCA GCG TTC CTG-3’ for AChET (Boudreau-Larivière et al. 2000), 5’-AAC GGA TTT GGC CGT ATT GG-3’ and 5’-CTT CCC GTT CAG CTC TGG G-3’ for GAPDH (Lee et al. 2004), 5’TGT GAT GCC CTT AGA TGT CC-3’ and 5’-GAT AGT CAA GTT CGA CCG TC3’ for 18S rRNA. The PCR products were examined by gel electrophoresis, and the identity of each amplicon was confirmed by DNA sequencing. For quantitation, real-time PCR was performed by SYBR Green Master mix and Rox reference dye, according to the manufacturer’s instructions (Applied Bioscience, Foster City, CA). The SYBR green signal was detected by Mx3000p™ multiplex quantitative PCR machine (Stratagene, La Jolla, CA). Transcript expression levels were quantified by using the ΔΔC t value method (Xie et al. 2007), where values were normalized to 18S rRNA as an internal control in the same sample. PCR products were analyzed by gel electrophoresis, and the specificity of amplification was confirmed by the melting curves.
AChE Activity and Sucrose Density Gradient Analysis
Tissues were homogenized in the lysis buffer containing 10 mM HEPES, 5 mM EDTA, 5 mM EGTA, 0.1% Triton X-100, 1 mg/ml bacitracin, 10 μg/ml aprotinin, and 10 μg leupeptin. AChE enzymatic activity was determined according to the method of Ellman with the modification of adding 0.1 mM tetraisopropylpyrophosphoramide (iso-OMPA), an inhibitor of butyrylcholinesterase (Choi et al. 2008). Separation of the various molecular forms of AChE was performed by sucrose density gradient analysis, as described previously (Xie et al. 2007). In brief, sucrose gradients (5% and 20%) in lysis buffer (10 mM HEPES, pH 7.5, 1 M NaCl, 1 mM EDTA, 1 mM EGTA, and 0.5% Triton X-100) were prepared in 12 ml polyallomer ultra-centrifugation tubes with a 0.4 ml cushion of 60% sucrose on the bottom. Cell extracts (0.2 ml) mixed with sedimentation markers (alkaline phosphotase, 6.1S; β-galactosidase, 16S) were loaded onto the gradients and centrifuged at 38,000 rpm in a Beckman SW41 rotor at 10°C for 16 h. Approximately 45 fractions were collected to determine the AChE enzymatic activity. The amount of PRiMA-linked AChE between muscle and spinal cord was determined by summation of the enzymatic activities corresponding to the peaks of G4 AChE in the sedimentation profile, and normalized by the difference of total AChE activity in the crude lysate.
Sciatic Nerve Section
Two-month-old Sprague–Dawley (SD) rats weighing ∼250 g were anesthetized by isoflurane. A ∼3-mm portion of the sciatic nerve located around the upper thigh, was removed by an aseptic surgical technique (Xie et al. 2007; Leung et al. 2009). Rats were sacrificed according to the instructions of Animal Care Facility of The Hong Kong University of Science and Technology. Spinal cord (lumbar) and tibialis muscles were collected 7 days after denervation. Samples were frozen in liquid nitrogen immediately after dissection and stored at −80°C for RNA and protein extraction and for confocal Microscopy. Control experiments were performed by sham operation on different rats.
Immuno-Fluorescent Staining
Tibialis muscles were dissected from sham-operated and denervated rats and frozen in an isopentane/liquid nitrogen bath, and sectioned by a cryostat with 20 μm thickness. Tissue sections were fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) for 10 min at room temperature followed by quenching with 50 mM ammonium chloride for 25 min. Blocking was performed in PBS containing 5% normal goat serum and 0.1% Triton X-100 for 1 h. The sections were then triple-stained by Alexa 633conjugated α-bungarotoxin (Invitrogen; 1:1,000), anti-AChE antibody (Santa Cruz Biotechnology; clone E-19; 1:500) and anti-PRiMA antibody (Xie et al. 2007; Leung et al. 2009; 2 μg/ml) in the blocking solution for 48 h at 4°C. Followed by 4× PBS washing for 10 min each, slides were incubated with Alexa 488-conjugated anti-rabbit and Alexa 555-conjugated anti-goat secondary antibodies (Invitrogen; 1:1,000) for 3 h. After 4× PBS washing for 10 min each, sections were sequentially dehydrated by ethanol (50%, 75%, and 100%) and mounted by fluorescent mounting medium (DakoCytomation, Carpinteria, CA). Confocal laser scanning microscopy was used for slide examination with Ex 488/Em 500–535 nm, Ex 543/Em 560–615 and Ex 643/Em 660–750 for Alexa 488, 555, and 633 fluorophores, respectively.
Protein Assay
Protein concentrations were measured routinely by Bradford's method (Bradford 1976) using a kit from Bio-Rad Laboratories (Hercules, CA).
Results
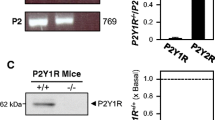
To analyze the participation of PRiMA-linked G4 AChE at rat nmjs, the existence of PRiMA mRNA, was at first measured. Tissues of skeletal muscle (tibialis) and spinal cord from adult rat were collected to perform RT-PCR. As a control, whole brain (cerebrum and cerebellum), containing the transcripts encoding all the subunits of AChET, PRiMA I, and PRiMA II was used (Fig. 1a). In line with our previous findings (Xie et al. 2007), only PRiMA I but not PRiMA II isoform was present in skeletal muscle. Interestingly, adult spinal cord possessed both PRiMA isoforms, with the majority being PRiMA I mRNA. GAPDH and 18S rRNA served as internal controls for cDNA input. By using quantitative PCR, the amounts of AChET and PRiMA (both I and II) were determined in muscle and spinal cord during development. The level of AChET mRNA increased markedly in both muscle and spinal cord during development. In contrast, the level of PRiMA mRNA decreased in adult muscle, whereas high levels remained in the spinal cord.
Differential expression of transcripts encoding PRiMA I and II in muscle and spinal cord. a Tissues of whole brain (cerebrum + cerebellum), skeletal muscle (tibialis), and spinal cord were collected from adult rat. Total RNAs were extraction for RT-PCR analysis of transcript encoding AChET (671 bp), PRiMA I (145 bp), and II (302 bp). GAPDH and 18S rRNA were used as the internal controls. Representative photo were shown, n = 4. b Different developmental stages (postnatal 1 to 60) of tibialis and spinal cord were collected. Total RNAs were extracted and subjected to real-time PCR analysis for measuring the change of AChET and PRiMA mRNAs. Data were expressed as x basal where the value of postnatal day 1 was set as 1, mean ± SEM, n = 3
In view of the co-existence of AChET and PRiMA I/II mRNAs in spinal cord, the characterization of PRiMA-linked G4 AChE was of interest. Total lysates from spinal cord, muscle, and brain were assayed for AChE activity in order to estimate the relative amount of the enzyme. Results indicated that among the three tissues, total brain contained the highest enzymatic activity. The spinal cord possessed high AChE activity, while that of muscle contained the lowest amount (Fig. 2a). To reveal the molecular forms, sucrose density gradient analysis was performed. Muscle expressing the PRiMA-linked G4 AChE and ColQ-linked asymmetric AChE was used as a positive control (Fig. 2b). In the spinal cord, the dominant molecular form was the G4 enzyme, with small proportion of G1 AChE (Fig. 2b). To determine the relative amount of PRiMA-linked G4 AChE to other AChE forms in muscle and spinal cord, the total area under the peak of G4 fractions was integrated and normalized to the total AChE activity in all peaks as shown in Fig. 2a. The ratio of G4 enzyme between muscle and spinal cord was ∼1:5 (Fig. 2c), which indicated that spinal cord contained a higher abundance of PRiMA-linked G4 AChE than the muscle. Within the spinal cord, the motor neuron is the major cell type expressing AChET and PRiMA (Wan et al. 1997; Leung et al. 2009).
Spinal cord contains more PRiMA-linked G4 AChE than that of muscle. a Tissues of brain, muscle, and spinal cord were homogenized for protein extraction. AChE enzymatic activity was performed by Ellman method. Data were expressed as arbitrary unit. b Total lysates from (a) were subjected to sucrose density gradient analysis to reveal the molecular forms. AChE activity was plotted as a function of the sedimentation value (S), estimated from the position of the sedimentation markers. Enzymatic activities are expressed in arbitrary units, and representative sedimentation profiles are shown. Data were expressed as arbitrary unit. c The amount of G4 AChE in muscle and spinal from (b) was quantified by summation of the enzymatic activities corresponding to the peaks of G4 AChE in the sedimentation profile. Data were normalized by the total AChE activity in (a) and expressed as arbitrary unit, mean ± SEM, n = 4
Although the above study demonstrated the existence of PRiMA mRNA and G4 enzyme in muscle and spinal cord, the precise location of PRiMA-linked G4 AChE at the nmjs was unknown. Therefore, an immuno-fluorescent staining was performed to identify the location of PRiMA in muscle. The sections of adult tibialis were triple-stained to visualize acetylcholine receptor (AChR), AChET, and PRiMA. Results showed that PRiMA was found aggregated and localized at the nmjs; the signal for PRiMA overlapped with that of AChR and AChET (Fig. 3). In addition, PRiMA-linked AChE staining at the nmjs was reduced following denervation of the sciatic nerve, which suggested that the major contribution of PRiMA-linked G4 AChE in the nmjs was from the pre-synaptic nerve.
Localization of AChE and PRiMA at the nmjs. Tibialis were collected from sham-operated and denervated rats, and sectioned for immuno-histofluorescent staining by Alexa 633-conjugated α-bungarotioxin (pseudo color) to locate the nmjs, anti-AChE antibody/Alexa 555-conjugated anti-goat secondary antibody (red), and anti-PRiMA antibody/Alexa 488-conjugated anti-rabbit secondary antibody (green). The triple-stained sections were examined by confocal microscopy to visualize the localization of PRiMA. Representative photo were shown, n = 4
Discussion
At the nmjs, the ColQ-associated AChE, attached onto basal lamina (McMahan et al. 1987), is the predominant molecular form controlling the muscle contraction process. On the other hand, PRiMA-linked G4 AChE that anchors into plasma membrane (Perrier et al. 2002) is the major form in brain. This evidence may suggest the separation of different forms of AChE in central and peripheral nervous systems. Interestingly, the current study demonstrated that both skeletal muscle and spinal cord expressed PRiMA and the G4 enzyme, and at least part of the PRiMA-linked G4 AChE targeted to the nmjs (Fig. 4).
Contribution of synaptic G4 AChE at the nmjs. At the nmjs, muscle fiber can synthesize different AChE subunits, including AChET, PRiMA, and ColQ. They are assembled to form PRiMA-linkded G4AChE and ColQ-associated A-form AChE, and anchored onto plasma membrane and basal lamina, respectively. In addition to the contribution by muscle, motor neurons also express AChET and PRiMA, which the functional G4 enzymes are targeted to nerve terminus. Therefore, both of the neuron- and muscle-derived PRiMA-linked G4 AChE may co-operate with ColQ-associated AChE for the proper control of muscle contraction process
There are three different cell types that can provide and produce AChE at the nmjs, namely motor neuron, muscle fiber, and Schwann cell. Muscle indeed is the dominant source for different molecular forms of AChE during development (De la Porte et al. 1986; Massoulié 2002). The Schwann cell, on the other hand, might only provide AChE in limited amount at the nmjs (Anglister 1991). According to our results and a previous study (Tsim et al. 1997), motor neurons in the spinal cord might be another source of the synaptic G4 AChE. The expression of PRiMA in neurons was reported to be tightly controlled by neuronal differentiation: PRiMA mRNA, protein, and the G4 enzyme were up-regulated during maturation of cultured cortical neurons (Xie et al., 2009). In response to growth factor, muscle-derived calcitonin gene-related peptide and its downstream cAMP-dependent signaling pathway have been shown to increase the transcriptional activity of AChET in motor neurons (Jiang et al. 2003), which is in accordance with the vital role of muscle-derived factors in controlling the expression of pre-synaptic proteins at the nmjs (Loeb and Fischbach, 1997). Such a cAMP-mediated signal cascade was also demonstrated to stimulate the amount of AChET, PRiMA, and G4 AChE in neuronal PC12 cells (Choi et al. 2008). Therefore, the gene expression of AChET and PRiMA in motor neurons may contribute the synaptic localization of PRiMA at the neuromuscular junctions, which suggests a critical role of PRiMA-linked G4 AChE in regulating the muscle contraction process.
References
Anglister, L. (1991). Acetylcholinesterase from the motor nerve terminal accumulates on the synaptic basal lamina of the myofiber. Journal of Cell Biology, 115, 755–764.
Bacou, F. (1982). Acetylcholinesterase forms in fast and slow rabbit muscle. Nature, 296, 661–664.
Boudreau-Larivière, C., Chan, R. Y., Wu, J., & Jasmin, B. J. (2000). Molecular mechanisms underlying the activity-linked alterations in acetylcholinesterase mRNAs in developing versus adult rat skeletal muscles. Journal of Neurochemistry, 74, 2250–2258.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Choi, R. C. Y., Mok, M. K., Cheung, A. W., Siow, N. L., Xie, H. Q., & Tsim, K. W. K. (2008). Regulation of PRiMA-linked G4 AChE by a cAMP-dependent signaling pathway in cultured rat pheochromocyoma PC12 cells. Chemico-Biological Interactions, 175, 76–78.
De la Porte, S., Vallette, F. M., Grassi, J., Vigny, M., & Koenig, J. (1986). Presynaptic or postsynaptic origin of acetylcholinesterase in heterologous nerve-muscle cocultures. Developmental Biology, 116, 69–77.
Falasca, C., Perrier, N., Massoulié, J., & Bon, S. (2005). Determinants of the t peptide involved in folding, degradation and secretion of acetylcholinesterase. Journal of Biological Chemistry, 280, 878–886.
Jiang, J. X. S., Choi, R. C. Y., Siow, N. L., Lee, H. H. C., Wan, D. C. C., & Tsim, K. W. K. (2003). Muscle induces neuronal expression of acetylcholinesterase in neuron-muscle co-culture: Transcriptional regulation mediated by cAMP-dependent signaling. Journal of Biological Chemistry, 278, 45435–45444.
Lee, H. H., Choi, R. C., Ting, A. K., Siow, N. L., Jiang, J. X., Massoulié, J., et al. (2004). Transcriptional regulation of acetylcholinesterase-associated collagen ColQ: Differential expression in fast and slow twitch muscle fibers is driven by distinct promoters. Journal of Biological Chemistry, 279, 27098–27107.
Leung, K. W., Xie, H. Q., Chen, V. P., Mok, M. K. W., Chu, G. K. Y., Choi, R. C. Y., et al. (2009). Restricted localization of proline-rich membrane anchor (PRiMA) of globular form acetylcholinesterase at the neuromuscular junctions: Contribution and expression from motor neurons. FEBS J, 276, 3031–3042.
Loeb, J. A., & Fischbach, G. D. (1997). Neurotrophic factors increase neuregulin expression in embryonic ventral spinl cord neurons. Journal of Neuroscience, 17, 1416–1424.
Lyles, J. M., Silman, I., & Barnard, E. A. (1979). Developmental changes in levels and forms of cholinesterases in muscles of normal and dystrophic chickens. Journal of Neurochemistry, 33, 727–738.
Massoulié, J. (1993). Molecular and cellular biology of cholinesterases. Progress in Neurobiology, 41, 31–91.
Massoulié, J. (2002). The origin of the molecular diversity and functional anchoring of cholinesterases. Neurosignals, 11, 130–143.
Massoulié, J., Bon, S., Perrier, N., & Falasca, C. (2005). The C-terminal peptides of acetylcholinesterase: Cellular trafficking, oligomerization and functional anchoring. Chemico-Biological Interactions, 157–158, 3–14.
McMahan, U. J., Sanes, J. R., & Marshall, L. M. (1987). Cholinesterase is associated with the asal lamina at the neuromuscular junction. Nature, 271, 172–174.
Perrier, A. L., Massoulié, J., & Krejci, E. (2002). PriMA: The membrane anchor of acetylcholinesterase in the brain. Neuron, 33, 275–285.
Perrier, N. A., Khérif, S., Perrier, A. L., Dumas, S., Mallet, J., & Massoulié, J. (2003). Expression of PRiMA in the mouse brain: Membrane anchoring and accumulation of ‘tailed’ acetylcholinesterase. European Journal of Neuroscience, 18, 1837–1847.
Tsim, K. W. K., Choi, R. C. Y., Dong, T. T. X., & Wan, D. C. C. (1997). A globular, not asymmetric, form of acetylcholinesterase is expressed in chick motor neurons: Down-regulation toward maturity and after denervation. Journal of Neurochemistry, 68, 479–487.
Wan, D. C., Ng, Y. P., Choi, R. C. Y., Cheung, P. W., Dong, T. T., & Tsim, K. W. K. (1997). Denervation decreases the ipsilateral expression of AChE in chick lumbric motor neurons. Neuroscience Letters, 232, 83–86.
Xie, H. Q., Choi, R. C. Y., Leung, K. W., Siow, N. L., Kong, L. W., Lau, F. T. C., et al. (2007). Regulation of a transcript encoding the proline-rich membrane anchor of globular muscle acatelycholinesterase. The suppressive roles of myogenesis and innervating nerves. Journal of Biological Chemistry, 282, 11765–11775.
Xie, H. Q., Choi, R. C. Y., Leung, W. K. W., Chen, V. P., Chu, G. K. Y., & Tsim, K. W. K. (2009). Transcriptional regulation of proline-rich membrane anchor (PRiMA) of globular form acetylcholinesterase in neuron: An inductive effect of neuron differentiation. Brain Res, 1265, 13–23.
Acknowledgements
The research was supported by grants from Research Grants Council (HKUST6419/06M, 662407, 662608, and N_HKUST629/07) of the Hong Kong SAR to KWKT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Proceedings of the XIII International Symposium on Cholinergic Mechanisms
Rights and permissions
About this article
Cite this article
Tsim, K.W.K., Leung, K.W., Mok, K.W. et al. Expression and Localization of PRiMA-Linked Globular Form Acetylcholinesterase in Vertebrate Neuromuscular Junctions. J Mol Neurosci 40, 40–46 (2010). https://doi.org/10.1007/s12031-009-9251-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-009-9251-2