Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by loss of motor neurons in the cerebral cortex, brain stem, and spinal cord. Most cases (90%) are classified as sporadic ALS (sALS). The remainder 10% are inherited and referred to as familial ALS, and 2% of instances are due to mutations in Cu/Zn superoxide dismutase (SOD1). Using cDNA microarray on postmortem spinal cord specimens of four sALS patients compared to four age-matched nonneurological controls, we found major changes in the expression of mRNA in 60 genes including increase of cathepsin B and cathepsin D (by the factors 2 and 2.3, respectively), apolipoprotein E (Apo E; factor 4.2), epidermal growth factor receptor (factor 10), ferritin (factor 2), and lysosomal trafficking regulator (factor 10). The increase in the expression of these genes was verified by quantitative reverse transcriptase polymerase chain reaction. Further analysis of these genes in hSOD1-G93A transgenic mice revealed increase in the expression in parallel with the deterioration of motor functions quantified by means of rotorod performance. The comparability of the findings in sALS patients and in the hSOD1-G93A transgenic mouse model suggests that the examined genes may play a specific role in the pathogenesis of ALS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease, characterized by the loss of motor neurons in the cerebral cortex, brain stem, and spinal cord. To date, six mutated genes have been reported to cause or predispose individuals to ALS (for review see Pasinelli and Brown 2006; Gros-Louis et al. 2006). The most widely studied are the mutations in the Cu–Zn-superoxide dismutase-1 gene (SOD1), located on chromosome 21, which has been identified in 15–20% of familial ALS (fALS) cases (Cudkowicz et al. 1997). Sporadic and familial forms are clinically and pathologically similar except for different age at onset and disease duration, and in both diseases there is marked involvement of the spinal cord including anterior horn cells. Moreover, several studies have demonstrated no major differences between fALS and sporadic ALS (sALS) (Bruijn et al. 2004) in terms of calcineurin-mediated pathways of signal transduction (Ferri et al. 2004), activity of CuZn–SOD (Jacobsson et al. 2001), glutamate toxicity, lipid peroxidation, and oxidative damage (Rothstein et al. 1992; Benotti et al. 2001; Howland et al. 2002; Pedersen et al. 1998; Gurney et al. 1996; Ferrante et al. 1997; Liu 1996). Expression of mutant SOD1 genes in transgenic mice causes progressive paralysis resembling ALS (Gurney et al. 1994) and provides insight into the mechanisms of motor neuron degeneration.
Several putative pathogenetic mechanisms have been discussed in sALS and the SOD1 ALS mice models, such as free toxic radicals (Liu 1996; Ferrante et al. 1997), lipid peroxidation (Pedersen et al. 1998; Guerney et al. 1996), glutamate toxicity (Rothstein et al. 1992; Bendotti et al. 2001; Howland et al. 2002; Guerney et al. 1996), lack of calcium-binding proteins, and inflammatory processes (P. L. McGeer and E. G. McGeer 2002). Several studies have found morphologic and biochemical changes suggesting programmed cell death (apoptosis) (Guégan and Przedborski 2003; Mu et al. 1996; Li et al. 2000; Embacher et al. 2001; Pasinelli et al. 2000; Martin 1999; Pedersen et al. 2000).
Although extensive research promotes our understanding of the pathophysiological mechanisms of ALS, the cause of the disease remains unknown. Most of the ALS studies looking for biochemical or genetic changes are based on mechanistic hypotheses and their outcome is theory-dependent. The aim of the current study was to screen spinal cord tissue of sALS patients using cDNA gene array approach for alterations in gene expression potentially related to motor neuron degeneration and to find out whether comparable changes occur in the hSOD1-G93A mouse model. We identified genes that were upregulated both in sALS spinal cord tissue and in the hSOD1-G93A mouse model of ALS.
Material and Methods
Tissues from Human Control and ALS Patients
The study was approved by the Tel-Aviv University and the Ministry of Health local ethical committee. Autopsies and human postmortem studies were conducted according to Austrian law. Blocks of cervical spinal cord from four nonneurological control persons and four patients with sALS were obtained at autopsy (Table 1). The study included tissue specimen of patients described previously (ALS cases numbers 2 and 5, controls numbers 1 and 7; Embacher et al. 2001). All sALS patients fulfilled both the clinical and the neuropathological criteria of ALS (Embacher et al. 2001). Part of the tissue was frozen at −30°C in methyl-butane, transferred in dry ice, and stored at −80°C. The remaining tissue was immersed in formalin and paraformaldehyde as described previously (Embacher et al. 2001).
Animal Model
The colony of TgN(SOD1-G93A)1Gur transgenic mice (Gurney et al. 1994) was obtained from the Jackson Laboratory (USA). The mice were bred in CSJLF1, and at 1 month of age, offspring were genotyped by polymerase chain reaction (PCR) analysis, using the following PCR primers: IL2 CTAGGCCACAGAATTGAAAGATCT and GTAGGTGGAAATTCTAGCATCATCC, SOD1 CATCAGCCCTAATCCATCTGA and CGCGACTAACAATCAAAGTGA. The annealing temperature is 53°C, the PCR products 236 bp (hSOD1), 324 bp (IL2). Mice used in this study were healthy until 3 months of age and become progressively paralyzed after 4 to 5 months of age onwards. The motor function test was performed on Rotorod (San Diego Industries, San Diego, CA, USA) 16 rpm speed. The time until the mouse fall or maximum 2 min was measured three times for each mouse and the average was calculated. Thirty two transgenic and wild-type mice were sacrificed at the ages of 30, 80, and 120 days. The whole spinal cords were removed and stored at −80°C. The mRNA of six selected genes [cathepsin B and D, Apo E, epidermal growth factor (EGF) receptor (EGFR), ferritin, and lysosomal trafficking regulator (LYST)] was measured.
Preparation of mRNA and Gene Array Analysis
Total RNA from spinal cord sections of sALS patients, nonneurological controls, and mice expressing the human mutant SOD1 was extracted using the Atlas pure total RNA labeling system kit (Clontech, Mountain View, CA, USA). RNA was quantified by spectrophotometer and separated on 1% agarose formaldehyde-denaturing gel electrophoreses to verify its integrity. Two micrograms of DNase I (RNase-free) treated total RNA from each spinal cord tissue was processed according to the Clontech protocol and hybridized with the Clontech cDNA-expression arrays human 3.6 (3 membranes containing 1,176 human specific probes each). The hybridization signals were measured with a phosphor imager (Cyclone, Packard, Pangbourne, UK) and the Clontech Image Software. Calculation of each cDNA expression ratio was based on normalization to the global signal background.
Reverse Transcription Reaction
Reverse transcription (RT) was carried out on 0.5-μg RNA samples using the 10-U enzyme RT-superscript II (Gibco-BRL, Gaithersburg, MD, USA) in a mixture containing 2 μM Random primer (Invitrogen), 1xBuffer supplied by the manufacturer, 10 mM dithiothreitol, 20 μM deoxyribonucleotide triphosphates, and RNase inhibitor (RNAguard, Amersham Pharmacia Biotech, Buckinghamshire, UK). RT was performed in a Thermocycler: 25°C for 10 min, 42°C for 2 h, 70°C for 15 min, and 95°C for 15 min.
Real-Time Quantitative RT-PCR
RT-PCR was performed in an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA, USA) using Syber green PCR master mix and specific primers (see Table 2) at final concentrations of 500 nmol/L. We used amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for stimulated conditions for sample normalization. The amplification protocol was 40 cycles of 94°C for 15 s followed by 60°C for 1 min each. Quantification of gene expression relative to GAPDH was calculated by the protocols \( 2^{{ - \Delta \Delta _{{CT}} }} \) method and from standard curve method.
Protein Preparation and Western Blot Analysis
Protein preparation was performed as previously described (Guegan et al. 2001). Tissue was gently homogenized with a glass–glass homogenizer in 10 vol (w/v) of cold buffer containing 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 2 mM EDTA, 20 mM HEPES, and protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN, USA). Homogenates were centrifuged (10,000×g, 10 min, in 4°C) and supernatants were collected. Protein concentration was determined with the BCA protein assay (Pierce, Rockford, IL, USA). The proteins were separated on 12% sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene difluride membrane (Bio-Rad, Hercules, CA, USA). Blots were probed with the mouse anti-heavy neurofilament (NF) (Sigma, St. Louis, MO, USA) diluted at 1:2,000 in 5% nonfat milk in Tris-buffered saline plus Tween 20 (TBS-T). Equal loadings of proteins were probed with mouse monoclonal antibody to actin (Chemicon, Temecula, CA, USA) diluted at 1:1,000 in 5% nonfat milk in TBS-T. The filters were then exposed to horseradish-peroxidase conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, USA) diluted 1:20,000, detected by SuperSignal® chemiluminescent (Pierce) and exposed to medical x-ray film (Fuji Photo Film, Tokyo, Japan). X-ray film densitometry was done in Versa Doc® imaging system (Bio-Rad).
Immunohistochemistry (IHC)
To verify morphological correlates of the biochemical studies and to find out whether our results corresponded to the literature (Masliah et al. 1996; Kikutchi et al. 2003), immunohistochemistry was performed on transverse 5-μm, formalin-fixed and paraffin-embedded, slide-mounted sections through the cervical spinal cord of two ALS patients and two controls using rabbit polyclonal IgG ABs to cathepsin D (catalog #. ab826, dilution 1:200) and horseradish-peroxidase secondary antibodies. The methods corresponded to those described previously (Embacher et al. 2001).
Statistical Analysis
Results are presented as mean ± standard errors of the mean; p values were calculated using two-tailed unpaired Student t test. In all tests, significance was assigned when p < 0.05.
Results
Immunohistochemistry of sALS spinal cords and nonneuronal controls shows a motor neuron reduction (Fig. 1a). Western blot analysis on those spinal cord extracts with anti-heavy NF antibodies demonstrated significantly lower levels in the sALS patients as compared to the four nonneurological control persons (minus 47%; Fig. 1b). The 200-kD bands density of the heavy NF from normal controls and the sALS patients was compared to the actin parallel bands. This specific reduction suggests loss of neurons and axons, which is characteristic for ALS.
The number of motor neurons in the anterior horn was reduced in ALS and part of the neurons appeared shrunken (b) as compared to matched controls (a). Statistically lower levels of heavy NF protein (HNF) in the spinal cords of ALS patients seen in Western blot analysis (asterisk p < 0.05) (c). The graph represents x-ray film densitometry of heavy NF calculated vs. the actin densitometry
To further analyze and compare the gene expression profiles of human sALS spinal cords with those of normal controls, we used the Clontech gene array containing 3,600 selected genes. We synthesized cDNAs from spinal cords of four ALS patients and four matched controls and hybridized them with the applied probes on the filters. First, we normalized the signal in each gene array with the expression of the housekeeping genes. Then, the ratio between the mean signals from the sALS patients and the normal controls were calculated. Table 3 presents all genes (N = 60) that showed a more than twofold increase or a less than 0.5-fold decrease in the sALS group compared to the control. The changes in all these presented genes were statistically significant (p < 0.05). Changes in transcription levels were found in a wide spectrum of gene groups, as classified by Clontech, including apoptosis, protein turnover, cell cycle functions, extracellular carrier/transport functions, cytoskeletal proteins, intracellular proteins, and transcription and translation proteins.
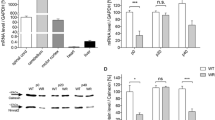
We decided to further analyze six genes that demonstrated either a dramatic elevation in expression levels in sALS patients or genes that were previously linked to the disease. The levels of the selected genes in sALS patient’s mRNA as compared to matched controls were cathepsin B (×2), cathepsin D (×2.3), apolipoprotein E (Apo E; ×4.2), EGFR [×(10 Ferritin; ×2)], and LYST (×10, Fig. 2). We also confirmed statistically increased expression of these genes by real-time PCR preformed on the same cDNA preparations, taken spinal cord tissues of healthy and ALS patients. We found indeed that the results from the gene array experiment and the real-time PCR analysis were comparable (Fig. 2).
As the cDNA preparations were taken postmortem from patients in the end stage of the disease, we intended to study the association of the transcripts levels and the disease progression. Therefore, we used the mice overexpressing human mutant SOD1, which are widely used as the animal model for ALS. The SOD1 Tg mice (A93G) developed progressive paresis until death at the age of 120–160 days (Fig. 3a,b). We isolated the RNA from the spinal cords on days 30, 80, and 120 and measured the mRNA expression of these six selected genes (cathepsin D and B, Apo E, EGFR, ferritin, and LYST).
a SOD-1 transgenic mice survival curve. b Transgenic and wild-type mice rotorod performance at different ages. c Apo-E mRNA expression is upregulated in spinal cord of human mutant SOD1 transgenic mice. d Cathepsin D mRNA expression is upregulated in spinal cord of human mutant SOD1 transgenic mice. e Cathepsin B mRNA expression is upregulated in spinal cord of human mutant SOD1 transgenic mice. f Ferritin mRNA expression is higher in SOD1 transgenic mice during all their life. g LYST mRNA expression is upregulated in spinal cord of human mutant SOD1 transgenic mice. h EGFR mRNA expression is upregulated in spinal cord of human mutant SOD1 transgenic mice. Asterisk p < 0.05
Apo-E expression was found to be low in the first 80 days of age (asymptomatic phase), whereas later it increased dramatically in parallel to the development of the motor symptoms (p < 0.05) (Fig. 3c). Similarly, cathepsin-B and cathepsin-D EGFR and LYST increased during disease progression and paralysis development, peaking on day 120, just before death (p < 0.05) (Fig. 3d,e,g,h). Ferritin level was stable along the 120 days but was higher than the wild type (p < 0.05).
Discussion
cDNA array is an established method to monitor changes in gene expression on a global scale in different pathological conditions. We found 60 genes that were differently expressed in the spinal cords of sALS patients as compared to controls. Some of the differently expressed genes, such as astrocyte glial fibrillary acidic protein (GFAP), cathepsin B, and Apo E, are know to be associated with ALS (Masliah et al. 1996; Lacomblez et al. 2002; Malaspina et al. 2001), whereas other genes such as LYST, EGFR, and ferritin are reported here for the first time in association with ALS. We therefore focused on EGFP and LYST due to their extraordinary upregulation (10-fold in the cDNA gene array). In addition, we looked at cathepsin D and ferritin because they seem to play an important role in other neurodegenerative diseases, such as Alzheimer’s disease (Papassotiropoulos et al. 2002; Nakanishi et al. 1997; Callahan et al. 1999; Nakamura et al. 1991; Simmons et al. 2007).
We found threefold higher mRNA levels of GFAP in the spinal cords of ALS patients as compared to the control.
Elevated expression of GFAP (×3), a marker for activate astrocytes, suggests astrogliosis in the late stage of the disease, as was already reported for advanced stage of ALS (Hoyaux et al. 2002). Selective loss of NF, as demonstrated in our study by Western blot, is also a typical hallmark of both sALS and fALS (Menzies et al. 2002).
Apo E is a cargo protein that participates in cell trafficking signals and localizing of the lysosomal system. Apo-E is highly produced by microglia and is known to play a central role in the mobilization of lipids and cholesterol in injured neural tissue including neurodegeneration (Masliah et al. 1996). It has been shown that ApoE plasma levels correlate with the rate of deterioration and survival time in ALS (Lacomblez et al. 2002). Additionally, knock-out of ApoE was found to be neuroprotective in the SOD-1 mouse model for ALS (Olsen et al. 2001). Whether the Apo-E ɛ4 allele is a risk factor for ALS needs to be clarified in further studies (Moulard et al. 1996; Drory et al. 2001). However, ApoE isoforms were not analyzed in our study. Here we show that total Apo E mRNA was up-regulated both in sALS patients and in advanced motor dysfunction in hSOD1 G93A tg mice. Our data are in agreement with a previous report by Haasdijk et al. (2002).
To our knowledge, we report here for the first time that LYST mRNA level is markedly increased in the spinal cords of sALS patients (×>10) and also in advanced motor dysfanction in hSOD1-G93A transgenic mice. LYST is known to modulate lysosomal exocytosis and vesicular trafficking and thus plays an important role in cellular homeostasis. Mutations in the 429-kDa LYST cytoplasmic protein lead to disruption of lysosome-related organelle function, resulting in immune insufficiency and albinism (Chediak–Higashi syndrome, Certain et al. 2000). The mechanism by which LYST regulates lysosome function, however, is unknown, as is its role in ALS.
The mRNAs of cathepsin D and cathepsin B, two lysosomal proteases, were at least two times higher in sALS spinal cords as compared to controls and increased with disease progression in hSOD1 G93A transgenic mice. Cathepsin B and D seem to play an important role in neuronal cell and are associated with the pathology of neurodegenerative diseases such as Alzheimer’s disease (Papassotiropoulos et al. 2002; Nakanishi et al. 1997; Callahan et al. 1999; Nakamura et al. 1991). It was suggested that cathepsin B and D are involved in protein turnover and serve as proapoptotic enzymes in various models of apoptosis (Kikuchi et al. 2003; Kagedal et al. 2000; Foghsgaard et al. 2001; Kingham and Pocock 2001; Kohda et al. 1996; Takuma et al. 2003). Moreover, increase of cathepsin B has recently been reported in sALS patients mainly in degenerating anterior horn neurons and in astrocytes of the lateral anterior horn (Kikuchi et al. 2003). In our study we found that cathepsin B is elivated in SOD mice on day 90, before the development of motor impairment. This might suggest a role of cathepsin B in the early stage of the disease.
Upregulation of EGFR in sALS and in mSOD mice is also a novel finding. EGFR is expressed in neurons of the hippocampus, cerebellum, and cerebral cortex and in other regions of the central nervous system. It binds to the mitogenic factor EGF that stimulates the proliferation of different types of cells, especially fibroblasts, epithelial cells, and neurons. Moreover, EGFR is involved in the migration, invasion, and proliferation of astrocytes (Kajiwara et al. 2003). EGFR activation triggers astrocytes to become reactive astrocytes (review Liu and Neufeld 2007). Therefore, the more than 10-fold increase in EGFR mRNA in sALS patients compared to control could be related to the astrogliosis seen in these patients. EGFR immunoreactivity has previously also been found in neuritic plaques in Alzheimer’s disease, which could also relate to gliosis (Birecree et al. 1988). On the other hand, it was reported that transgenic mice lacking the EGFR develop neurodegeneration and die within the first month after birth (Threadgill et al. 1995; Kornblum et al. 1998; Sibilia 1998). In the adult CNS, the EGFR pathway is absent in astrocytes but is up-regulated and activated following neuronal injury (Liu et al. 2006). The robust effects and the fact that other EGFR inhibitors are also in clinical use in cancer treatments make these drugs particularly attractive candidates for clinical trials in spinal cord injury (Erscbamer et al. 2007). Thus, although the role of EGFR in the pathophysiology of ALS is yet unclear and further studies are needed, EGFR inhibitor might be a possible treatment for ALS.
We also found an increase in ferritin L transcript in the sALS spinal cords and in the spinal cords of SOD transgenic mice. Ferritin L is one of the proteins known to play a key role in iron metabolism and is found to be associated with the pathophysiology of neurodegenerative diseases, including Huntington’s disease (Simmons et al. 2007). It was suggested that the elevated level of ferritin L might reflect an adaptive response to the intracellular iron released due to mitochondrial dysfunction (Olsen et al. 2001). Interestingly, transferrin, another iron-binding protein, was found to accumulate in Bunina bodies of ALS patients (Mizuno et al. 2006), again suggesting the pathological role of iron in ALS.
In conclusion, our study confirms, albeit in a small number of humans with sALS, a gene expression pattern related to inflammatory reaction followed by demyelination, astrogliosis, and motor neuron apoptosis. Part of these genes has previously been reported in human ALS patients or in a mouse model of ALS, whereas others are described here for the first time. Comparison of these selected genes demonstrates similarity between sALS and the fALS models in mice so that, with respect to these genes, experiments in the hSOD1-G93A model might allow conclusions for human ALS. Further analysis of these genes might increase our understanding on the disease etiology and progression and might provide selective targets for future therapies.
References
Bendotti, C., Tortarolo, M., Suchak, S. K., Calvaresi, N., Carvelli, L., Bastone, A., et al. (2001). Transgenic SOD1 G93A mice develop reduced GLT-1 in spinal cord without alterations in cerebrospinal fluid glutamate levels. Journal of Neurochemistry, 7(4), 737–746.
Birecree, E., Whetsell, W. O. Jr., Stoscheck, C., King, L. E. Jr., & Nanney, L. B. (1988). Immunoreactive epidermal growth factor receptors in neuritic plaques from patients with Alzheimer’s disease. Journal of Neuropathology and Experimental Neurology, 47(5), 549–560.
Bruijn, L. I., Miller, T. M., & Cleveland, D. W. (2004). Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annual Review of Neuroscience, 27, 723–749.
Callahan, L. M., Vaules, W. A., & Coleman, P. D. (1999). Quantitative decrease in synaptophysin message expression and increase in cathepsin D message expression in Alzheimer disease neurons containing neurofibrillary tangles. Journal of Neuropathology and Experimental Neurology, 58(3), 275–287.
Certain, S., Barrat, F., Pastural, E., Le Deist, F., Goyo-Rivas, J., Jabado, N., et al. (2000). Protein truncation test of LYST reveals heterogenous mutations in patients with Chediak–Higashi syndrome. Blood, 95(3), 979–983.
Cudkowicz, M. E., McKenna-Yasek, D., Sapp, P. E., Chin, W., Geller, B., Hayden, D. L., et al. (1997) Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Annals of Neurology, 49(1), 213–222.
Drory, V. E., Birnbaum, M., Korczyn, A. D., & Chapman, J. (2001). Association of APOE epsilon4 allele with survival in amyotrophic lateral sclerosis. Journal of the Neurological Sciences, 190(1–2), 17–20.
Embacher, N., Kaufmann, W. A., Beer, R., Maier, H., Jellinger, K. A., Poewe, W., et al. (2001). Apoptosis signals in sporadic amyotrophic lateral sclerosis: an immunocytochemical study. Acta Neuropathologica, 102(5), 426–434.
Erschbamer, M., Pernold, K., & Olson, L. (2007) Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. Journal of Neuroscience, 27(24), 6428–6435.
Ferrante, R. J., Browne, S. E., Shinobu, L. A., Bowling, A. C., Baik, M. J., MacGarvey, U., et al. (1997). Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. Journal of Neurochemistry, 69(5), 2064–2074.
Ferri, A., Nencini, M., Battistini, S., Giannini, F., Siciliano, G., Casali, C., et al. (2004). Activity of protein phosphatase calcineurin is decreased in sporadic and familial amyotrophic lateral sclerosis patients. Journal of Neurochemistry, 90, 1237–1242.
Foghsgaard, L., Wissing, D., Mauch, D., Lademann, U., Bastholm, L., Boes, M., et al. (2001). Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. Journal of Cell Biology, 153(5), 999–1010.
Gros-Louis, F., Gaspar, C., & Rouleau, G. A. (2006). Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochimica et Biophysica Acta, 1762(11–12), 956–972.
Guegan, C., & Przedborski, S. (2003). Programmed cell death in amyotrophic lateral sclerosis. Journal of Clinical Investigation, 111(2), 153–161.
Guégan, C., Vila, M., Rosoklija, G., Hays, A. P., & Przedborski, S. (2001). Recruitment of the mitochondrial-dependent apoptotic pathway in amyotrophic lateral sclerosis. Journal of Neuroscience, 21(17), 6569–6576.
Gurney, M. E., Cutting, F. B., Zhai, P., Doble, A., Taylor, C. P., Andrus, P. K., et al. (1996). Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Annals of Neurology, 39, 147–157.
Gurney, M. E., Pu, H., Chiu, A. Y., Dal Canto, M. C., Polchow, C. Y., Alexander, D. D., et al. (1994). Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science, 264, 1772–1775.
Haasdijk, E. D., Vlug, A., Mulder, M. T., & Jaarsma, D. (2002). Increased apolipoprotein E expression correlates with the onset of neuronal degeneration in the spinal cord of G93A-SOD1 mice. Neuroscience Letters, 335(1), 29–33.
Howland, D. S., Liu, J., She, Y., Goad, B., Maragakis, N. J., Kim, B., et al. (2002). Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proceedings of the National Academy of Sciences of the United States of America, 99, 1604–1609.
Hoyaux, D., Boom, A., Van den Bosch, L., Belot, N., Martin. J. J., Heizmann, C. W., et al. (2002). S100A6 overexpression within astrocytes associated with impaired axons from both ALS mouse model and human patients. Journal of Neuropathology and Experimental Neurology, 61(8), 736–744.
Jacobsson, J., Jonsson, P. A., Andersen, P. M., Forsgren, L., & Marklund, S. L. (2001). Superoxide dismutase in CSF from amyotrophic lateral sclerosis patients with and without CuZn-superoxide dismutase mutations. Brain, 124, 1461–1466.
Kagedal, K., Johansson, U., & Ollinger, K. (2000). The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB Journal, 15(9), 1592–1594.
Kajiwara, Y., Yamasaki, F., Hama, S., Yahara, K., Yoshioka, H., Sugiyama, K., et al. (2003). Expression of survivin in astrocytic tumors: correlation with malignant grade and prognosis. Cancer, 97(4), 1077–1083.
Kikuchi, H., Yamada, T., Furuya, H., Doh-ura, K., Ohyagi, Y., Iwaki, T., et al. (2003). Involvement of cathepsin B in the motor neuron degeneration of amyotrophic lateral sclerosis. Acta Neuropathologica, 105(5), 462–468.
Kingham, P. J., & Pocock, J. M. (2001). Microglial secreted cathepsin B induces neuronal apoptosis. Journal of Neurochemistry, 76(5), 1475–1484.
Kohda, Y., Yamashima, T., Sakuda, K., Yamashita, J., Ueno, T., Kominami, E., et al. (1996). Dynamic changes of cathepsins B and L expression in the monkey hippocampus after transient ischemia. Biochemical and Biophysical Research Communications, 228(2), 616–622.
Kornblum, H. I., Hussain, R., Wiesen, J., Miettinen, P., Zurcher, S. D., Chow, K., et al. (1998). Abnormal astrocyte development and neuronal death in mice lacking the epidermal growth factor receptor. Journal of Neuroscience Research, 53(6), 697–717.
Lacomblez, L., Doppler, V., Beucler, I., Costes, G., Salachas, F., Raisonnier, A., et al. (2002). APOE: a potential marker of disease progression in ALS. Neurology, 58(7), 1112–1114.
Li, M., Ona, V. O., Guegan, C., Kaul, M., Tenneti, L., Zhang, X., et al. (2000). Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science, 288(5464), 335–339.
Liu, D. (1996). The roles of free radicals in amyotrophic lateral sclerosis. Journal of Molecular Neuroscience, 7(3), 159–167.
Liu, B., Chen, H., Johns, T. G., & Neufeld, A. H. (2006). Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. Journal of Neuroscience, 26(28), 7532–7540.
Liu, B., & Neufeld, A. H. (2007). Activation of epidermal growth factor receptors in astrocytes: From development to neural injury. Journal of Neuroscience Research, 85(16), 3523–3529.
Malaspina, A., Kaushik, N., & de Belleroche, J. (2001). Differential expression of 14 genes in amyotrophic lateral sclerosis spinal cord detected using gridded cDNA arrays. Journal of Neurochemistry, 77(1), 132–145.
Martin, L. J. (1999). Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. Journal of Neuropathology and Experimental Neurology, 58(5), 459–471.
Masliah, E., Mallory, M., Veinbergs, I., Miller, A., & Samuel, W. (1996). Alterations in apolipoprotein E expression during aging and neurodegeneration. Progress in Neurobiology, 50, 493–503.
McGeer, P. L., & McGeer, E. G. (2002). Inflammatory processes in amyotrophic lateral sclerosis. Muscle & Nerve, 26(4), 459–470.
Menzies, F. M., Grierson, A. J., Cookson, M. R., Heath, P. R., Tomkins, J., Figlewicz, D. A., et al. (2002). Selective loss of neurofilament expression in Cu/Zn superoxide dismutase (SOD1) linked amyotrophic lateral sclerosis. Journal of Neurochemistry, 82(5), 1118–1128.
Mizuno, Y., Amari, M., Takatama, M., Aizawa, H., Mihara, B., & Okamoto, K. (2006). Transferrin localizes in Bunina bodies in amyotrophic lateral sclerosis. Acta Neuropathologica, 112, 597–603.
Moulard, B., Sefiani, A., Laamri, A., Malafosse, A., & Camu, W. (1996). Apolipoprotein E genotyping in sporadic amyotrophic lateral sclerosis: evidence for a major influence on the clinical presentation and prognosis. Journal of the Neurological Sciences, 139, 34–37 (Suppl).
Mu, X., He, J., Anderson, D. W., Trojanowski, J. Q., & Springer, J. E. (1996). Altered expression of bcl-2 and bax mRNA in amyotrophic lateral sclerosis spinal cord motor neurons. Annals of Neurology, 40(3), 379–386.
Nakamura, Y., Takeda, M., Suzuki, H., Hattori, H., Tada, K., Hariguchi, S., et al. (1991). Abnormal distribution of cathepsins in the brain of patients with Alzheimer’s disease. Neuroscience Letters, 130(2), 195–198.
Nakanishi, H., Amano, T., Sastradipura, D. F., Yoshimine, Y., Tsukuba, T., Tanabe, K., et al. (1997). Increased expression of cathepsins E and D in neurons of the aged rat brain and their colocalization with lipofuscin and carboxy-terminal fragments of Alzheimer amyloid precursor protein. Journal of Neurochemistry, 68(2), 739–749.
Olsen, M. K., Roberds, S. L., Ellerbrock, B. R., Fleck, T. J., McKinley, D. K., & Gurney, M. E. (2001). Disease mechanisms revealed by transcription profiling in SOD1-G93A transgenic mouse spinal cord. Annals of Neurology, 50(6), 730–740.
Papassotiropoulos, A., Lewis, H. D., Bagli, M., Jessen, F., Ptok, U., Schulte, A., et al. (2002). Cerebrospinal fluid levels of beta-amyloid(42) in patients with Alzheimer’s disease are related to the exon 2 polymorphism of the cathepsin D gene. Neuroreport, 13(10), 1291–1294.
Pasinelli, P., & Brown, R. H. (2006). Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nature Reviews Neuroscience, 7(9), 710–723 (review).
Pasinelli, P., Houseweart, M. K., Brown, R. H., & Cleveland, D. W. (2000). Caspase 1 and 3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotorophic lateral sclerosis. Proceedings of the National Academy of Sciences of the United States of America, 97, 13901–13906.
Pedersen, W. A., Fu, W., Keller, J. N., Markesbery, W. R., Appel, S., Smith, R. G., et al. (1998). Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Annals of Neurology, 44(5), 819–824.
Pedersen, W. A., Luo, H., Kruman, I., Kasarskis, E., & Mattson, M. P. (2000). The prostate apoptosis response-4 protein participates in motor neuron degeneration in amyotrophic lateral sclerosis. FASEB Journal, 14(7), 913–924.
Rothstein, J. D., Martin, L. J., & Kuncl, R. W. (1992). Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. New England Journal of Medicine, 326(22), 1464–1468.
Sibilia, M., Steinbach, J. P., Stingl, L., Aguzzi, A., & Wagner, E. F. (1998). A strain-independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO Journal, 17(3), 719–731.
Simmons, D. A., Casale, M., Alcon, B., Pham, N., Narayan, N., & Lynch, G. (2007). Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington’s disease. Glia(Galaţi), 55(10), 1074–1084.
Takuma, K., Kiriu, M., Mori, K., Lee, E., Enomoto, R., Baba, A., et al. (2003). Roles of cathepsins in reperfusion-induced apoptosis in cultured astrocytes. Neurochemistry International, 42(2), 153–159.
Threadgill, D. W., Dlugosz, A. A., Hansen, L. A., Tennenbaum, T., Lichti, U., Yee, D., et al. (1995). Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science, 269(5221), 230–234.
Acknowledgments
The project was partially supported by the National Parkinson Foundation, Miami, FL, USA; the Norma and Alan Aufzein Chair for Research in Parkinson’s disease, Tel Aviv University, Israel; and the Scientific and Technical Cooperation Austria–Israel, project #III/7 1999.
We are very grateful to Prof. Dr. Manfred Gerlach, Department of Clinical Neurochemistry, Medical Faculty, Würzburg, Germany, for providing us with spinal cord tissue of a patient who died from sALS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Offen, D., Barhum, Y., Melamed, E. et al. Spinal Cord mRNA Profile in Patients with ALS: Comparison with Transgenic Mice Expressing the Human SOD-1 Mutant. J Mol Neurosci 38, 85–93 (2009). https://doi.org/10.1007/s12031-007-9004-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-007-9004-z