Abstract
N-acetyl-l-aspartic acid (NAA), an amino acid synthesized and stored primarily in neurons in the brain, has been proposed to be a molecular water pump (MWP) whose function is to rapidly remove water from neurons against a water gradient. In this communication, we describe the results of a functional 1H proton magnetic resonance spectroscopy (fMRS) study, and provide evidence that in the human visual cortex, over a 10-min period of visual stimulation, there are stimulation-induced graded changes in the NAA MRS signal from that of a preceding 10-min baseline period with a decline in the NAA signal of 13.1% by the end of the 10-min stimulation period. Upon cessation of visual stimulation, the NAA signal gradually increases during a 10-min recovery period and once again approaches the baseline level. Because the NAA MRS signal reflects the NAA concentration, these changes indicate rapid focal changes in its concentration, and transient changes in its intercompartmental metabolism. These include its rates of synthesis and efflux from neurons and its hydrolysis by oligodendrocytes. During stimulation, the apparent rate of NAA efflux and hydrolysis increased 14.2 times, from 0.55 to 7.8 μmol g−1 h−1. During recovery, the apparent rate of synthesis increased 13.3 times, from 0.55 to 7.3 μmol g−1 h−1. The decline in the NAA signal during stimulation suggests that a rapid increase in the rate of NAA-obligated water release to extracellular fluid (ECF) is the initial and seminal event in response to neurostimulation. It is concluded that the NAA metabolic cycle in the visual cortex is intimately linked to rates of neuronal signaling, and that the functional cycle of NAA is associated with its release to ECF, thus supporting the hypothesis that an important function of the NAA metabolic cycle is that of an efflux MWP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
N-acetyl-l-aspartic acid (NAA) is an unusual amino acid present in high concentration in brain that is synthesized by, and stored primarily in neurons. It is an N-acetylated derivative of l-aspartic acid (Asp), synthesized in neuron mitochondria from acetyl-coenzyme A (AcCoA), the two carbon acetate (Ac) portion of which is predominantly derived from the oxidation of glucose (Glc), a product of ongoing mitochondrial Glc energy metabolism (Patel et al. 2004). The only known anabolic pathway for synthesis of NAA is from AcCoA and Asp, and its rate of synthesis is therefore a function of the rate of Glc oxidation. Although NAA is synthesized by neurons, it cannot be catabolized by neurons. Thus, every NAA molecule synthesized by neurons must be liberated to extracellular fluid (ECF) for further processing (Baslow 2000). Because the level of NAA in brain is a functional constant (K 3), its concentration reflects a balance between its rates of synthesis (S), efflux (E), and hydrolysis (H) (Baslow 2002). In the steady state, S = E = H, and most measurements of NAA levels made are static, reflecting steady-state conditions.
Although the mechanism of NAA release is as yet unknown, its efflux is not passive, but a highly controlled process attested to by the high intracellular/extracellular NAA gradient (>200:1) maintained by neurons. Thus, the role played by changes in rates of NAA release down its high gradient (E), rather than (H) or (S), may be the critical component for understanding the function of neuronal NAA. The NAA released to ECF diffuses to oligodendrocytes, the only source of its catabolic enzyme, amidohydrolase II, where it is rapidly hydrolyzed, the Ac moiety primarily taken up by oligodendrocytes, and the Asp recycled in large part back to neurons (Baslow 2003). The synthesis, efflux to ECF, and then hydrolysis of NAA have been described in terms of a human data-based Glc-driven dynamic metabolic NAA model (Baslow 2002; Baslow and Guilfoyle 2006).
Whereas the functions of neuronal NAA are still unclear (Moffett et al. 2007), it has been proposed that an important role is that of a neuronal molecular water pump (MWP) (Baslow 1999, 2002; Baslow and Guilfoyle 2006) where each NAA molecule with a minimum of 32 obligated water molecules (Baslow and Guilfoyle 2002) is released by neurons to ECF down the steep NAA gradient, effectively transporting the water to ECF. The result of the intercompartmental NAA metabolic cycle is that neuronal metabolic and other water can be rapidly transported out of neurons, and liberated as “free water” in ECF upon hydrolysis of NAA, from where it can then pass down an osmotic gradient “Starling’s relationship” (Kimelberg 2004) into the vascular system for removal from the brain via the abundant vascular capillary endothelial-free water-selective aquaporin 4 channels (Kobayashi et al. 2001).
In addition to increased production of neuronal metabolic water from visual stimulation-induced higher rates of Glc metabolism (Frahm et al. 1996), there are other quantitative changes in cell and tissue water associated with neuron stimulation. These can include an increase in water influx as a result of Glc MWP activity (Meinild et al. 1998) because of the movement of Glc-obligated water into brain ECF via the vascular endothelial Glc transporter 1 (Escartin et al. 2006). In addition, there is a rapid (<2.4 s) accumulation of water in neurons as a function of neuron swelling (Le Bihan et al. 2006) resulting from the inward movement of Na+-water during the axonal depolarization process (Masson et al. 2006).
Animal studies of NAA in brain using proton magnetic resonance spectroscopy (1H MRS), followed by chemical analysis by high-pressure liquid chromatography, have demonstrated that the NAA MRS signal reflects the NAA concentration (Sager et al. 1999; Fatouros et al. 2000). In addition, phantom studies have shown that the NAA MRS signal reflects the NAA concentration and is linear from 0–50 mM (Duc et al. 1998). The acetyl methyl group of NAA produces a very strong nuclear magnetic resonance (NMR) signal at 2.02 ppm (Vrenken et al. 2005) that can be measured using MRS. N-acetylaspartylglutamate (NAAG), an anabolic product of NAA and glutamate, is also present in brain. In the visual cortex of rats it is present at about 2% of the level of NAA (Battistuta et al. 2001), and produces a much weaker singlet resonance signal at 2.04 ppm (Vrenken et al. 2005) that cannot easily be separated from the NAA signal at most NMR magnetic strengths used in human studies. Therefore, the NAAG MRS signal is generally incorporated into the total absolute or relative values usually presented for NAA (tNAA). In this investigation, we use the term “NAA” synonymously with “tNAA,” although recognizing that the NAAG signal may only be a minor component of tNAA in the human visual cortex. Because the NAA MRS signal reflects the concentration of NAA, we anticipated that any transient changes in rates of hydrolysis or synthesis of NAA during neurostimulation resulting in changes in NAA concentrations would be evident as changes in the NAA MRS signal. In this study, we used both the “standard” single subject (Traber et al. 2006) and multiple subject (Sarchielli et al. 2005) techniques, and present the results of a functional MRS (fMRS) study of the human visual cortex in response to visual stimulation.
For the NAA system to operate as a MWP, its metabolism must be tightly coupled to neurostimulatory events. The results of this dynamic NAA study attest to an intimate association between neuronal stimulation and NAA metabolism, and provide empirical evidence of large stimulation-related transient changes in NAA levels and in its apparent rates of efflux, hydrolysis, and synthesis. These results also identify NAA-obligated water efflux across the neuronal plasma membrane as the initial step in its functional cycle, supporting the hypothesis that the intercompartmental NAA metabolic cycle functions as a neuronal efflux MWP. Lastly, these results describe a previously unknown neuronal function, the direct relationship between neurostimulation and NAA release that may be a useful dynamic measurement tool for further understanding brain activation.
Materials and Methods
Subjects
The pool of subjects consisted of 6 volunteers, 5 men and 1 woman, ranging in age from 31 to 73 years with a mean age of 48.3 years. All showed normal MR anatomical brain scans and normal neurological function based on casual observation. Four of the six subjects were visual-stimulation-tested; one subject once, two subjects twice during the same session, and one subject, four separate times over 15 days, using several stimulation protocols. Based on these protocols, all were tested with eyes closed without stimulation during a 10-min baseline period. In addition, two of the four that were visual stimulation tested, and two others that were not stimulation tested, were also tested once each under extended baseline conditions. Two of these were tested for 20 min and two others for 30 min. Specific ages and genders of individual subjects represented in tables and figures are nos. 1 (m, 73), 2 (m, 66), 3 (m, 47), 4 (m, 34), 5 (f, 31), and 6 (m, 39). The number of MRS trials by subject were nos. 1 (4), 2 (2), 3 (2), 4 (1), 5 (1),and 6 (1). Of these, subjects 5 and 6 were baseline tested only.
Design of the Experiment
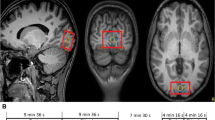
The general areas of activation in the primary visual cortex in response to experimental stimulation were first identified in several volunteers using a blood oxygenation level-dependent (BOLD) technique (Baslow and Guilfoyle 2007). For BOLD measurements, a repeated 3-min on/off visual stimulus was used. The visual stimulus was a black and white radial checkerboard pattern, with flicker at 8 Hz. For MRS, a single 2 × 2 × 2 cm voxel (8 cm3) was selected in the left brain hemisphere (Traber et al. 2006) in a visual stimulus BOLD-responsive region of interest (ROI), and a corresponding anatomical location was selected in all subjects.
The standard experimental protocol (A) consisted of three 10-min periods: a baseline period, a stimulation period, and a recovery period. The subjects had their eyes closed during the baseline period (Maddock et al. 2006) and were audibly cued to the beginning of the visual stimulation period 30 s before its start, so that eyes could be opened. At the end of the stimulation period, eyes were again closed during the recovery period. Other protocols were used to determine the variability of the NAA MRS signal within the standard 30-min protocol. In one protocol (B), there was 20 min of dark with no stimulation. In protocol (C), 30 min of dark with no stimulation. In a longer protocol (D), 30 min of dark was followed by a 10-min stimulation period. In a second long protocol (E), 10 min of dark was followed by 10 min of stimulation, 10 min of dark, and another 10 min of stimulation. Before any experimental protocol, anatomical scans were acquired and a single voxel was shimmed. This took about 15 min, so that the entire procedure was completed in about 45–50 min for the standard protocol, and 50–55 min for the longer protocols.
Statistical Analysis
An important analytical feature in the present study is that only relative changes in each individual’s NAA MRS signal was used, thus avoiding the use of ratios with other metabolites or water, and of external absolute measures based on NAA phantom methods. Therefore, for evaluation of changes in the NAA MRS signal in the stimulation and recovery periods, relative changes were computed for each subject using that individual’s 10-min baseline NAA MRS signal as control. Values normalized to this baseline are presented as plus or minus (percent) of the baseline control. These values were then analyzed by paired t tests to compare individual responses, and also used to compute changes in NAA levels (K 3), and in apparent rates of NAA (H) and (S) based on the dynamic NAA human metabolic model.
Whereas the method of paired t tests is very straightforward, it has some limitations. First, its significance level does not account for multiple comparisons performed simultaneously. Second, it assumes that NAA concentrations were sampled at equal time intervals in all subjects and experimental sessions. Therefore, we implemented a more rigorous analysis using the R language (R Development Core Team 2006). First, we assessed data normality from the histogram and a normal quantile–quantile plot of the baseline condition. To obtain significance levels and estimates for the apparent rates of NAA synthesis and hydrolysis, we constructed a linear model with three categorical variables representing the effects of individual subjects, experimental sessions, and stimulus condition. A single continuous variable represented the time within each stimulus condition. The significance levels of these effects were tested by analysis of variance and the effects not significant at level p of at least 0.01 were eliminated from the model. Finally, 95% confidence intervals (CI) were constructed for the estimated rates of apparent NAA synthesis and hydrolysis.
1H MRS Methods
Visual Stimulation Experiments
The data were acquired on a 3.0-T Magnex (Yarnton, U.K.) 80-cm bore magnet connected to a head dedicated spectrometer system (M.R.R.S. Guildford U.K., formerly S.M.I.S. Ltd). This system used a 38-cm ID gradient coil with a gradient strength of 40 mT m−1, rise time of 280 μs, and a 30-cm ID transmission line RF coil (Morris Instruments). Early in this investigation, an occipital surface receiver coil (Nova Medical) was acquired, providing a larger signal to noise ratio (SNR) that allowed for a change in the sampling protocol so that a single 1H spectrum required only 15 averages over 30 s rather than 100 averages over 200 s necessary with the standard head coil. A position resolved spectroscopy sequence (PRESS) was used (Bottomley 1987), with an echo time (TE) of 135 ms, and repetition time (TR) of 2 s. The sequence using the surface coil provided one averaged spectrum in each 30-s acquisition period, and the sequence using the standard head coil provided one averaged spectrum in each 200-s acquisition period. For t test comparisons between sets, the 30-s values were further averaged to obtain a single value for a longer interval.
The NAA MRS signal was computed as the area integral under the peak in arbitrary units. The three periods in standard protocol (A) were 600 s each. Within each period, there were three intraperiod samples. Because of the change in acquisition protocol during this investigation, intraperiods 1 and 2 were either 200 s or 210 s in duration, and intraperiod 3 was either 180 or 200 s in duration. For tabular data presentation, we report the three intraperiod points in each period as 200-s subperiod averages. For graphic presentations of multisubject data, the baseline data point represents the average of the preceding 600-s baseline period, and for stimulation and recovery data, each point represents the average of the preceding 200-s subperiod. This was done to emphasize that data collection occurred throughout each 10-min period and coincided with the abrupt changes in the 10-min on/off visual stimulation program. For graphic presentations of a single subject’s data, each point represents the average for 30 s, and is placed at the midpoint of that 30-s acquisition period.
MR Phantoms
Two MRS phantoms were used to verify instrument stability. Each was 2 l in volume. The first contained NaCl 3.6 g l−1, NAA 10 mM and D-Glc 1.1 mM, and the second contained NaCl 3.6 g l−1, NAA 20 mM and D-Glc 0.6 mM.
Results
NAA MRS Baseline Measurements in the Human Visual Cortex
Long-term baseline measurements for 10 to 30 min without stimulation demonstrated that in the selected visual ROI there were no trends in NAA MRS signal fluctuations. Mean variations in 200-s subperiods ranged between −2.7 and +7.8% of the first 10 min in the baseline period (baseline-1) with an overall standard deviation (SD) of ±4.0 (%). These results are shown in Table 1. Similar results were reported for the visual cortex by Sarchielli et al. (2005), where over a period of 36.7 min without stimulation, NAA MRS signal values had a SD from baseline of ±4.3 (%). In brain frontal and temporal lobe studies in humans at 3 T, it has also been observed that scans made 14 days apart showed variability of less than 10% for tNAA MRS signals with no systematic differences (Wellard et al. 2005). In addition, no right-left brain differences were apparent.
Long-term NAA MRS Signal Measurements in Phantoms
Long-term measurements of the NAA MRS signal for 60 min in phantoms indicated instrument stability over this period was satisfactory, with a variation of <0.5%.
Effect of Visual Stimulation on the NAA MRS Signal
In stimulation studies, the effect of visual stimulation on the NAA MRS signal was a graded decline in signal over a 10-min stimulation period. At the end of the stimulation period, signal had declined on average by 13.1%. During a subsequent 10-min no-stimulation recovery period, the NAA MRS signal rose and again approached prestimulation baseline levels. The decline in NAA MRS signal was observed whether the first stimulation was applied after 10 or after 30 min of dark baseline conditions. Similar declines occurred when stimulation was applied for a second time starting at 30 min, immediately after the 10-min recovery period. The results of these multisubject visual stimulation studies are presented in Table 2, and in graphic form showing the sequential results of consecutive first and second stimulation periods in Fig. 1.
Graphic representation of mean changes in NAA MRS signal in nine trials, including seven first and two second stimulation periods. Data from Table 2
In Fig. 2, the effects of visual stimulation on the NAA MRS signal at 30-s intervals (80 data points) in subject 2 (Fig. 2a) and subject 3 (Fig. 2b) for stimulation trials 1 and 2 during the same MRS session are shown.
In this figure, whereas the stimulation-induced responses are presented in greater temporal detail, they still show that NAA MRS signal changes occur gradually over a period of minutes, and then return to nearly prestimulation levels over a period of minutes after the stimulation has been terminated. Importantly, they show that repeated stimulations result in similar responses.
“R” Statistical Language Analysis
In these analyses, advantage was taken of the fact that the difference in SNR between the two RF coils used (volume coil vs surface coil) was compensated for by appropriate adjustment of the signal averaging in the scanner hardware (200 vs 30 s averaging intervals, corresponding to 100 vs 15 averaged TR periods). The baseline condition histogram and normal quantile–quantile plots showed that the assumption of data normality was reasonable. The standard deviation of the normalized NAA baseline samples was 7.2%, calculated across all subjects and experimental sessions.
The tests based on a linear statistical model showed that the effects of individual subjects and experimental sessions were not significant. The most significant effects (p < 0.001) were associated with the stimulus condition (baseline, first visual stimulus, recovery, and second visual stimulus), and linear regression lines fitted separately for these conditions differed significantly from each other in their intercepts and slopes. The baseline condition regression line did not have any significant slope and was not significantly offset from 100%. The first visual stimulus (n = 7) regression line had its intercept close to 100% (normalized baseline value) but its downward slope was significantly different from 0 (p ≪ 0.001). This slope provided an estimate of the apparent NAA (H), 1.5% min−1, with a CI from 0.9 to 2.1% min−1. Subsequent recovery of the NAA signal was slower, with an apparent NAA (S) of 0.8% min−1, with a 95% CI from 0.2–1.5% min−1, significant at p = 0.05. The t test analysis presented in Table 2 for this group was similar and values fell within the CI limits, 1.4% min−1 for apparent (H) and 1.3% min−1 for apparent (S).
Based on sessions where a second visual stimulus (n = 2) followed the 10-min recovery period, the NAA regression line was also negative, with an apparent (H) of 0.7% min−1, but not significant at p = 0.05. This probably results from two factors. First, only two subjects underwent a second visual stimulation, thus reducing the statistical power. Second, because of incomplete recovery from the first stimulus, the NAA signal level at the stimulus onset was already significantly below the 100% baseline (p = 0.05). However, both the linear statistical model and t test analyses generally agree and provide for similar interpretations of important aspects of the data.
Discussion
Comparison of the Results of this Investigation with Other Dynamic fMRS Studies of NAA in the Human Visual Cortex
In this dynamic study, it is shown that the NAA MRS signal level in the visual cortex is very responsive to a visual stimulation of sufficient magnitude and duration, varying inversely with, and showing graded responses to stimulation during 10-min stimulation periods, as well as during subsequent 10-min recovery periods. There are few other dynamic human studies of the relationship between the NAA MRS signal and visual stimulation that have been published.
A Dynamic Study Reporting Measurable Effects of Visual Stimulation on the NAA MRS Signal
The results of the present study are in agreement with the results recently reported by Sarchielli et al. (2005) that showed, for a group of normal subjects, that there was a gradual reduction in the NAA MRS signal in the visual cortex during periods of visual stimulation. As observed in the present study, these reductions were then followed by gradual increases in the NAA MRS signal during subsequent recovery periods. In that study, the control subjects consisted of 5 men and 5 women with an average age of 33.3 years. The protocol was 3.7 min dark baseline, 25.7 min light stimulation, followed by 7.4 min of recovery. Visual stimulations resulted in an attenuation of the NAA MRS signal, reaching an average level of about −12% after 11 min of stimulation, and then leveling off and maintaining that level of signal reduction until the end of the stimulation period. This was followed by a return to baseline signal levels by 7.4 min after cessation of stimulation.
Other Dynamic Studies Reporting no Significant Effects of Visual Stimulation on the NAA MRS Signal
Several other dynamic investigations of the effects of visual stimulation on the NAA MRS signal in the human visual cortex, using a variety of different techniques and protocols, have also been performed, none of which reported significant changes in the NAA MRS signal. In each of these cases, for a variety of different technical reasons, the relationship between stimulation and NAA attenuation may have been obscured.
Katz-Brull et al. (2006) performed a visual stimulation study using 9 subjects, 5 men and 4 women from 28 to 57 years of age, with a protocol that consisted of repetitive 32 s of light stimulation, followed by 118 s of dark. Based on the results of the present study, and that of Sarchielli et al. (2005), the stimulation period was probably too short, and recovery period between stimulations too long to observe significant stimulation-induced NAA signal changes.
Urrila et al. (2006), tested 8 female subjects from 20–25 years of age, with a protocol that consisted of a preliminary 30-min dark adaptation period, followed by a 4-min and 24-s dark baseline, and then photic stimulation for 13 min 12 s. No recovery period values were recorded. In this study, the water MRS signal was used as a standard and the NAA MRS results provided as NAA to water ratios.
Maddock et al. (2006) tested 6 subjects, 4 men and 2 women with a mean age of 24 years, with a total of 9.6 min of baseline, and then a 11.2-min stimulation period, but with no recovery period. The NAA MRS signal results are not comparable to the present study as they were provided as ratios to other brain metabolites.
Boucard et al. (2005) performed experiments on 4 subjects, ages 21 to 40 years, using 14 min stimulation and control time blocks, but with relatively large anterior-posterior brain ROIs, 9 × 7 × 2, 5 × 7 × 2, and 3 × 3 × 3 cm, that may have influenced their ability to identify focal changes in NAA signal occurring in the primary visual cortex.
Lastly, Zhu and Chen (2001) performed a study using 6 subjects, 2 men and 4 women with an average age of 32 years. They used 6.4-min baseline, 12.8-min stimulation, and 6.4-min recovery periods. However, they processed NAA spectral peak heights rather than areas under the peaks, which are sensitive to local susceptibility changes. Reporting a 2.5% rise in the NAA peak heights during stimulation, they attributed the rise to an extravascular BOLD effect.
Evidence that Rates of NAA Catabolic and Anabolic Metabolism are Intimately Associated with Neurostimulatory Events
From the temporal changes in the NAA MRS signal observed in this investigation, it is possible to calculate apparent changes in rate of efflux and hydrolysis of NAA during stimulation periods, and in its apparent rate of synthesis during the recovery periods based on the NAA metabolic model (Table 3).
By the end of 10-min periods of visual stimulation, average NAA MRS signal levels in focal areas of the visual cortex had decreased by 13.1%, and then increased to near baseline levels once again by the end of 10-min recovery periods. Based on the correspondence of NAA MRS signal and NAA concentration (Sager et al. 1999; Fatouros et al. 2000), and using a nominal value for brain NAA of 10 μmol g−1 (10 mM), a 13.1% loss in signal by the end of the 10-min stimulation period corresponds to an NAA loss of 1.3 μmol g−1 10 min−1 or 7.8 μmol g−1 h−1. From the model, the average rate of NAA hydrolysis in brain is 0.55 μmol g−1 h−1, so that the apparent rate of NAA catabolism (H–S) is 14.2 times that of nonstimulated brain. In whole bovine brain, a calculated maximum rate of NAA hydrolysis is about 6 μmol g−1 h−1 (Kaul et al. 1991). If similar in human brain, and based on the juxtaposition of neurons and oligodendrocytes, this suggests that (H) capacity will almost always be greater than (E) within the range of NAA reductions observed in the present study, so that any NAA released to ECF would not be expected to persist for very long, nor to build up in ECF.
In nonstimulated brain the turnover (T) of NAA is about 16–18 h. Based on this experiment, it would appear that NAA turnover (T = K 3/H) in the selected visual ROI (about 8 g of brain) is reduced to 1.3 h, indicating that whereas the average turnover of NAA in brain appears relatively slow and constant, focal areas of stimulation may exhibit wide temporal variations in rates of NAA catabolism and turnover. In this investigation, NAA signal values also recovered almost to baseline levels at the end of the recovery period. Using the same reasoning, the difference from −13.1 to −0.9% was +12.2% in 10 min, an increase in NAA of 1.2 μmol g−1 in 10 min or 7.3 μmol g−1 h−1. Thus, the apparent rate of synthesis (S–H) during recovery was 13.3 times the brain average rate, indicating an NAA turnover (T = K 3/S) of about 1.4 h.
Evidence in Support of the Hypothesis that NAA Functions as a Neuronal Efflux MWP
A Real-time Neurostimulation NAA–Water Efflux Connection
From the NAA model, the average rate of Glc oxidation in cortical gray matter is 22.2 μmol g−1 h−1 under nonstimulation conditions, and the rate of production of metabolic water is 133 μmol g−1 h−1. During stimulation of the visual cortex, focal brain Glc levels decrease by about 50% in approximately 5 min (Frahm et al. 1996; Merboldt et al. 1992) resulting from an increase in brain Glc oxidation of 22% over the nonstimulated condition (Chen et al. 1993). At a 22% increase in Glc oxidation to 29.3 μmol g−1 h−1, there would be an associated increase in the synthesis of metabolic water for a total of 162 μmol g−1 h−1, 80% of which (130 μmol g−1 h−1) is considered to be because of neuronal activity (Patel et al. 2004). From the present study, it is observed that the apparent rate of NAA efflux (=H) during the 10-min visual stimulation period increased to 7.8 μmol g−1 h−1. At a minimum ratio of 32 waters to each NAA (Baslow and Guilfoyle 2002), this level of NAA efflux could transport 250 μmol g−1 h−1 of water into extracellular space (ECS), an amount adequate to pump the equivalent of all of the neuronal metabolic water resulting from the visual neurostimulation protocol into ECS.
Evidence of the Operation of the NAA Metabolic Cycle as a MWP
Based on the NAA model, under steady-state conditions E = H = S, so that if E increases, then H must also increase, and subsequently S must increase to maintain NAA at its functional level (K 3). Thus, the model predicts that any increase in E will be followed in order, first by an increase in H, and then in S. For NAA levels to fall during stimulation, E (=H) must be larger than S, and in order for NAA levels to rise during recovery, E (=H) must be less than S.
Using the empirical results presented in Table 2 for observed changes in the NAA MRS signal in all stimulations (n = 9), and elements of the NAA metabolic model presented in Table 3, the temporal operation of the NAA intercompartmental cycle as a MWP can be evaluated. In Fig. 3, we have incorporated these results with NAA model values, graphically illustrating that the expected theoretical relationships between E, H, and S correspond with the in vivo data, indicating that an increase in (E) is the first step in the process, and supporting the hypothesis that the NAA MWP operates in a timely fashion to remove neuronal water to ECF in the stimulated human visual cortex.
Demonstration of the NAA metabolic cycle operating as a MWP in the stimulated visual cortex. Data from Table 2, all stimulations. Water efflux = E × K 2
The neuronal content of NAA (with neurons at 50% of brain volume) is about 20 mM, providing a large NAA reserve capacity available to rapidly respond to constantly changing neuronal stimulation-associated water fluctuations, without requiring an immediate increase in the rate of NAA synthesis. Thus, there is a reasonable explanation, based on the NAA MWP hypothesis, for both the unusually high cytosolic NAA levels, and the steep intracellular/ECF NAA gradients that are maintained by neurons. We believe that the results of this study demonstrate NAA MWP activity in the human visual cortex. In this experiment, we “forced” the system by providing a prolonged (600 s) and high level of stimulation to observe the MWP function. However, during “normal” brain activity we believe that this system operates continuously, but does so on a millisecond time scale reflecting stimulatory and signaling events that occur at typical frequencies of 0.1 to 500 Hz, with the small changes in NAA levels only evident as a continuous NAA background “noise.”
Conclusions
The results of this dynamic study of the stimulated human visual cortex provide evidence of an important piece in the puzzle regarding the function of neuronal NAA, that of its metabolism in relationship to the rate of neuronal activity. These results indicate an intimate association of NAA metabolism and neurostimulation, with metabolic changes initiated within seconds of stimulation, resulting in significantly reduced NAA levels in minutes. From this observation, we can reconstruct the NAA-related metabolic sequence that occurs during visual stimulatory events.
As a result of stimulation, neuronal NAA and its obligated water is liberated down its high gradient into ECF, from where it diffuses to, and is rapidly catabolized by oligodendrocyte enzymatic activity. The first event in this sequence is an increase in (E), with (H) following second. During the stimulatory period, the rate of NAA synthesis is apparently lower than its rates of efflux and hydrolysis resulting in reduced neuronal NAA levels. During the subsequent recovery period, the relationship is reversed, and the rate of synthesis is apparently higher than its rates of efflux and hydrolysis so that neuronal NAA levels again rise to baseline levels.
We hypothesize that the increase in rate of NAA synthesis is initiated within the stimulatory period and continues into the recovery period, paralleling the increase in rate of mitochondrial Glc oxidation used for resynthesis of the adenosine triphosphate (ATP) expended in the neuron repolarization process that is a direct result of increased neuronal signaling. Because Glc oxidation is also the major source of AcCoA used for mitochondrial NAA synthesis, the rates of Glc use for ATP resynthesis and for NAA resynthesis are seen to be temporally, spatially, structurally, and metabolically linked within neuron mitochondria. A by-product of Glc oxidation for neuronal energy production is metabolic water that must be removed from neurons. The relationships between these interactive ongoing metabolic processes occurring simultaneously in neuronal mitochondria (with neurons representing 80% of whole brain metabolism) are summarized in the following bioenergetic equation (Eq. 1).
ATP is the neuron’s primary source of energy, and utilization of extant ATP energy stores to run the Na+–K+ ATPase pump for membrane repolarization produces ADP and P, driving this complex of reactions to the right. Importantly, any build-up of mitochondrial or cellular water would shift the equilibrium to the left, reducing the rate of ATP production. The simultaneous increase in rate of efflux of NAA and its obligated water into ECF during neurostimulatory energy-requiring events and its rapid hydrolysis result in the timely transport of neuronal water to ECS, thus supporting a high rate of ATP production, and at the same time performing the initial step in a process that is required for removal of water from the brain.
Whereas the results of this investigation provide an insight into how neurons maintain their osmotic and functional integrity, and support the MWP hypothesis, they do not prove that the only role of neuronal NAA is that of a MWP. However, they do demonstrate a dynamic relationship between the NAA metabolic cycle and neurostimulatory events, and focus attention on an increase in rate of NAA transport across the neuronal plasma membrane into ECF as the primary and seminal event in response to stimulation, and the likely site of its functional role. Importantly, these results describe a previously unknown neuronal function, the direct relationship between rates of stimulation and NAA release.
Summary of Findings
-
1.
Robust stimulation in a ROI in the primary visual cortex results in a graded loss of NAA MRS signal in that area over a period of 10 min.
-
2.
During a subsequent 10-min recovery period, the NAA MRS signal gradually returns to near baseline level.
-
3.
These changes are reproducible in individuals tested days or weeks apart, and by sequential activations in the same individual immediately after a 10-min recovery period.
-
4.
The loss of NAA MRS signal during stimulation and subsequent recovery poststimulation indicate that NAA metabolism is intimately linked to neurostimulatory events, with large stimulation-induced temporal changes in its focal neuronal content and in its focal rates of (E), (H), and (S).
-
5.
The initial effect of neuron stimulation on neuronal NAA appears to be a rapid release of stored NAA down its steep gradient into ECF, indicating that “(E)” is the first step in its functional cycle. Because (H) capacity resides in a different cell, it can only follow a change in (E).
-
6.
The rate of release of NAA-obligated water to ECF corresponds with the increased rate of Glc oxidation and increased rate of production of metabolic water by stimulated neurons in the primary visual cortex. NAA release can account for removal of the equivalent of all metabolic water to ECF during the stimulation event.
Abbreviations
- Ac:
-
acetate
- AcCoA:
-
acetyl-coenzyme A
- Asp:
-
aspartic acid
- ATP:
-
adenosine triphosphate
- AQP4:
-
aquaporin 4
- BOLD:
-
blood oxygen level-dependent imaging
- CI:
-
confidence interval
- ECF:
-
extracellular fluid
- ECS:
-
extracellular space
- fMRS:
-
functional magnetic resonance spectroscopy
- Glc:
-
glucose
- GLUT 1:
-
Glc transporter 1
- 1H MRS:
-
proton magnetic resonance spectroscopy
- MRS:
-
magnetic resonance spectroscopy
- NAA:
-
N-acetyl-l-aspartic acid
- NAAG:
-
N-acetylaspartylglutamic acid
- NMR:
-
nuclear magnetic resonance
- tNAA:
-
total NAA + NAAG
- ROI:
-
region of interest
- SNR:
-
signal to noise ratio
References
Baslow, M. H. (1999). The existence of molecular water pumps in the nervous system. A review of the evidence. Neurochemistry International, 34, 77–90.
Baslow, M. H. (2000). Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain. Role in glial cell-specific signaling. Journal of Neurochemistry, 75, 453–459.
Baslow, M. H. (2002). Evidence supporting a role for N-acetyl-L-aspartate as a molecular water pump in myelinated neurons in the central nervous system. An analytical review. Neurochemistry International, 40(4), 295–300.
Baslow, M. H. (2003). N-acetylaspartate in the vertebrate brain: Metabolism and function. Neurochemical Research, 28(6), 941–953.
Baslow, M. H., & Guilfoyle, D. N. (2002). Effect of N-acetylaspartic acid on the diffusion coefficient of water: A proton magnetic resonance phantom method for measurement of osmolyte-obligated water. Analytical Biochemistry, 311(2), 133–138.
Baslow, M. H., & Guilfoyle, D. N. (2006). Functions of N-acetylaspartate and N-acetylaspartylglutamate in brain: Evidence of a role in maintenance of higher brain integrative activities of information processing and cognition. Advances in Experimental Medicine & Biology, 576, 95–112.
Baslow, M. H., & Guilfoyle, D. N. (2006). Using proton magnetic resonance imaging and spectroscopy to understand brain “activation.” Brain and Language (in press). DOI 10.1016/s.band1.2006.06.119.
Battistuta, J., Bjartmar, C., & Trapp, B. D. (2001). Postmortem degradation of N-acetyl aspartate and N-acetyl aspartylglutamate: An HPLC analysis of different rat CNS regions. Neurochemical Research, 26, 695–702.
Bottomley, P. A. (1987). Spatial localization in NMR spectroscopy in vivo. Annals of the New York Academy of Science, 508, 333–348.
Boucard, C. C., Mostert, J. P., Cornelissen, F. W., De Keyser, J., Oudkerk, M., & Sijens, P. E. (2005). Visual stimulation, 1H MR spectroscopy and fMRI of the human visual pathways. European Radiology, 15, 47–52.
Chen, W., Novotny, E. J., Zhu, X.-H., Rothman, D. L., & Shulman, R. G. (1993). Localized 1H NMR measurement of glucose consumption in the human brain during visual stimulation. Proceedings of the National Academy of Sciences of the United States of America, 90, 9896–9900.
Duc, C. O., Weber, O. M., Trabesinger, A. H., Meier, D., & Boesiger, P. (1998). Quantitative 1H MRS of the human brain in vivo based on the simulation phantom calibration strategy. Magnetic Resonance in Medicine, 39, 491–496.
Escartin, C., Valette, J., Lebon, V., & Bonvento, G. (2006). Neuron–astrocyte interactions in the regulation of brain energy metabolism: A focus on NMR spectroscopy. Journal of Neurochemistry, 99, 393–401.
Fatouros, P. P., Heath, D. L., Beaumont, A., Corwin, F. D., Signoretti, S., Al-Samsam, R. H., et al. (2000). Comparison of NAA measures by MRS and HPLC. Acta Neurochirurgica, 76, 35–37.
Frahm, J., Kruger, G., Merboldt, K. D., & Kleinschmidt, A. (1996). Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magnetic Resonance in Medicine, 35, 143–148.
Katz-Brull, R., Alsop, D. C., Marquis, R. P., & Lenkinski, R. E. (2006). Limits on activation-induced temperature and metabolic changes in the human primary visual cortex. Magnetic Resonance in Medicine, 56, 348–355.
Kaul, R., Casanova, J., Johnson, A. B., Tang, P., & Matalon, R. (1991). Purification, characterization, and localization of aspartoacylase from bovine brain. Journal of Neurochemistry, 56, 129–135.
Kimelberg, H. K. (2004). Water homeostasis in the brain: Basic concepts. Neuroscience, 129, 851–860.
Kobayashi, H., Minami, S.-I., Itoh, S., Shiraishi, S., Yokoo, H., Yanagita, T., et al. (2001). Aquaporin subtypes in rat cerebral microvessels. Neuroscience Letters, 297, 163–166.
Le Bihan, D., Urayama, S.-I., Aso, T., Hanakawa, T., & Fukuyama, H. (2006). Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proceedings of the National Academy of Sciences of the United States of America, 103(21), 8263–8268.
Maddock, R. J., Buonocore, M. H., Lavoie, S. P., Copeland, L. E., Kile, S. J., Richards, A. L., et al. (2006). Brain lactate responses during visual stimulation in fasting and hyperglycemic subjects: A proton magnetic resonance spectroscopy study at 1.5 Tesla. Psychiatry Research, 148, 47–54.
Masson, J.-B., Sauviat, M.-P., Martin, J.-L., & Gallot, G. (2006). Ionic contrast terahertz near-field imaging of axonal water fluxes. Proceedings of the National Academy of Sciences of the United States of America, 103(13), 4808–4812.
Meinild, A.-K., Klaerke, D. A., Loo, D. D. F., Wright, E. M., & Zeuthen, T. (l998). The human Na+-glucose cotransporter is a molecular water pump. Journal of Physiology, 508(1), l5–21.
Merboldt, K. D., Bruhn, H., Hanicke, W., Michaelis, T., & Frahm, J. (1992). Decrease of glucose in the human visual cortex during photic stimulation. Magnetic Resonance in Medicine, 25, 187–194.
Moffett, J. R., Ross, B., Arun, P., Madhavarao, C.N., & Namboodiri, A. M. A. (2007). N-acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Progress Neurobiology, 81, 89–131.
Patel, A. B., de Graaf, R. A., Mason, G. F., Kanamatsu, T., Rothman, D. L., Shulman, R. G., et al. (2004). Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activation. Journal of Cerebral Blood Flow and Metabolism, 24(9), 972–985.
R Development Core Team (2006). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, ISBN 3-900051-07-0.
Sager, T. N., Laursen, H., Fink-Jensen, A., Topp, S., Stennsgaard, A., Hedehus, M., et al. (1999). N-acetylaspartate distribution in rat brain striatum during acute brain ischemia. Journal of Cerebral Blood Flow and Metabolism, 19, 164–172.
Sarchielli, P., Tarducci, R., Presciutti, O., Gobbi, G., Pelliccioli, G. P., Stipa, G., et al. (2005) Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage, 24, 1025–1031.
Traber, F., Block, W., Freymann, N., Gur, O., Kucinski, T., Hammen, T., et al. (2006). A multicenter reproducibility study of single-voxel 1H-MRS of the medial temporal lobe. European Radiology, 16, 1096–1103.
Urrila, A. S., Hakkarainen, A., Heikkinen, S., Huhdankoski, O., Kuusi, T., Stenberg, D., et al. (2006). Preliminary findings of proton magnetic resonance spectroscopy in occipital cortex during sleep deprivation. Psychiatry Research, 147, 41–46.
Vrenken, H., Barkhof, F., Uitdehaag, B. M. J., Castelijns, J. A., Polman, C. H., & Pouwels, P. J. W. (2005). MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magnetic Resonance in Medicine, 53, 256–266.
Wellard, R. M., Briellmann, R. S., Jennings, C., & Jackson, G. D. (2005). Physiologic variability of single-voxel proton MR spectroscopic measurements at 3T. American Journal of Neuroradiology, 26, 585–590.
Zhu, X.-H., & Chen, W. (2001). Observed BOLD effects on cerebral metabolite resonances in human visual cortex during visual stimulation: A functional 1H MRS study at 4 T. Magnetic Resonance in Medicine, 46, 841–847.
Acknowledgement
This study was supported in part by a grant from the National Institutes of Health, National Institute of Biomedical Imaging and Bioengineering (1 R21 EB004727-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baslow, M.H., Hrabe, J. & Guilfoyle, D.N. Dynamic Relationship Between Neurostimulation and N-Acetylaspartate Metabolism in the Human Visual Cortex. J Mol Neurosci 32, 235–245 (2007). https://doi.org/10.1007/s12031-007-0049-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-007-0049-9