Abstract
Background
Worldwide, gastric carcinoma (GC) is the 5th most common malignancies in both sexes representing 6.8% of the total fatalities and is the 3rd leading cause of cancer death representing 8.8% of total fatalities. In Egypt, GC considers the 12th leading cause of cancer death representing 2.2% of the total cancer mortality. A growing body of evidence supports that cancer stem cells (CSCs) are resistant to chemotherapy or radiation, and the cell adhesion molecule CD44 has been identified as a cell surface marker associated with cancer stem cell in several types of tumors including gastric cancer. CD44 regulates gastric stem cell proliferation by increasing cyclin D1 expression which represents an important regulatory protein in the cell cycle transition from G1 phase to S phase. This study aimed to investigate whether cyclin D1 and CD44 can be used as prognostic indicators in gastric cancer.
Material and Methods
Forty formalin-fixed and paraffin-embedded gastric tissues, obtained from patients who underwent endoscopic resection or surgical resection, constituted the group of our study. The immunohistochemical expression of cyclin D1 and CD44 was examined and correlated with clinical-pathological parameters and outcome of the patients.
Results
Overexpression of CD44 and cyclin D1 was noted (in of 55 and 50% respectively). Cyclin D1 and CD44 positive expressions in GC were positively correlated with tumor differentiation (p = 0.020, p = 0.004 respectively), TNM stage (p < 0.001 for both), poor survival (p < 0.001 for both), and with increased rate of recurrence (p = 0.020, p = 0.005 respectively).
Conclusion
CD44 and cyclin D1 were associated with poor prognosis in gastric cancer, and so, they comprise an attractive target for anticancer drug development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, gastric carcinoma (GC) is the 5th most common malignancies in both sexes representing 6.8% of the total fatalities and is the 3rd leading cause of cancer death representing 8.8% of total fatalities [1].
In Egypt, GC is the 12th most common cancer in both sexes representing 1.6% of total cancer and is considered the 12th leading cause of cancer death representing 2.2% of the overall cancer mortality [1, 2]. Many Egyptian population-based cancer registries approved the previous data [3].
The median age of GC in the Egyptians is 56 years [2]. The pathogenesis of GC has not been fully understood, and the identification of new tumor markers and therapeutic targets have an important clinical significance for the treatment of gastric cancer [4].
A growing body of evidence supports that only a small population of cells within a solid tumor has “stem-like” characteristics. These cancer stem cells (CSCs), distinct from non-malignant stem cells, show higher self-renewal capacity, propensity to differentiate into active proliferating tumor cells, and resistance to chemotherapy or radiation, and so many cancer patients experience recurrence owing to failure to target the cancer stem cells [5] adequately.
Nishii et al. [6] have identified cancer stem cells in gastric adenocarcinoma and demonstrated that GC stem cells may be the metastasis precursors and that CD44 was one of the molecules that was significantly overexpressed in the cancer stem cell population constituting the bulk of peritoneal metastasis.
A cluster of differentiation 44 (CD44) is the major cell surface receptor for hyaluronate encoded on the short arm of chromosome 11 in humans. CD44 is composed of three domains: extracellular, transmembrane, and intracellular. Its extracellular portion binds to the ligand hyaluronan (HA), where their interaction promotes cell migration and maintains proliferation and differentiation of cancer stem cells (CSCs). CD44 exists in a standard form (CD44s) and 10 distinct isoforms (CD44v) [7, 8].
Takaishi et al.’s [9] study was the first that demonstrated the existence of CD44 positive cells endowed with stem cell properties in gastric adenocarcinoma and also approved that CD44 (+) gastric cancer cells showed increased resistance to chemotherapy or radiation therapy.
CD44 regulates gastric stem cell proliferation by increasing cyclin D1 expression through directly interacting with the active signal transducer and activator of transcription 3 (STAT3), that were reported to have carcinogenic effects [10].
The cyclin D1 proto-oncogene is a regulatory protein in the cell cycle transition from G1 phase to S phase in many different cell types. Together with its binding partner’s cyclin-dependent kinase 4 and 6 (CDK4 and CDK6), cyclin D1 forms active complexes that promote cell cycle progression by phosphorylating and inactivating the retinoblastoma protein (RB). The cyclin D1 protein has been shown to be unstable with a short half-life (~24 min) [11].
High activity of cyclin D1 leads to premature cell passage through the G1–S transition, resulting in the propagation of unrepaired DNA damage and accumulation of genetic errors, therefore leading to a selective advantage for abnormal cell proliferation [12].
Cyclin D1 overexpression is believed to play an essential role in the tumorigenesis through modulating the tumor cell proliferation, apoptosis, invasion, metastasis, and immune escape [13].
Material and Methods
Tissue Sample
Paraffin-embedded tumor tissue was obtained from 40 GC that was diagnosed at the pathology department and treated at Zagazig University Hospital during the period from 2012 to December 2016. All patients were followed up every 4–6 months for 3 years until December 2016 with a median follow-up of [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] months. The clinicopathological and follow-up data were available from the patient’s reports. The Ethics Committee of Zagazig University Hospital authorized the collection of specimens.
The hematoxylin and eosin stained slides were reviewed and graded by Lauren classification: intestinal type GC corresponds to well or moderately differentiated tumor and diffuse type corresponds with poorly differentiated tumors. Pathologic stage was reassigned according to the 2010 American Joint Committee on Cancer TNM staging system (7th edition) [14].
Immunohistochemical Analyses
Immunohistochemical staining of tissue sections was conducted using the avidin-biotin complex (ABC) method The formalin-fixed, paraffin-embedded (FFPE) sections were cut into 5 um thick. Sections were deparaffinized with xylene and dehydrated through graded concentrations of alcohol. Incubation with 0.3% H 2 O 2 in methanol for 30 min was done to block the endogenous peroxidase activity. The sections were then treated with microwave radiation for 10 min for antigen retrieval, and to prevent intrinsic antibody binding, they were reacted with normal serum (mouse IgG) for 10 min at room temperature. The sections were then incubated with primary antibodies (monoclonal antibody against CD44 Std./HCAM AB-4 (0.7 ml. of antibody prediluted 0.05 mol/L Tris-HCl, pH 7.6 containing stabilizing protein and 0.015 mol/L sodium azide—Thermo Fisher Scientific. UK) and Rabbit monoclonal anti-cyclin D1 antibody (Cat. from Thermo Scientific/Lab Vision Corporation, Fermont, USA, and clone: EPR2764. 0.09% sodium azide. Dilution 1:100), with appropriate negative and positive controls. The sections were incubated with biotinylated anti-mouse antibody at room temperature for 20 min, followed by peroxidase-labeled streptavidin for 20 min. Binding was detected using DAB (Dako). Sections were counterstained with Mayer’s hematoxylin before mounting.
Brown nuclear staining was regarded as a positive result for cyclin D1 and scored as the following: negative, 1 + (weak) = less than 10%, 2 + (moderate) = 11 to 50%, and 3 + (strong) = more than 50% nuclear tumor cells stained positive [15]. Positive immunoreactivity in the cytoplasm for cyclin D1 was considered aberrant. Positivity for CD44 was reported as cytoplasmic and membranous staining or negative with the percentage of positive cells [16]. The intensity of CD44 expression was scored based on the percentage of positive cells, as follows: negative (−), < 10%; weak positive (+), 10–25%; moderate positive (++), 25–50%; and strong positive (+++), > 50% [17].
Controls
Sections from a tonsillar tissue and breast cancer were used as a positive control for CD44 and CD1 respectively. Negative controls were obtained by replacing the primary antibody with a non-immunized rabbit or mouse serum.
Statistical Analyses
Continuous variables were expressed as the mean ± SD and median (range), and the categorical variables were expressed as a number (percentage). Percentage of categorical variables was compared using Pearson’s chi-square test or Fisher’s exact test when appropriate. The trend of change in the distribution of relative frequencies of ordinal data was analyzed using the Chi-square test for direction. Disease-free survival (DFS) was calculated as the time from the time of surgery to relapse or the most recent follow-up in which patient was free from relapse. Overall survival (OS) was calculated as the time from diagnosis to death or the most recent follow-up contact (censored). Stratification of DFS and OS was done according to immunohistochemical markers. These time-to-event distributions were estimated using the method of Kaplan-Meier plot and compared using two-sided exact log-rank test. All tests were two-sided. A p value < 0.05 was considered significant. All statistics were performed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA) and MedCalc windows (MedCalc Software bvba 13, Ostend, Belgium).
Results
-
A—Clinical characteristics and pathological findings
The age of the 40 patients ranged from (41–60) years (mean 52.57 ± 5.25 years). There were 25 (62.5%) males and 15 (37.5%) females. The majority of cases 35 (87.5%) were of the intestinal type, and only 5 (12.5%) were of diffuse-type adenocarcinoma. All the data were illustrated in Table 1. No significant correlation was found between patient’s age or, sex, initial site, histopathological subtype, or size of the tumor with both markers expression (Table 2).
-
B—Expression of cyclin D1 and CD44 in GC (Table 1, Figs. 1 and 2)
Fifty percent (20/40) of the cases were positive for cyclin D1, and 55 % (22/40) were positive for CD44.
-
C—Correlation between cyclin D1 and CD44 expression in GC (Table 2)
Both markers showed a significant positive correlation with each other (p < 0.001)
-
D—Correlation between cyclin D1 and CD44 expression and pathological features (Table 2)
Cyclin D1 was significantly correlated with higher tumor grade, high incidence of LN metastases, and advanced stage of the tumor (p = 0.020, p = 0.020, and p < 0.001 respectively). Also, CD44 overexpression was significantly positively correlated with grade, LN metastases and stage of the tumor (p = 0.004, p = 0.005, and p < 0.001 respectively).
-
E—Correlations between cyclin D1 and CD44 expression and outcome of the patients (Table 3, Fig. 3).
-
I—Out of 40 patients, 32 (80%) died and 8 (20%) were alive at the last follow-up. Positive expression of cyclin D1 and CD44 was significantly associated with shortened disease-free survival (DFS) and overall survival (OS) (p < 0.001 for both).
-
II—A positive expression of both cyclin D1 and CD44 was significantly associated with an increase the incidence of tumor recurrence (p = 0.020, p = 0.005 respectively).
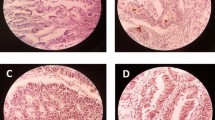
Expression of cyclin D1 and CD44 in gastric cancer d Well-differentiated gastric adenocarcinoma with cytoplasmic and membranous CD44 staining ×200. e Moderately differentiated gastric adenocarcinoma with cytoplasmic CD44 staining. f High-grade gastric adenocarcinoma with membranous and cytoplasmic CD44 staining 400
Discussion
According to the National Cancer Institute, gastric cancer ranks among worst malignancies in prognosis, with less than 30% of patients surviving for 5 years after diagnosis [18].
Accumulating evidence indicates that CD44 is a cancer stem cell (CSC) markers and critical players in regulating the properties of CSCs [19].
CD44 regulates the CSC via STAT3-cyclin D1 pathway [10]. Both CD44v and CD1 have been shown to be highly expressed in gastric adenocarcinoma [20, 21]. Cyclin D1 overexpression has been reviewed in many studies as a poor prognostic marker of colorectal, esophagus, stomach, pancreas, and liver cancers [29]. CD44 has been found to function as a prognostic marker in many other tumors, including lung, colorectal, breast, hepatocellular, head, neck, and hypopharyngeal squamous cell carcinoma [19].
Our work aims to evaluate the prognostic value of cyclin D1 and CD44 expression in gastric adenocarcinoma.
In this study, cyclin D1 was positive in 50% of gastric cancer. In the previous report done by Begnami et al. [22], the incidence was 50%. However, Casasola et al. [23] showed cyclin D1 expression in only 29.2% of gastric cancer.
In our study, we found that cyclin D1 overexpression was associated with reduced differentiation in gastric adenocarcinoma. It was in agreement with the studies of Feakins et al. [21] and Casasola et al. [23], but some studies as that of Begnami et al. [22] who found no relationship between cyclin D1 expression and histological differentiation.
In the present study, cyclin D1 was correlated with lymph node metastasis and TNM stage, and these were in disagreement with a survey by Arici et al. [24] who found a nonsignificant correlation between cyclin D1 expression and depth of invasion and lymph node metastasis; also. Takano et al. [25] approved that cyclin D1 did not have prognostic significance and showed no significant differences as regards the grade of differentiation and lymph node state.
Analysis of the disease-free survival (DFS) and overall survival (OS) showed that cyclin D1 overexpression is correlated with shorter cancer patient survival. This result was in agreement with Jares et al. [26] who reported that disease-free survival (DFS) and overall survival (OS) were significantly more reduced for cyclin D1-positive patients. However, this result was in contrast to Ahn et al.’s [27] study, which showed no correlation with disease-free survival or overall survival.
Tumor survival and metastasis are controlled by the balance between angiogenesis stimulators and inhibitors [28], and cyclin D1 may play a role in the maintenance of VEGF expression [29].
Cyclin D1 overexpression can downregulate Fas expression, leading to increased chemotherapeutic resistance and protection from apoptosis [30]. Also, Yoon et al. [31] has demonstrated the resistance of gastric cancer cases that were positive CD44 to 5-fluorouracil and cisplatin chemotherapy.
In our study, the frequency of CD44-positive expression in tumor samples was 55%. Near to the result, Dhimgra et al. [32] found that CD44 expression was 51% in the tumor. Yamaguchi et al. [33], in a study of 95 cases of gastric carcinoma, reported that the expression of CD44 was 47.3%.Unlike in the studies of Ghafarzadegan et al. [16], Kim et al. [34], and Nosrati et al. [20], the incidence of CD44 in gastric cancers was 65, 11.4, and 60% respectively.
In this study, the CD44 expression seems to correlate with the degree of tumor differentiation and this was in agreement with the review of Wang et al. [35]. However, Yamaguchi et al. [33] found that the expression of the CD44 protein was significantly higher in differentiated adenocarcinoma than in diffuse-type carcinoma.
This variation may contribute to the use of various antibodies having subtle differences in specificity and thus increasing the possibility of cross-reactivity between the antibodies. Another reason for such discrepancies is probably the comparison of results having different techniques [36].
In the study of Chen et al. [37], the CD44 expression was positively correlated with advanced stage and has been implicated in the development of lymph node metastasis, which is in harmony with our results and in contrast to Cao et al.’s [38] study which proved that there is no significant difference in CD44 expression level in relation to TNM stage. Cancer cells expressing CD44 utilize a camouflage function against lymphocytes to escape identification and destruction of the human immune system, thus, enabling easier metastasis [35].
Among all the examined 40 cases in this study, the overall survival rate was significantly lower in those whose tumors express CD44 than in those with tumors that did not show it. Similarly, Cao et al. [38], Yan et al. [19], and Chen et al. [37] demonstrated that CD44 expression was associated with reduced overall survival rates. Moreover, no relationship between CD44 and recurrence could be approved by Yong et al.’s [39] study, and this result was in disagreement with the present study.
The higher expression of CD44 in gastric cancer cells may represent a more top percent of CSCs from which a weaker survival was explained [38, 40]. Therapies that specifically target CSC may give a great promise for improving survival for cancer patients [19].
The results of the current study are consistent with previous findings, showing that CD44 and cyclin D1expression is associated with poor prognosis in gastric cancer. So, the critical role of CD44 as a cancer stem cell (CSC) marker in tumor initiation, metastasis, and chemo-radioresistance comprise an attractive target for anticancer drug development that destroys the CSC population. Also, there is an ample evidence that cyclin D1 is valuable prognostic markers in various types of tumors. Therefore, therapies that target cyclin D1 hold excellent promise for the cure of cancers. One of the most reliable points of our study is the prospective nature of our work, but it lacks follow-up of the cases with the same tissue markers after chemotherapy. Also, non-studying the association between chemoradiotherapy and tissue makers is another weak point of the present work.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet].Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. Accessed on day/month/year.
Zeen eldein AA, Ramadan H, El Gamal MM, Saber MM, Elgamal D, Sherisher MA. Gastric carcinoma at Tanta Cancer Center: a comparative retrospective clinicopathological study of the elderly versus the non-elderly. J Egypt Natl Canc Inst. 2014;26:127–37.
The National Cancer Registry Program of Egypt (NCRPE). Reports and Statistics: Aswan, Damietta & El-Minia [Internet]. [cited 2014 Feb 22]. Available from: http://www.Cancerregistry.gov.eg/oops.aspx?aspxerrorpath=/publications.aspx.
Zhang X, Zhou C, Gu H, Yan L, Zhang G. Original article correlation of RKIP, STAT3 and cyclin D1 expression in the pathogenesis of gastric cancer. Int J Clin Exp Pathol. 2014;7(9):5902–8.
Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. Proc Am Soc Clin Oncol. 2008;26(17):2795–9.
Nishii T, Yashiro M, Shinto O, Sawada T, Ohira M, Hirakawa K. Cancer stem cell-like SP cells have a high adhesion ability to the peritoneum in gastric carcinoma. Cancer Sci. 2009;100(8):1397–402.
Naor D, Sionov R, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241–319.
Negi LM, Talegaonkar S, Manu Jaggi FJA, Iqbal Z, Khar RK. Role of CD44 in tumor progression and strategies for targeting. J Drug Target. 2012;20(7):561–73.
Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27(5):1006–20.
Liu D, Sun J, Zhu J, Zhou H, Zhang X, Zhang Y. Expression and clinical significance of colorectal cancer stem cell marker EpCAMhigh/CD44+ in colorectal cancer. Oncol Lett. 2014;7:1544–8.
Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12(22):3499–511.
Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67–108.
Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. https://doi.org/10.1245/s10434-010-0985-4.
Khoo ML, Beasley NJ, Ezzat SJ, Freeman LASL. Overexpression of cyclin D1 and underexpression of p27 predicts lymph node metastases in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2002;87:1814–8.
Ghaffarzadehgan K, Jaffarzadhen M, Raziel H, Sima H, Esmaili E, Hosseinnezhad H, et al. Expression of cell adhesion molecule Cd44 in gastric adenocarcinoma and its prognostic importance. World J Gastroenterol. 2008;14(41):6376–81.
Wang Y, Liu Y, Xiao B. Rapid and reliable detection of CD44 variants in gastric carcinoma using a nested reverse transcription-polymerase chain reaction. Oncol Lett. 2015;10(5):2962–6.
Minarikova P, Benesova L, Halkova T, Belsanova B, Tuckova I, Belina F, et al. Prognostic importance of cell cycle regulators cyclin D1 (CCND1) and cyclin-dependent kinase inhibitor 1B (CDKN1B/p27) in sporadic gastric cancers. Gastroenterol Res Pract. 2016;2016:9408190.
Yan Y, Zuo OX, We D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med. 2015;4(9):1033–43.
Nosrati A, Naghshvar F, Khanari S. Cancer stem cell markers CD44, CD133 in primary gastric adenocarcinoma. Int J Mol Cell Med. 2014;3(4):279–86.
Feakins RM, Nickols CD, Bidd H, Walton SJ. Abnormal expression of pRb, p16, and cyclin D1 in gastric adenocarcinoma and its lymph node metastases: relationship with pathological features and survival. Hum Pathol. 2003;34(12):1276–82.
Begnami MD, Fregnani JTG, Nonogaki S, Soares F. Evaluation of cell cycle protein expression in gastric cancer: cyclin B1 expression and its prognostic implication. Hum Pathol. 2010;41:1120–7.
Casasola SV, Menéndez MJ, Martínez OA, Rodríguez JM. Prognostic value of clinicopathologic factors Ki67, cyclin D1, cyclin D3 and CDK4 in gastric carcinoma. Oncologia. 2004;27(9):537–43.
Arici DS, Tuncer E, Ozer H, Sime KG, Koyuncu A. Expression of retinoblastoma and cyclin D1 in gastric carcinoma. Neoplasma. 2009;56:1.
Takano Y, Kato Y, Masuda M, Ohshima Y, Okayasu I. Cyclin D2, but not cyclin D1, overexpression closely correlates with gastric cancer progression and prognosis. J Pathol. 1999;189:194–200.
Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7(10):750–62.
Ahn MJ, Kim B, Jang SJ, Ki LM. Expression of cyclin D1 and cyclin E in human gastric carcinoma and its clinicopathologic significance. J Korean Med Sci. 1998;13(5):513–8.
Harlozinska A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res. 2005;25(5):3327–33.
Yasui M, Yamamoto H, Ngan C, Damdinsuren B, Sugita Y, Fukunaga H. Antisense to cyclin D1 inhibits vascular endothelial growth factor-stimulated growth of vascular endothelial cells: implication of tumor vascularization. Clin Cancer Res. 2006;12(15):4720–9.
Shintani M, Okazaki A, Masuda T, Kawada M, Ishizuka M, Doki Y, et al. Overexpression of cyclin DI contributes to malignant properties of esophageal tumor cells by increasing VEGF production and decreasing Fas expression. Anticancer Res. 2002;22(2A):639–47.
Yoon C, Park DJ, Schmidt B, Thomas NJ, Lee HJ, Kim TS, et al. CD44 expression denotes a subpopulation of gastric cancer cells in which hedgehog signaling promotes chemotherapy resistance. Clin Cancer Res. 2014;20(15):3974–88. https://doi.org/10.1158/1078-0432.CCR-14-0011.
Dhingra S, Feng W, Brown RE, Zhou Z, Khoury T, Zhang R, et al. Clinicopathological significance of putative stem cell markers, CD44 and nestin, in gastric adenocarcinoma. Int J Clin Exp Pathol. 2011;4(8):733–41.
Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, et al. Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol. 2002;79(4):230–5.
Kim JY, Bae BN, Kim KS, Shin E, Kl P. Osteopontin, CD44, and NF-kappa B expression in gastric adenocarcinoma. Cancer Res Treat. 2009;41(1):29–35.
Wang T, Ong C, Shi J, Srivastava S, Yan B, Cheng C, et al. Sequential expression of putative stem cell markers in gastric carcinogenesis. Br J Cancer. 2011;105:658–65.
Zavrides HN, Zizi-Sermpetzoglou A, Panousopoulos D, Athanasios G, Elemenoglou I, Peros G. Prognostic evaluation of the CD44 expression in correlation with BCL-2 and p53 in colorectal cancer. Folia Histochem Cytobiol. 2005;43(1):31–6.
Chen Y, Fu Z, Xu S, Xu Y, Xu P. The prognostic value of the CD44 expression in gastric cancer: a meta-analysis. Biomed Pharmacother. 2014;68:693–7.
Cao X, Cao D, Jin M, Jia Z, Kong F, Ma H, et al. CD44 but not CD24 expression is related to poor prognosis in non-cardia adenocarcinoma of the stomach. BMC Gastroenterol. 2014;14:157.
Yong C, Yang C, Chou Y, Liao C, Lee CW, Lee CC. Cd44\Cd24 expression in recurrent gastric cancer: a retrospective analysis. BMC Gastroenterol. 2012;12:95.
Chen W, Zhang X, Chu C, Cheung W, Ng L, Lam S, et al. Identification of Cd44 positive cancer stem cell in human gastric cancer. Hepato-Gastroenterology. 2013;60(124):949–54.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Hanaa M. Ibrahim and Amr Ibrahim. (II) Administrative support: Abeer M. Abdelbary and Salem Y Mohamed. (III). Provision of study materials or patients: Hanaa M. Ibrahim, Abeer M Abdelbary, and Mohamed I Abdelhamid (IV). Collection and assembly of data: Hanaa M. Ibrahim and Salem Y Mohamed. (V) Data analysis and interpretation: All authors. (VI) Manuscript writing: All authors. (VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ibrahim, H.M., AbdElbary, A.M., Mohamed, S.Y. et al. Prognostic Value of Cyclin D1 and CD44 Expression in Gastric Adenocarcinoma. J Gastrointest Canc 50, 370–379 (2019). https://doi.org/10.1007/s12029-018-0079-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-018-0079-2