Abstract
The current antidepressant drugs are ineffective in 30 to 40% of the treated patients; hence, the pathophysiology of the disease needs to be further elucidated. We used the chronic mild stress (CMS) paradigm to induce anhedonia, a core symptom of major depression, in rats. A fraction of the animals exposed to CMS is resistant to the development of anhedonia; they are CMS resilient. In the CMS-sensitive animals, the induced anhedonic state is reversed in 50% of the animals when treating with escitalopram, whereas the remaining animals are treatment resistant. We used the microarray and the real-time quantitative reverse transcription polymerase chain reaction technique, as well as the ingenuity pathway analysis software to identify the differential gene expression pathways, which are associated with the occurrence of the treatment resistance and the stress-resilient rats. In the hippocampus, we found a significant upregulation of apoptotic pathways in the treatment-resistant animals and significantly increased expression levels of genes involved in hippocampal signaling in the CMS-resilient rats. We hypothesize that sensitivity to the stress-induced anhedonia in rats is correlated with the impairment of hippocampal neurogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unipolar depression is the most common psychiatric disease with a prevalence of 15%. At least one of the two cardinal symptoms, depressed mood or anhedonia, i.e., inability to experience pleasure, must be present in addition to a number of minor symptoms for fulfilling the diagnostic criteria of a depressive episode (Reus 2006).
Chronic mild stress (CMS) in rodents has been proposed to model some of the environmental factors that contribute to the induction of depressive disorders in humans (Willner 1997; Willner 2005). The model fulfills face, predictive, and construct validity criteria of animal disease models (Willner 1997). Sequential exposure to a variety of mild stressors causes behavioral deficits in different paradigms that measure sensitivity to rewards. Thus, CMS suppresses the consumption of and preference for palatable sweet solutions (Willner et al. 1987) and the rewarding properties of food pellets, sweet solutions, and amphetamine, as assessed by the place preference conditioning test (Papp et al. 1991). CMS furthermore increases thresholds for ventral tegmental self-stimulation, indicating a decreased responsiveness to rewarding stimuli (Moreau et al. 1995). This decrease in sensitivity to reward is assumed to model human anhedonia.
The stress-induced anhedonia has been reversed by antidepressants with virtually all different kinds of mechanisms of action (Moreau et al. 1993; Moreau et al. 1995; Muscat et al. 1992; Sanchez et al. 2003). In particular, the antidepressant drug, escitalopram, has been shown to more effective, i.e., faster onset of action, than other selective serotonin reuptake inhibitors (Sanchez et al. 2003). All antidepressants tested in the CMS model were effective at low to moderate dosages (5–10 mg/kg per day), and a chronic drug exposure for 3–5 weeks was a prerequisite for obtaining full recovery (Jayatissa et al. 2006).
Previous studies have shown that the decrease in consumption of sweet solutions takes place during a few weeks of exposure to CMS and does not occur in all of the animals. There is a subgroup of CMS resistant rats, denoted as CMS-resilient rats (Jayatissa et al. 2006; Strekalova et al. 2004). Additionally, chronic, i.e., 3 to 4 weeks, but not acute antidepressant therapy reverts the anhedonic effects for approximately 50% of the anhedonic test subjects. Control groups are unaffected by antidepressant treatment (Jayatissa et al. 2006).
Hence, the CMS model not only simulates the chronic aspect of clinic depression, but it also models the fact that conventional antidepressant therapy is without any effect in a subgroup of treated patients (Berman et al. 1997; Fava 2003). Thus, the CMS model provides a unique opportunity to study the discrepancies in gene expression between the treatment responders and the treatment nonresponders and between the stress-sensitive and the stress-resilient animals. Taken together, the subgroup segregation enhances the validity of CMS as a model of clinical depression. We used the microarray and the real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) technologies, to analyze the subgroup segregations. The Ingenuity software was assessed to structure the gene expression data. These data indicate certain pathways to be involved in the brain processes of anhedonic development and antidepressant drug responsiveness.
Experimental Procedures
Animals
Male Wistar rats were purchased from Taconic, Denmark. Animal weight was approximately 200 g, when adaptation for sucrose consumption was initiated and approximately 350 g at the start of stress regime. The animals were singly housed, except when grouping was applied as a stress parameter. Food and water was available ad libitum except when food or/and water deprivation was applied as a stress parameter. The standard 12-h light/dark cycle was only changed in the course of stress regime.
Sucrose Consumption Test
For the first 5 weeks, the animals were trained to consume a palatable sucrose solution (1.5%). The sucrose test consisted of 1-h exposure to a single bottle with sucrose solution. To follow the sucrose intake for individual animals, the bottles were weighted before and after the test. In the training period, the sucrose test was made twice a week during the first 3 weeks and once a week during the last 2 weeks. During the stress period, the sucrose consumption test was performed once a week. The animals were food and water deprived 14 h before the test.
Chronic Mild Stress Protocol
On the basis of sucrose intakes in the three final baseline tests, the animals were divided into two matched groups and placed in separate rooms. The stress group was exposed to an initial 5 weeks of chronic mild stressors, and the control group was left undisturbed. The unchallenged control group was food and water deprived 14 h before sucrose consumption test; otherwise, food and water was freely available.
The stress procedure used was described previously (Jayatissa et al. 2006). Based on the endpoint sucrose intake, the animals were finally subdivided into six groups: 1: CMS-sensitive, escitalopram responders (N = 7), 2: CMS-sensitive, escitalopram nonresponders (N = 7), 3: CMS-sensitive, vehicle treated (N = 7), 4: Unchallenged escitalopram (N = 10), 5: Unchallenged vehicle (N = 10), and 6: CMS resilient (N = 8). Stress was continued during the entire period of treatment. The treatment with continued stress lasted for 25 days. Afterward, the animals were decapitated, and the hippocampi were isolated and immediately frozen in RNAlater (Ambion) for subsequent ribonucleic acid (RNA) isolation.
Drug Administration
Drug or vehicle was administrated intraperitoneally daily in the morning. Escitalopram (Lundbeck, Denmark) was dissolved in physiological saline given in a dose of 5 mg/kg.
RNA Isolation
The hippocampal tissue was homogenized in RNAlater (Ambion), and the RNA was isolated using the GeneElute Mammalian Total RNA kit (Sigma) according to the manufacturer’s instructions. According to these instructions, further DNase treatment should not be necessary. The RNA was divided in two equally sized pools, one for the microarray experiment and one for RT-PCR. The purity of the RNA was verified, and the RNA concentration was quantified by determining absorbance at 260 nm. Finally, the RNA for microarray analysis was pooled within the six groups considered, and purity and concentrations were again determined. This pooling was necessary to ensure sufficient RNA for the chip analysis.
Microarray
Complementary RNA Preparation and In vitro Transcription
Five micrograms of total RNA was used as starting material for the complementary deoxyribonucleic acid (cDNA) preparation. The first and second strand cDNA synthesis was performed using the SuperScript II System (Invitrogen) according to the manufacturer’s instructions except using an oligo-dT primer containing a T7 RNA polymerase promoter site. Labeled complementary RNA (cRNA) was prepared using the BioArray High Yield RNA Transcript Labeling Kit (Enzo). Biotin Hybridi cytidine triphosphate and uridine triphosphate (Enzo) were used in the reaction together with unlabeled nucleoside triphosphates. After the in vitro transcription reaction, the unincorporated nucleotides were removed using Rneasy columns (Qiagen).
Array Hybridization and Scanning
Fifteen micrograms of cRNA was fragmented at 94°C for 35 min in a fragmentation buffer containing 40 mM Tris–acetate pH 8.1, 100 mM KOAc, 30 mM MgOAc. Before hybridization, the fragmented cRNA in a 6× sodium-citrate-phosphate–ethylenediamine tetraacetic acid–Tris (SSPE-T) hybridization buffer (1 M NaCl, 10 mM Tris pH 7.6, 0.005% Triton), was heated to 95°C for 5 min and subsequently to 45°C for 5 min before loading onto the Affymetrix HG_U133 2.0 probe array cartridge. The probe array was then incubated for 16 h at 45°C at constant rotation (60 rpm). The washing and staining procedure was performed in the Affymetrix Fluidics Station 450. The probe array was exposed to ten washes in 6× SSPE-T at 25°C followed by four washes in 0.5× SSPE-T at 50°C. The biotinylated cRNA was stained with a streptavidin–phycoerythrin conjugate, final concentration 2 mg/ml (Molecular Probes, Eugene, OR) in 6× SSPE-T for 30 min at 25°C followed by ten washes in 6× SSPE-T at 25°C An antibody amplification step followed using normal goat IgG as blocking reagent, final concentration 0.1 mg/ml (Sigma), and biotinylated anti-streptavidin antibody (goat), final concentration 3 mg/ml (Vector Laboratories). This was followed by a staining step with a streptavidin–phycoerythrin conjugate, final concentration 2 mg/ml (Molecular Probes, Eugene, OR) in 6× SSPE-T for 30 min at 25°C and ten washes in 6× SSPE-T at 25°C. The probe arrays were scanned at 560 nm using a confocal laser-scanning microscope (Affymetrix Scanner 3000). The readings from the quantitative scanning were analyzed by the Affymetrix Gene Expression Analysis Software. All data were log2 transformed and normalized to minimize variations related to the microarray technology and not biological variation (Smyth and Speed 2003).
Real-time qRT-PCR
Total RNA was reverse transcribed into cDNA using SuperScript II reverse transcriptase (Invitrogen). RT-PCR was performed on the iCycler (BioRad). IQ™ SYBR® Green Supermix was used for the PCR. All primers were designed using Net Primer Software (www.premierbiosoft.com). All primers were subsequently verified by checking the sequences against the nucleotide sequence available on National Center for Biotechnology Information Blast resources. This included checking that primers spanned at least one intron to be able to distinguish between amplicons from the cDNA template and possible genomic DNA contamination. All primer sequences are presented in Table 1. To normalize variation, beta-actin was run in parallel with each set of primers for all samples. Results were calculated as ratios of relative expression level to beta-actin expression. The iCycler software provided by BioRad performed PCR efficiency and threshold cycle calculation automatically. PCR conditions were optimized for each set of primers before experiments.
A dissociation (melt) curve analysis was performed at the end of all PCRs to verify the specificity of the PCR products. All PCR products were also subjected to gel electrophoresis (1% agarose) as an additional control of specificity.
Pathway Analysis
All gene identifiers and corresponding fold changes were uploaded into Ingenuity Pathway Analysis (IPA, Ingenuity® System, http://www.Ingenuity.com) to identify molecular pathways and functions related to stress resilience and drug resistance in CMS.
The number of genes eligible for generating networks represents those unique genes that meet the specified parameters, have associated molecular interaction information within the Ingenuity Pathways Knowledge Base, and thus may be incorporated into networks. Identifiers corresponding to genes not residing within the Ingenuity Pathways Knowledge Base, and most expressed sequence tags are not included in analyses.
IPA calculates a significance score for each network. The network score is generated using a p value calculation and is displayed as the negative log of that p value. This score indicates the likelihood that the assembly of a set of focus genes in a network could be explained by random chance alone. A score of 2 indicates that there is a 1 in 100 chance that the focus genes are together in a network because of random chance. Therefore, networks with scores of 2 or higher have at least a 99% confidence of not being generated by random chance alone (see http://www.Ingenuity.com).
Cluster Analysis
The cluster analysis was performed with TreeView software (Page 1996). The microarray data was reduced to 89 genes by three sequential steps. First, all data were log2 transformed and normalized. Then, they were median centered, and finally, all genes with standard deviations of observed values smaller than 0.5 were removed. The 89 sequences remaining was then used for hierarchical clustering analysis and visualized.
Statistical Analysis
Sigma Stat 3.0 was used for the statistical analysis. The sucrose data was analyzed by one-way analysis of variance followed by pairwise multiple comparison tests (Student–Newman–Keuls method) of specific differences between individual groups. Student’s t test was used for the analysis of the RT-PCR data. The significance level was set at p < 0.05.
Ethics
All procedures involving animals were accepted by the Danish National Committee for Ethics in Animal Experimentation (2002/561-575). Experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). All possible measures were taken to minimize animal pain and discomfort.
Results
CMS Models Antidepressant Drug Resistance and Stress Resilience
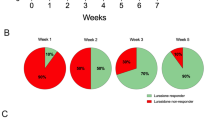
Exposure to CMS caused a significant decrease in sucrose drinking in about two thirds of the CMS-sensitive stressed animals, compared to unchallenged controls, indicating a stress-induced decrease in sensitivity to reward (Fig. 1). One third of the animals exposed to CMS did, however, not decrease sucrose intake and were denoted CMS resilient (CMS-RS). The operational cutoff value for animals being anhedonic was set at 50%; that is, anhedonic animals decreased their sucrose intake by more than 50% compared to baseline levels.
Sucrose intake as measure of anhedonia in rats subjected to 63 days of continuous exposure to chronic mild stress (CMS) and 25 days of escitalopram treatment. Data are presented as means ± SEM. The stress was initiated at day 0 (arrow Stress). No difference in sucrose intake between animals was present here at baseline (F 2, 24 = 2.203; p = 0.134). During the next 35 days of treatment, the animals exposed to CMS segregated into two subgroups: two thirds of the stressed animals, denoted CMS sensitive, decreased their sucrose consumption compared to an unchallenged group, not exposed to CMS (F 2, 15 = 40.001; p < 0.001), and one third of the stressed animals, denoted CMS resilient, did not decrease their sucrose consumption compared to the unchallenged group (F 2, 15 = 40.001; p > 0.05). The segregation into two subgroups was highly significant (F 2, 15 = 40.001; p < 0.001). After 38 days of stress, daily treatments with escitalopram or vehicle (NaCl) were started (arrow Treatment). Because sucrose drinking was measured once a week, 3 days passed from the last measurement (35 days) before treatment, to start of treatment (day 38). Hence, the first sucrose measure after treatment (42 days) was after 4 days of treatment, not 7 days. The CMS-resilient group was not injected but was continuously exposed to CMS. The unchallenged group was divided in two equally sized subgroups, injected with escitalopram (U-Es) and vehicle (U-V), respectively. The CMS-sensitive animals subjected to antidepressant treatment segregated into two subgroups during the 25 days of treatment. At endpoint (day 63), there was segregation in two equally sized groups. One group of animals: CMS-escitalopram-responders (CMS-Es-R), which increased their sucrose intake upon treatment, and one group: CMS-Es-NR, not increasing sucrose intake, The segregation was highly significant (F 5, 18 = 30.317; p = 0.002). For a detailed statistical analysis of this difference, see also Jayatissa et al. 2006. The vehicle-treated CMS-sensitive group (CMS-V) did not increase sucrose intake, during treatment, compared to CMS-Es-R (F 5, 18 = 30.317; p = 0.006) and was not statistically different from the CMS-Es-NR (F 5, 18 = 30.317; p = 0.382). Sucrose intake of the CMS-V group was highly significantly decreased compared to the CMS-RS (F 5, 18 = 30.317; p < 0.001), U-V (F 5, 18 = 30.317; p < 0.001), U-Es (F 5, 18 = 30.317; p < 0.001). There was no statistical difference between CMS-RS and the unchallenged groups. The decline in sucrose intake for the unchallenged groups, U-V and U-Es, at week 6 was caused by the stressing effects of injections and handling; after a while, rats adapt to this. CMS-Es-NR Chronic mild stress-sensitive escitalopram nonresponders, CMS-Es-R chronic mild stress-sensitive escitalopram responders, CMS-RS, chronic mild stress resilient, CMS-V chronic mild stress-sensitive vehicle, U-Es unchallenged escitalopram, U-V unchallenged vehicle
Chronic (25 days) treatment with escitalopram was initiated in the CMS-sensitive group, after 38 days of CMS exposure, and reversed the decrease in sucrose intake in 50% of the treated CMS rats, during the treatment period. These animals were denoted CMS-sensitive, escitalopram responders (CMS-Es-R). The remaining escitalopram-treated animals did not increase sucrose intake and were denoted CMS-sensitive, escitalopram, nonresponders (CMS-Es-NR). A third subgroup of CMS sensitive animals were treated with vehicle and did not increase sucrose intake (CMS-V). The definition of an escitalopram responder was a minimum 10% increase in sucrose intake compared to anhedonic levels.
The unchallenged group was divided in two at day 38 and treated with escitalopram (U-Es) and vehicle (U-V), respectively, for the 25 days of treatment.
At the endpoint, after 63 days of CMS combined with 25 days of treatment, the segregation between the CMS-V and the CMS-RS groups and the segregation between the CMS-Es-R and the CMS-Es-NR groups were highly significant. In both cases, the p value was less than 0.001.
Microarray Analysis of the Hippocampal RNA
The TreeView cluster analysis (Fig. 2) provides an overview of the genes differentially regulated in the six experimental groups. The cluster analysis showed that the three groups sensitive to CMS (CMS-Es-R, CMS-Es-NR, CMS-V) clustered all together and were dissimilar to the two unchallenged groups (U-Es, U-V). Moreover, the resilient (CMS-RS) group was quite dissimilar to the CMS-V group and closely related to the U-V group. The CMS-V group was closer related to the escitalopram nonresponder (CMS-Es-NR) group than to the escitalopram responder (CMS-Es-R) group. Altogether, the cluster analysis corresponds very well with the sucrose drinking data and thus confirms the segregation of the CMS escitalopram-treated animals into the responder (CMS-Es-R) and the nonresponder (CMS-Es-NR) subgroups (Fig. 1).
Cluster analysis of mRNA gene expression from hippocampal tissue visualized by TreeView software. Note how the CMS-sensitive groups (CMS-Es-R, CMS-Es-NR, CMS-V) are clustered together and are dissimilar to the unchallenged groups (U-Es, U-V). Note also the great difference between the CMS resilient (CMS-RS) and CMS-sensitive, vehicle-treated group (CMS-V). CMS-Es-NR Chronic mild stress-sensitive escitalopram nonresponders, CMS-Es-R chronic mild stress-sensitive escitalopram responders, CMS-RS chronic mild stress resilient, CMS-V chronic mild stress-sensitive vehicle, U-Es unchallenged escitalopram, U-V unchallenged vehicle
We used a cutoff at twofold of differential regulation, to construct an overview of the number of genes differentially regulated, when the groups of interest were analyzed in a pairwise fashion (Fig. 3). Sixteen genes were upregulated in the CMS-Es-NR group compared to the CMS-Es-R group. One hundred and forty-nine genes were upregulated, and six genes were downregulated in the CMS-RS group compared to the CMS-V group. Moreover, 12 genes were upregulated, and 267 genes were downregulated in the CMS-V group compared to the U-V group. Two genes were upregulated, and 11 were downregulated in the CMS-V group compared to the CMS-Es-R group (Fig. 3, Table 4).
Overview of the microarray data. The boxes represent the six animals groups. The three groups on the left-hand side are the escitalopram-treated animals, whereas the three groups on the right-hand side were either vehicle or untreated (CMS resilient). Only genes/sequences scored as twofold changes or higher were included. Interpretation of arrows: An arrow from box a to box b means that the given number of genes displays more than twofold upregulation in group a compared to group b. Note, specifically, that 149 sequences were twofold or more upregulated in the CMS-RS group compared to the CMS-V group, and six sequences similarly downregulated. Furthermore, note the similarity of the U-V and CMS-RS compared to the CMS-V group. Similarly, 16 sequences were twofold or more upregulated in the CMS-Es-NR group compared to the CMS-Es-R group. The U-Es group was left out of this analysis. CMS-Es-NR Chronic mild stress-sensitive escitalopram nonresponders, CMS-Es-R chronic mild stress-sensitive escitalopram responders, CMS-RS chronic mild stress resilient, CMS-V chronic mild stress-sensitive vehicle, U-Es unchallenged escitalopram, U-V unchallenged vehicle
The number of genes differentially regulated between the groups was in agreement with the sucrose drinking and the cluster analysis; for example, only 44 sequences were differentially regulated between U-V and CMS-RS, whereas 279 sequences were different between CMS-V and the U-V group.
The expression of a set of housekeeping genes, e.g., beta-actin, showed a stable expression pattern in the six groups considered (not shown). Moreover, no differences were found in genes directly related to the stress system, i.e., the serotonergic system nor the glucocorticoid or mineralocorticoid receptors.
Drug Resistance Experiment
Sixteen sequences displayed more than twofold differential regulation between the CMS-Es-NR and CMS-Es-R groups (Table 2). Ten of these were eligible for network analysis by the Ingenuity software (see “Experimental Procedures”; Table 3). The Ingenuity software clustered these significantly differentially regulated genes in a pathway surrounding tumor necrosis factor (TNF) and amyloid beta precursor protein (APP).
All 16 genes that were upregulated in the CMS-Es-NR group compared to the CMS-Es-R were also significantly upregulated in the CMS-V group compared to the CMS-Es-R and to the U-V groups (results not shown).
The results were confirmed by qRT-PCR (Fig. 4) for 7 of the 16 genes (p < 0.05). Three of the genes showed fold change in the same direction as micorarray data but with p > 0.05. For the remaining six genes, reproducible data could not be produced. Alternative primers were constructed for five of these (there was no DNA sequence available for Pb1 messenger RNA [mRNA]), but results remained irreproducible (not shown).
RT-PCR and microarray gene expression levels of the CMS-Es-NR group compared to the CMS-Es-R group. The cutoff at twofold regulation by microarray analysis was used. Because the microarray experiment was only conducted once because of limitations of hippocampal tissue, it was not possible to generate p values for these data. PCR results are average results of two experiments. Data are presented as mean + SEM of log2-transformed fold changes. For one of the sequences, Pb1, no DNA sequence was available, hence no PCR was performed. For 5 of the 16 genes considered (F5, Slc4a5, Clic6, Tmem27, Bmpr2), quantifiable expression levels could not be determined. For the remaining genes, all RT-PCR showed the same direction of change as found by microarray analysis, i.e., upregulation in the nonresponding group. Seven sequences (Sostdc-1, Klotho, Enpp2, Ttr, Mfrp, Claudin-2, Igf-2) were found to be significantly different (p < 0.05) using Student’s t test. The remaining three (App, Narg1, Pb1) were not significantly different (p > 0.05) yet showed an increase in fold change comparable to what was seen by the microarray experiment. CMS-Es-NR, Chronic mild stress-sensitive escitalopram nonresponders, CMS-Es-R chronic mild stress-sensitive escitalopram responders
Of specific interest were 12 genes that were either downregulated in the CMS-V group compared to the U-V group and then reversely upregulated by escitalopram or vice versa (Table 4). The Ingenuity software was only able to score three of these genes to networks. These were categorized as related to cell death/apoptosis and immune response by IPA (Table 5). Analysis of functions was highly correlated to upregulation of APP, i.e., CMS-induced, escitalopram-reversible apoptotic processes.
The results were confirmed by qRT-PCR for 7 of 12 sequences (p < 0.05; see Fig. 5). All fold changes found by RT-PCR showed the same direction of regulation as found by microarray. Five sequences could not be quantified by PCR; however, one (Zfhx1b) was already covered by another sequence (Zfhx1b_b).
qRT-PCR and microarray gene expression levels of the genes downregulated by stress (CMS-V) and again upregulated in the escitalopram responding group (CMS-Es-R) or vice versa (APP). The cutoff at twofold regulation by microarray analysis was used. Because the microarray experiment was only conducted once because of limitations of hippocampal tissue, it was not possible to generate p values for these data. PCR results are average results of two experiments. Data are presented as mean + SEM of log2-transformed fold changes. For 5 of the 12 genes considered (Zfhx1b, Zbtb20, Unknown2, C9orf41, Unknown3), quantifiable expression levels could or was not determined. For the remaining seven genes (Ddx6, Ubn1, Unknown1, Phip, Rgd1563119, Zfhx1b_b, App), the RT-PCR verified the microarray data well. Note two sequences are from the same mRNA (Zfhx1 and Zfhx1_b; PCR was run only on Zfhx1_b). All fold changes were in the same direction as predicted by microarray and all but two t tests were significant with p < 0.05. CMS-Es-R, Chronic mild stress-sensitive escitalopram responders; CMS-V chronic mild stress-sensitive vehicle
Stress Resilience Experiment
The Ingenuity analysis of CMS-V vs CMS-RS genes showed 78 of the 156 more than twofold differentially regulated genes scored as eligible for generating six networks in the Ingenuity software (Table 6). Similarly, 30 of the 44 genes more than twofold differentially regulated between the U-V and CMS-RS groups were scored as eligible for network generation (Table 7). The six networks from the CMS-V vs CMS-RS are commented as follows.
Network 1
Many genes related to the immune response were downregulated in the CMS-V group compared to the CMS-RS group, e.g., CCL13, FOS, IL6ST. Several of these genes were also more than twofold differentially regulated in the U-V group, when compared to the CMS-RS (see Table 7). The APP gene was more than twofold upregulated in the CMS-V group compared to both the CMS-RS and the U-V.
Network 2
A diverse group of genes related to signal transduction in relation to the normal function of the nervous system (e.g., the α-amino-3-hydroxy-5-methylisoxazole-4- propionic acid [AMPA] receptor subunits GRIA2 and GRIA3) and development. These genes were both upregulated in the U-V and the CMS-RS groups compared to the CMS-V group.
Network 3
As in network 1, several genes related to the immune response (ORM2, PSMB, CCL7, CXCL13) were at least twofold upregulated in the CMS-RS group compared to the CMS-V and to the U-V group.
The remaining genes were related to growth and cellular proliferation, e.g., kinases as BMP2 and CSNK2A1 and transcriptional regulators, e.g., NCOR1. These genes were not differentially regulated between the CMS-RS and the U-V groups.
Network 4
Glycam1, LCN2, and ORM2 were significantly upregulated in the CMS-RS compared to the CMS-V and to the U-V groups. These genes are relevant for cellular adhesion, apoptosis, and immune response, respectively.
Networks 5 and 6
In general, the genes involved in cellular growth, proliferation, development, and damage repair were downregulated in the CMS-V group compared to the CMS-RS and to the U-V groups.
A second analysis of the CMS-V vs CMS-RS experiment performed by the Ingenuity software categorized genes according to their function. The top five categories are given in Table 8. These results fully confirm the previous results from the network analysis.
Discussion
In the present study, we used the highly validated CMS animal model of depression to analyze the genetic mechanisms in the rat hippocampus, which are responsible for development of the resistance to the selective serotonin reuptake inhibitor, escitalopram. Moreover, we analyzed the mechanisms in the hippocampus, which could possibly explain why some individuals are less susceptible to the development of anhedonia upon chronic stress than others. No previous gene expression studies have been done to characterize the CMS-resilient and the treatment-resistant animals in the CMS model.
The CMS model has been optimized and repeatedly run with high reproducibility in our laboratory (Jayatissa et al. 2006). The procedure used at different laboratories varies but often involves severe stressors, e.g., electrical foot shock or restraint stress (Willner 2005). It is our experience that milder stress forms are preferable in the development of anhedonia, which has also been stated previously (Porsolt and Papp 1998).
It is essential to use the sucrose consumption test to track the anhedonic status of the animals in particular because during antidepressant drug treatment, the animals segregate into responders and nonresponders. This segregation to treatment responders and nonresponders is highly reproducible for escitalopram and other drugs like sertraline, citalopram, and duloxetine (not published). The distribution is close to 50% in each of the two subgroups using conventional antidepressants. Moreover, we also reproducibly find about 30% of initially stressed animals that never decrease the intake of sucrose; that is, they are CMS resilient. This phenomenon has also been described by others (Strekalova et al. 2004; Strekalova et al. 2006).
This is the first report on gene expression profiling of the resilience group with comparison to the CMS-sensitive group. No gene expression analysis of CMS escitalopram responders vs CMS escitalopram nonresponders have been published previously either.
The cluster analysis (Fig. 2) of the hippocampal mRNA profiles of the six animal groups was in great accordance with the sucrose-drinking data (Fig. 1). The CMS-sensitive groups clustered together rather distantly related to the unchallenged control and CMS-resilient groups. Moreover, the mRNA profile of the CMS-sensitive, vehicle-treated group was more similar to the CMS-sensitive, escitalopram-nonresponding group than to the CMS-sensitive, escitalopram-responding group. The same conclusion could be made from the pairwise analysis of animal groups (Fig. 3). The CMS-sensitive, vehicle-treated group showed a very different gene expression profile, when compared to the unchallenged vehicle and CMS-resilient groups, which displayed great similarity in mRNA expression levels.
We thus conclude that the cluster analysis of gene expression profiles for CMS escitalopram responders and CMS escitalopram nonresponders and the CMS sensitive (vehicle-treated) and CMS resilient strongly support the segregation of the animals into the different subgroups according to their sucrose intake. The constant expression levels seen for the set of housekeeping genes is strong evidence that changes in gene expression levels found were due to true changes in mRNA levels and not, e.g., due to loss of hippocampal neurons as an effect of the induced stress.
It was surprising to see that no difference in gene expression was found for genes directly related to the stress system, i.e., the serotonergic system nor the glucocorticoid and mineralocorticoid receptors.
In the microarray study, 16 genes were found to be more than twofold differentially upregulated in the CMS escitalopram-nonresponding group compared to the CMS escitalopram-responding group. The same sequences were also upregulated in the CMS-sensitive animals compared to the unchallenged animals and CMS escitalopram responders. We found the genes to be related to apoptotic hippocampal pathways including the proapoptotic factors APP (Dore et al. 1997; Marcon et al. 1999) and TNF (Cacci et al. 2005).
Eleven genes were downregulated in CMS-sensitive animals and reversibly upregulated in CMS escitalopram responders. APP displayed opposite regulation. The Ingenuity software was unable to cluster most of these genes into networks, yet APP and apoptotic processing were again upregulated by CMS.
Conclusively, CMS seems to induce escitalopram-reversible apoptotic processes on total hippocampal RNA profiling. In the CMS escitalopram-nonresponding animals, there seems to be an upregulation of apoptotic processes compared to the CMS escitalopram responders. Previous studies indicate chronic stress to slow down apoptotic as well as proliferative processes in the dentate gyrus of the hippocampus (Heine et al. 2004); however, regional differences exist. As we in our experiment have taken RNA from the entire hippocampus under consideration, results are not necessarily in conflict with the previous findings. Moreover, some of the remaining sequences found in this experiment have in some studies been shown to display antiapoptotic properties. Examples and reference to studies are insulin-like growth factor 2 (Dore et al. 1997), transthyretin, (Giunta et al. 2005), bone morphogenetic protein receptor 2 (McMurtry et al. 2007), and prolactin receptor (Walker 2005). Future studies should aim at isolation and gene/protein quantification of subareas of the hippocampus, e.g., the dentate gyrus, where adult neurogenesis is known to take place. Finally, escitalopram is likely to have effects on mRNA expression levels, unrelated to the therapeutic effect, confounding conclusions.
Results from the drug resistance study were confirmed by RT-PCR experiments with all primer sets showing the same direction of regulation as found by the microarray experiment. Most fold changes found were also significant with p < 0.05. In general, a verification rate of 70–90% of genes are expected for PCRs (Dallas et al. 2005; Wang et al. 2006). This criterion was met by our studies.
For most genes, the fold change found by PCR was larger than the fold change found by the microarray experiment, which is also consistent with previous findings (Yuen et al. 2002).
We found 156 genes to be more than twofold differentially regulated between CMS-sensitive and CMS-resilient animals. Ingenuity software sorting of the data showed that in general, the CMS-sensitive animals displayed lower hippocampal levels of genes important for “normal” cellular functioning, e.g., growth, proliferation, differentiation, metabolism, and maintenance. CMS is known to suppress hippocampal neurogenesis (Alonso et al. 2004; Jayatissa et al. 2006; Tanapat et al. 1998), synaptic plasticity (Holderbach et al. 2007), and survival of newborn cells (Lee et al. 2006). There is evidence that genes scored and grouped as highly different between stress sensitive and stress resilient are involved in functioning and synaptic transmission in hippocampal subareas. AMPA receptor currents (Gria2, Gria3) are positively correlated to dentate gyrus postnatal neurogenesis (Ye et al. 2005), and cell proliferation is stimulated by AMPA receptor agonists (Bai et al. 2003). Endothelin receptor type B is involved in neurogenesis of the enteric nervous system (Barlow et al. 2003) and necessary for migration of enteric neuroblasts in mice (Shin et al. 1999) and T cell lymphoma invasion and metastasis 1 is positively involved in neurite outgrowth (Matsuo et al. 2002).
Approximately 45 genes were also more than twofold differentially regulated between U-V and CMS-resilient animals. Several of these sequences were not differentially regulated between CMS-sensitive and unchallenged animals. These genes must be related to effects of CMS not leading to anhedonia. The difference is explained by upregulation of immune response related genes, e.g., chemokines (Adler et al. 2005), interleukins (Choi et al. 2003), and heat-shock proteins (Soti et al. 2005) in the CMS-resilient animals, which is not seen in CMS-sensitive or unchallenged animals.
A linkage between immune response, e.g., proinflammatory cytokines, and dentate gyrus neurogenesis has been suggested, yet the mechanism is virtually unexplored (Jacobs 2002). The recruitment of the immune system cells, T lymphocytes and microglia, to the dentate gyrus has been proven as important in the maintenance of adult hippocampal neurogenesis in rats (Ziv et al. 2006).
We hypothesise, that the CMS-sensitive animals may fail to induce a possibly protective immune response, which is initiated in the CMS-resilient animals and may serve to protect these animals from the development of anhedonia by some yet unknown mechanism.
We did not verify the stress resilience microarray study by RT-PCR because of limitations in RNA amounts. However, all genes verified by RT-PCR in the drug resistance experiment showed regulation in the same direction as found by microarray. Hence, it is a very likely assumption that similar findings would be made with the genes from the stress resilience study.
We used a fluorescent dye for quantification of the RT-PCR. This increases the risk of unspecific hybridization because the dye hybridizes to all double-stranded DNA. However, as suggested in Bustin (2000), we did successful post-PCR melting curve analysis and agarose gel runs to confirm the purity and correctness of the amplicon probe lengths. Hence, we are confident that the RT-PCR findings are reliable. It should be noted that the use of fluorescent dyes could lead to overestimation of longer amplicons than shorter ones, as more dye molecules will bind to longer stretches of DNA (Bustin 2000). No such correlation is evident from our PCR data, however.
In a very recent study, we have undertaken proteomic studies of the molecular mechanisms of escitalopram resistance and CMS resilience on the protein level on the tissue isolated from the ventral part of the hippocampus alone. We found escitalopram resistance to be correlated to dysregulation of proteins involved in axonal guidance and extension and maintenance of the plasticity of neuronal connections (Bisgaard et al. 2007). This is not contradictory to findings in the present study, although results are not quite similar. The comparison of mRNA, isolated from the whole hippocampus with protein levels, isolated from the ventral hippocampus could not necessarily be expected to give identical results.
We also found that the CMS resilience was correlated to upregulation of (soluble N-ethylmaleimide-sensitive fusion protein, attachment proteins) SNAP proteins in the CMS-sensitive animals compared to the CMS resilients. These proteins inhibit synaptic formation and maintenance and may cause increased glutamate excitotoxicity, which could compromise synaptic function and possibly neurogenesis (Bisgaard et al. 2007). Taken together, on both mRNA and protein level, sensitivity to CMS seem to be correlated to decreased synaptic function and possibly decreased neurogenesis, compared to the resilience to CMS.
Conclusion
We have undertaken the first microarray gene expression analysis study of the antidepressant drug resistance phenomenon and of the CMS-resilient animal population in the CMS model.
We hypothesize here that CMS-sensitive animals may fail to induce a possibly protective hippocampal immune response, which is triggered in the CMS-resilient animals. Moreover, molecular pathways related to normal cellular functioning, signaling, and neural development are downregulated in CMS-sensitive animals compared to CMS resilients and unchallenged controls. Because evidence from several animals studies has emerged that hippocampal neurogenesis could be an important mechanism of resistance to depression, it is interesting that the CMS-sensitive animals seem to be compromised in genes related to normal cellular development and signaling in the hippocampus.
CMS escitalopram-responding animals display lower levels of mRNAs related to apoptotic pathways than CMS-sensitive and CMS escitalopram-nonresponding animals. Hence, antidepressant resistance could be related to increased cellular turnover in the hippocampus, which again could be related to decreased hippocampal neurogenesis.
It is possible that apoptotic processes triggered acutely by CMS is compensated by antiapoptotic factors after some time of stress. The protective mechanism may just be insufficient in the CMS-sensitive animals compared to unchallenged controls and CMS resilients leading to anhedonia development. The CMS escitalopram responders may for some reason be able to compensate better against the stress-induced apoptotic processes and, hence, over a few weeks, normalizing the increased levels of apoptotic and antiapoptotic/proliferative processes.
Abbreviations
- APP:
-
amyloid beta precursor protein
- CMS:
-
chronic mild stress
- CMS-Es-R:
-
chronic mild stress sensitive escitalopram responders
- CMS-Es-NR:
-
chronic mild stress sensitive escitalopram non-responders
- CMS-RS:
-
chronic mild stress resilient
- CMS-V:
-
chronic mild stress sensitive vehicle
- IPA:
-
ingenuity pathway analysis
- qRT-PCR:
-
(real-time) quantitative reverse transcription-polymerase chain reaction
- RT-PCR:
-
reverse transcription-polymerase chain reaction
- TNF:
-
tumor necrosis factor
- U-Es:
-
unchallenged escitalopram
- U-V:
-
unchallenged vehicle
References
Adler, M. W., Geller, E. B., Chen, X., & Rogers, T. J. (2005). Viewing chemokines as a third major system of communication in the brain. AAPS Journal, 7, E865–E870..
Alonso, R., Griebel, G., Pavone, G., Stemmelin, J., Le, F. G., & Soubrie, P. (2004). Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Molecular Psychiatry, 9, 278–286, 224.
Bai, F., Bergeron, M., & Nelson, D. L. (2003). Chronic AMPA receptor potentiator (LY451646) treatment increases cell proliferation in adult rat hippocampus. Neuropharmacology, 44, 1013–1021.
Barlow, A., de, G. E., & Pachnis, V. (2003). Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron, 40, 905–916.
Berman, R. M., Narasimhan, M., & Charney, D. S. (1997). Treatment-refractory depression: definitions and characteristics. Depression and Anxiety, 5, 154–164.
Bisgaard, C. F., Jayatissa, M. N., Enghild, J. J., Sanchez, C., Artemychyn, R., & Wiborg, O. (2007). Proteomic investigation of the ventral rat hippocampus link DRP-2 to escitalopram treatment resistance and SNAP to stress resilience in the chronic mild stress rat model of depression. Journal of Molecular Neuroscience, 32, 132–144.
Bustin, S. A. (2000). Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. Journal of Molecular Endocrinology, 25, 169–193.
Cacci, E., Claasen, J. H., & Kokaia, Z. (2005). Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. Journal of Neuroscience Research, 80, 789–797.
Choi, J. S., Kim, S. Y., Park, H. J., Cha, J. H., Choi, Y. S., Kang, J. E., Chung, J. W., Chun, M. H., & Lee, M. Y. (2003). Upregulation of gp130 and differential activation of STAT and p42/44 MAPK in the rat hippocampus following kainic acid-induced seizures. Brain Research: Molecular Brain Research, 119, 10–18.
Dallas, P. B., Gottardo, N. G., Firth, M. J., Beesley, A. H., Hoffmann, K., Terry, P. A., Freitas, J. R., Boag, J. M., Cummings, A. J., & Kees, U. R. (2005). Gene expression levels assessed by oligonucleotide microarray analysis and quantitative real-time RT-PCR—how well do they correlate? BioMed Central Genomics, 6, 59.
Dore, S., Kar, S., & Quirion, R. (1997). Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proceedings of the National Academy of Sciences of the United States of America, 94, 4772–4777.
Fava, M. (2003). Diagnosis and definition of treatment-resistant depression. Biological Psychiatry, 53, 649–659.
Giunta, S., Valli, M. B., Galeazzi, R., Fattoretti, P., Corder, E. H., & Galeazzi, L. (2005). Transthyretin inhibition of amyloid beta aggregation and toxicity. Clinical Biochemistry, 38, 1112–1119.
Heine, V. M., Maslam, S., Zareno, J., Joels, M., & Lucassen, P. J. (2004). Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. European Journal of Neurosciences, 19, 131–144.
Holderbach, R., Clark, K., Moreau, J. L., Bischofberger, J., & Normann, C. (2007). Enhanced long-term synaptic depression in an animal model of depression. Biological Psychiatry, 62, 92–100.
Jacobs, B. L. (2002). Adult brain neurogenesis and depression. Brain, Behavior and Immunity, 16, 602–609.
Jayatissa, M. N., Bisgaard, C., Tingstrom, A., Papp, M., & Wiborg, O. (2006). Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology, 31, 2395–2404.
Lee, K. J., Kim, S. J., Kim, S. W., Choi, S. H., Shin, Y. C., Park, S. H., Moon, B. H., Cho, E., Lee, M. S., Choi, S. H., Chun, B. G., & Shin, K. H. (2006). Chronic mild stress decreases survival, but not proliferation, of new-born cells in adult rat hippocampus. Experimental and Molecular Medecine, 38, 44–54.
Marcon, G., Giaccone, G., Canciani, B., Cajola, L., Rossi, G., De, G. L., Salmona, M., Bugiani, O., & Tagliavini, F. (1999). A betaPP peptide carboxyl-terminal to Abeta is neurotoxic. American Journal of Pathology, 154, 1001–1007.
Matsuo, N., Hoshino, M., Yoshizawa, M., & Nabeshima, Y. (2002). Characterization of STEF, a guanine nucleotide exchange factor for Rac1, required for neurite growth. Journal of Biological Chemistry, 277, 2860–2868.
McMurtry, M. S., Moudgil, R., Hashimoto, K., Bonnet, S., Michelakis, E. D., & Archer, S. L. (2007). Overexpression of human bone morphogenetic protein receptor II does not ameliorate monocrotaline pulmonary arterial hypertension. American Journal of Physiology: Lung Cellular and Molecular Physiology, 292, L872–L878.
Moreau, J. L., Jenck, F., Martin, J. R., Mortas, P., & Haefely, W. (1993). Effects of moclobemide, a new generation reversible Mao-A inhibitor, in a novel animal model of depression. Pharmacopsychiatry, 26, 30–33.
Moreau, J. L., Scherschlicht, R., Jenck, F., & Martin, J. R. (1995). Chronic mild stress-induced anhedonia model of depression; sleep abnormalities and curative effects of electroshock treatment. Behavioral Pharmacology, 6, 682–687.
Muscat, R., Papp, M., & Willner, P. (1992). Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology (Berl), 109, 433–438.
Page, R. D. (1996). Treeview: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences, 12, 357–358.
Papp, M., Willner, P., & Muscat, R. (1991). An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl), 104, 255–259.
Porsolt, R. D., & Papp, M. (1998). Psychiatric models of disease: Depression. In S.J. Enna, M. Williams, J. W. Ferkany, T. Kenakin, P. Moser, & B. Ruggeri (Eds.) Current protocols in Pharmacology pp. 5.9.1–5.9.8. New York: Wiley.
Reus, V. I. (2006). Chapter 371: mental disorders. In D. L. Kasper, E. Braunwald, A. S. Fauci, S. L. Hauser, & D. L. Longo (Eds.) Harrison’s internal medicine (16st ed.). McGraw-Hill: New York.
Sanchez, C., Gruca, P., & Papp, M. (2003). R-citalopram counteracts the antidepressant-like effect of escitalopram in a rat chronic mild stress model. Behavioural Pharmacology, 14, 465–470.
Shin, M. K., Levorse, J. M., Ingram, R. S., & Tilghman, S. M. (1999). The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature, 402, 496–501.
Smyth, G. K., & Speed, T. (2003). Normalization of cDNA microarray data. Methods, 31, 265–273.
Soti, C., Nagy, E., Giricz, Z., Vigh, L., Csermely, P., & Ferdinandy, P. (2005). Heat shock proteins as emerging therapeutic targets. British Journal of Pharmacology, 146, 769–780.
Strekalova, T., Gorenkova, N., Schunk, E., Dolgov, O., & Bartsch, D. (2006). Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behavioural Pharmacology, 17, 271–287.
Strekalova, T., Spanagel, R., Bartsch, D., Henn, F. A., & Gass, P. (2004). Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology, 29, 2007–2017.
Tanapat, P., Galea, L. A., & Gould, E. (1998). Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. International Journal of Developmental Neuroscience, 16, 235–239.
Walker, A. M. (2005). Prolactin receptor antagonists. Current Opininion in Investigational Drugs, 6, 378–385.
Wang, Y., Barbacioru, C., Hyland, F., Xiao, W., Hunkapiller, K. L., Blake, J., Chan, F., Gonzalez, C., Zhang, L., & Samaha, R. R. (2006). Large scale real-time PCR validation on gene expression measurements from two commercial long-oligonucleotide microarrays. BioMed Central Genomics, 7, 59.
Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl), 134, 319–329.
Willner, P. (2005). Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology, 52, 90–110.
Willner, P., Towell, A., Sampson, D., Sophokleous, S., & Muscat, R. (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl), 93, 358–364.
Ye, G. L., Yi, S., Gamkrelidze, G., Pasternak, J. F., & Trommer, B. L. (2005). AMPA and NMDA receptor-mediated currents in developing dentate gyrus granule cells. Brain Research: Developmental Brain Research, 155, 26–32.
Yuen, T., Wurmbach, E., Pfeffer, R. L., Ebersole, B. J., & Sealfon, S. C. (2002). Accuracy and calibration of commercial oligonucleotide and custom cDNA microarrays. Nucleic Acids Research, 30, e48.
Ziv, Y., Ron, N., Butovsky, O., Landa, G., Sudai, E., Greenberg, N., Cohen, H., Kipnis, J., & Schwartz, M. (2006). Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature Neuroscience, 9, 268–275.
Acknowledgments
The authors would like to thank Ph.D. Arne Mørk at H. Lundbeck, Copenhagen for excellent advice on neuropsychopharmacology in general. We also thank Jan Torleif Pedersen and Karina Fog at H. Lundbeck, Copenhagen, for instructions on the use of the Ingenuity Pathway Analysis software. Specifically, we are grateful for the use of the Lundbeck license of IPA. Finally, we are grateful to Lundbeckfonden and the Danish Medical Research Council for supporting the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bergström, A., Jayatissa, M.N., Thykjær, T. et al. Molecular Pathways Associated with Stress Resilience and Drug Resistance in the Chronic Mild Stress Rat Model of Depression—a Gene Expression Study. J Mol Neurosci 33, 201–215 (2007). https://doi.org/10.1007/s12031-007-0065-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-007-0065-9