Abstract
Aim
The intensity and duration of surveillance for rectal cancer after surgical resection remain contentious. We evaluated the pattern of recurrences in a rectal cancer cohort followed up beyond 10 years.
Methods
An analysis was performed on a retrospective database of 326 patients with rectal cancer who underwent curative surgical resection from 1999 to 2007. The above study duration was chosen to ensure at least 10 years of follow-up. Data on patient demographics, peri-operative details, and follow-up outcomes were extracted from the database. The pattern of recurrences and investigative modality that detected recurrences was identified. Patients were followed up until either year 2016 or the day of their demise.
Results
Two hundred seventeen patients (66.6%) were male and 109 patients (33.3%) female. Median age was 64 years old. Close to a third of the patients received adjuvant therapy (34%). Among the 326 patients studied, 29.8% of (97/326) patients developed recurrence. 7.7% (25/326) had loco-regional recurrence while 22.1% (72/326) had distant metastasis. Median time to recurrence was 16 months (4–83) and 18 months (3–81), respectively. Computed tomography scan was the best modality to detect both loco-regional and distant recurrences (48% in loco-regional and 41.7% in distant metastasis). The most common site of distant metastasis is the lung (34.7%). The salvage rate for loco-regional and distant recurrences was 52 and 12.5%, respectively.
Conclusion
The predominant pattern of recurrence in rectal cancer is distant disease. Surveillance regimes may need to be altered to increase early detection of distant metastases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, with a lifetime risk of developing the disease within the range of 4–5% [1]. It is the second leading cause of cancer death and in the USA and is expected to account for about 49,190 deaths in 2016 [1]. There has been a sharp increase in age-incidence standardized incidence rate of newly diagnosed CRC between 1975 and 1994, but this rate has been plateauing and decreasing gradually recently in years. Survival has also seen an overall increase over the years [2]. It remains however, a substantial clinical problem in the country. Among all cases of new diagnosed colorectal cancers, rectal cancers constitute approximately 30% [3]. Rectal cancer is often regarded as a distinct disease as factors such as sphincter preservation and sexual dysfunction secondary to autonomic neuropathy are issues unique to rectal cancer. In addition, the anatomical confines of the pelvis further contribute to the technical challenge of rectal resection and hence local recurrence rates are significantly higher compared to colon cancer.
The evolution of rectal cancer treatment has expanded rapidly over the years and new surgical options range from minimally invasive options to local excision surgery. Furthermore, the appreciation of new histopathological parameters such as circumferential radial margins has led to better understanding of recurrence patterns and survival outcomes. In general, two thirds of patients newly diagnosed with rectal cancer are treated by surgery with curative intent [4]. The gold standard for curative rectal surgery remains a good total mesorectal excision (TME). The addition of neoadjuvant chemoradiotherapy in the last decade for locally advanced rectal cancers together with TME has also led to a major reduction of local recurrence rates [5,6,7,8,9]. However, despite these advances, overall survival has not been shown to improve with neoadjuvant chemoradiotherapy and both local and systemic recurrences after rectal cancer resection remain a major concern.
About 25 to 40% of patients with resected rectal cancer will develop recurrence. Common recurrence sites include the lung, liver, and loco-regional lymph nodes in the peri-tumoral drainage basin [10]. Current recommendations for surveillance include a combination of history, physical examination, tumor marker evaluation, imaging, and endoscopy with variable intensity depending on guidelines of various cancer organizations and countries, as well as stage of disease. Guidelines are created largely for the initial 5 years post curative resection and the intent largely is to detect curable recurrences amenable to salvage surgery. While it is well reported that most recurrences occur within 5 years, recurrences have been seen to develop after [5, 11]. Hence, the optimal follow-up duration for rectal cancer after resection remains unknown as it has been shown that recurrences can occur even as late as 7–8 years after curative surgery [12]. The optimal duration, frequency and modality of how the follow-up should be conducted remains unknown. In addition, it remains contentious whether long-term or intensive follow-up results in improved disease specific survival [13].
Thus, we aim to appraise the rectal cancer long-term follow-up outcomes of our institution and evaluate the patterns of recurrence in a bid to improve our surveillance protocols.
Methods
An analysis of a prospectively maintained database of all patients who underwent curative resection for rectal cancers from 1999 to 2007 in the Department of Colorectal Surgery at Singapore General Hospital was performed. Patients with clear resection margins (R0 resection) and microscopic residual disease (R1 resection) were included in the study. The above study duration was chosen as it ensured that all patients included in the study had at least 10 years’ duration of follow-up. While there were no standardizations of duration of follow-up, patients were routinely followed up for at least 5 years, and were followed up beyond as long as they were keen to remain in the institution. Patients were allowed to transfer care to another institution or discharged to primary health physicians if they requested, usually after 5 years of initial surveillance. Exclusion criteria of this study included patients with hereditary cancers or polyposis, stage 4 tumors, or patients who declined curative surgery on diagnosis even in stage I–III disease. We also excluded cases which had endoscopic removal of malignant rectal polyps or local/transanal excision of low rectal cancers to remove the uncertainty of under-staged tumors in our analysis. Furthermore, patients were considered to have non-curative resections if they had persistent gross local disease (R2) after initial surgical management and were also not included in analysis. The study protocol was approved by the Institutional Review Board of Singapore General Hospital.
Data on patient demographics, peri-operative details and follow-up outcomes were extracted from the database. The pattern of recurrences and investigative modality that detected recurrences was identified. Patients were followed up till end of 2016 or to the day of their demise. All the cases were performed by consultant surgeons in the department.
Staging and Pathologic Analysis
Stage of distant disease was evaluated by plain chest radiographs, ultrasound, and/or computed tomography (CT) of the chest, abdomen, and pelvis. Magnetic resonance imaging (MRI) of the pelvis or endo-rectal ultrasound was performed for all lower rectum tumors if possible. Upper rectum is defined as 11–15 cm, middle rectum as 6–10 cm, and lower rectum as 0–5 cm from the anal verge. These measurements were recorded by the consultant surgeon after digital rectal examination and endoscopy. In our institution, a selective neoadjuvant chemoradiation policy is adopted and this may be offered for locally advanced cancers such as ultrasound or MRI T3 or T4 staged tumors; the presence of positive nodes (N1/N2) on imaging or if circumferential radial margin (CRM) is positive (<2 mm). The majority of the cases in the study era did not undergo neoadjuvant treatment. Adjuvant therapy is offered to all medically fit patients with disease staged stage III and above. Pathologic staging of disease was performed according to American Joint Committee on Cancer (AJCC) Staging Manual, 7th edition after surgical resection with review of the resected specimen and investigations of distant metastases [14]. Distal margin (DM) was defined as the gross distance between the distal edge of the tumor to the distal mucosal resection margin. This is performed usually by pinning the surgical specimen and measuring the gross margins after formalin fixation.
Follow-up Details
The follow-up regime in our department is three monthly intervals for the first 2 years, six monthly for the next 3 years, and then yearly thereafter. At each consultation, CEA levels were measured and full history and physical examination (including digital rectal examination) were performed. Colonoscopy was performed within 6 months of surgery if initial complete evaluation was not possible pre-operatively due to tumor obstruction or stenosis. Those who had an initial complete colonic evaluation would undergo colonoscopy at the first year follow-up, and again at 3-yearly intervals post-operatively. Patients with suspicious symptoms and signs of rising CEA trend on follow-up will be evaluated earlier with colonoscopy and/or radiological imaging (including computerized tomography of the chest, abdomen and pelvis, bone scan and positron emission tomography scans if applicable).
Recurrence Pattern
Loco-regional recurrence was defined as the first clinical, radiologic, and/or pathologic evident tumor of the same histological type, within the true pelvis, at the anastomosis or the region of the anastomosis, or in the mesentery up to the root of the inferior mesenteric artery stump. Distant recurrence was defined as similar evidence of tumor detected outside the primary sites, such as the liver, lungs, bone, brain, and para-aortic region. Patients who were first diagnosed with loco-regional recurrence but later found to have more advanced disease upon completion of staging (i.e., distant recurrences) were classified under the more advanced metastatic category. Each recurrence subgroup was further analyzed based on the modality that first detected recurrence, median time to recurrence, and whether the recurrence eventually underwent salvage surgery.
Data Processing
Recurrence-free survival was calculated by subtracting the date of recurrence detection from the date of operation. For patients who had died due to disease progression, overall survival was computed by subtracting the date of death from the date of operation, while those who had died of other causes were censored at the date of death. For patients who were still alive, overall survival was calculated by subtracting the date of last review from the date of operation and censored at the date of last review.
Statistical Analysis
The survival functions for overall and recurrence-free survival were estimated using the Kaplan-Meier estimator. All statistical analyses were performed using Stata/MP Version 13.1 (College Station, TX, USA).
Results
A total of 326 patients were included in this study and all underwent curative resection for rectal cancer during the study duration. Demographic characteristics of the study cohort are illustrated in Table 1. Two hundred seventeen patients (66.6%) were male and median age of the patients was 64 years old. Two hundred ninety-six patients (90.8%) underwent open surgery while 30 patients (9.2%) had laparoscopic surgery. There were few neoadjuvant chemoradiotherapy cases in this study period as this was just being introduced in the institution. However, approximately 34% of patients received adjuvant chemotherapy or radiotherapy. The oncologic and peri-operative outcomes of the entire study cohort are presented in Table 2. Seventy-one patients (21.8%) were stage I, 106 patients (32.5%) were stage II, and 149 patients (45.7%) were stage III. Median length of hospitalization was 7 days. Median duration of follow-up was 45 months (0–134 months) and 97 patients (29.8%) of the study cohort had disease recurrence.
The Kaplan-Meier curve for overall survival disease-free survival of the study cohort is illustrated in Fig. 1.
Pattern of Recurrences
Loco-regional recurrence rate was 7.7% (25 out of 326) while distant recurrence rate was 22.1% (72 out of 326). Among the 97 patients who had recurrence, the majority were distant in nature (72 out of 97, 74.3%) while 25.8% (25 out of 97) were loco-regional recurrences. Close to 80% of all recurrences occurred within the first 3 years. Recurrences beyond the fifth year occurred in 1.2% (4 out of 326) of the entire population. The distribution of recurrence patterns is illustrated in Table 3.
Recurrence Outcomes With and Without Adjuvant Therapy
A total of 138 (42.3%) patients received adjuvant therapy. Recurrence rates among those who did and did not receive adjuvant therapy were 50.7 and 14.4%, respectively (p < 0.001). Among patients who received adjuvant therapy, loco-regional recurrence rates and distant recurrence rates were 8.7 and 42.1%, respectively. Among the patients who did not receive adjuvant therapy the corresponding figures were 2.7 and 11.7%.
Investigation Modality Which First Detected Recurrence
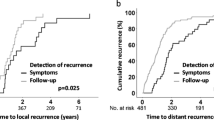
The investigative modality which first detected recurrence is illustrated in Table 4. Loco-regional recurrences were predominantly detected by computed tomography scans (12 out of 25, 48.0%) and colonoscopy (8 out of 25, 32.0%). In contrast, distant recurrences were predominantly detected via computed tomography scans (30 out of 72, 41.7%) and elevated carcinoembryonic antigen levels (26 out of 72, 36.1%).
The most common site of distant recurrence was the lung (25 out of 72, 34.7%) followed by the liver (17 out of 72, 23.6%). Majority of those with distant recurrences had disease confined to a single site (48 out of 72, 68.1%) while dual site metastases constituted 27.8% (20 out of 72).
Among those with loco-regional recurrence, 13 (52%) underwent subsequent curative surgery. Surgical procedures performed for these patients include local excision (2 patients), abdominal perineal resection (7 patients) and pelvic exenteration (4 patients).
The salvage metastectomy rate among patients with distal recurrence was 12.5% (9 out of 72). Four patients had hepatectomy for liver metastasis while 5 patients had lung metastectomy.
Overall and Recurrence-Free Survival
The 5-year overall and recurrence-free survival rates for the cohort were 70.8% (95% CI 64.4 to 76.2%) and 63.5% (95% CI 57.2 to 69.1%), respectively.
The 25th percentile duration for overall and recurrence-free survival were 49.9 and 26.5 months, respectively (median survival not reached).
Discussion
The management of rectal cancer remains challenging despite advances in surgical technique and adjuvant and neoadjuvant therapy [5,6,7]. Tumor recurrence often poses a technical challenge due to the anatomical confines of the pelvis. In addition, the presence of key structures in close proximity often renders recurrences either unresectable or resectable only at the expense of procedures which confer significant surgical morbidity.
Till date, the ideal duration of follow-up post curative rectal cancer resection remains unknown. Most published clinical practice guidelines recommend follow-up till 5 years post curative resection [15,16,17,18]. This was based on the rationale that 90% of recurrences occurred within the first 5 years following surgery [19]. However, these studies comprised predominantly of colon cancer patients and thus the findings may not necessarily be applicable to rectal cancer patients which may have different recurrence patterns as compared to colon cancer. Few studies have been done specifically looking at late recurrences beyond 5 years from index surgery. Data from a French and Australian population study have concluded that 5 to 12% of patients can relapse beyond 5 years of curative surgery [20, 21]. The study by Bouvier et al. was, however, done exclusively on a cohort of colon cancer patients while Broadbridge et al. included both colon and rectal cancer patients [20, 21]. A recent study by Cottet et al. was based on a French population registry and focused primarily on rectal cancer patients. It concluded that one out of 13 patients may develop recurrence between 5 to 10 years after initial curative surgery [22].
In our study, 95.8% (93 out of 97) of all recurrences occurred within the first 5 years after curative resection. Recurrences beyond the 5-year mark occurred among 4% of the recurrent population, which constituted 1.2% of the entire study cohort. These findings appear to be significantly different from that reported by Cottet et al. whereby 1 in 13 patients who were disease free at 5 years post-surgery developed a recurrence thereafter [22]. A potential explanation could be the study period whereby patients were recruited (1985–2000) in the study by Cottet et al. This was close to 15 years prior to the year where patients were first included in our study (1999). Thus, the disparity in findings may have been influenced by the inferior efficacy and inaccessibility of systemic therapy during the era of the 1980s and 1990s. This hypothesis is supported by the fact that the authors also found that late recurrences (beyond the 5-year mark) occurred more frequently in those diagnosed between 1985 and 1989 when compared to those diagnosed between 1995 and 2000 [22]. Nonetheless, these findings indicate that recurrences do occur beyond the 5-year mark.
The method of surveillance protocols within and beyond the traditional 5 years also remains undefined. In our study, the recurrence pattern of rectal cancer appears to be predominantly distant in nature, with distant recurrences accounting for 74.2% of all recurrences. Patients who received adjuvant therapy showed higher rates of recurrence which can be attributed to selection bias since more advanced and higher risks tumors were more likely to be offered adjuvant therapy. Nonetheless, the predilection of rectal cancer for distant recurrences persisted even on subset analysis of those who received adjuvant therapy. This is consistent with findings of a recent publication which similarly concluded that close to 80% of recurrences in rectal cancer were distant metastases [23]. This contrasted with publications in the pre-TME era whereby recurrence burden seemed to be predominantly loco-regional in nature [24, 25]. The widespread adoption of TME may have altered recurrence patterns in rectal cancer such that distant disease constitutes the predominant recurrence burden. Thus, our surveillance protocols may need to be altered to detect distant metastases at an early stage such that patients are amenable for curative metastectomy. This is particularly pertinent as only 12.5% of patients with distant metastases in our study cohort eventually underwent surgical resection. While this low rate of metastectomy may be partially explained by the lack of surgical expertise during that era, one can argue that the advancement in surgical metastectomy expertise over the years further reinforces the need for more intensive surveillance regimes to detect distant recurrences since that is no longer a limitation in the current era. This is consistent with the latest practice guidelines by the American Society of Colon and Rectal Surgeons on the surveillance of rectal cancer after curative resection [26]. Annual computed tomography imaging is now recommended for all rectal cancers which are stage two and above, with more frequent imaging intervals recommended if there are risk factors for recurrence such as N2 disease. However, it remains unknown if intensive surveillance truly translates to clinical benefits. While they were initially believed to improve curative resection rates and survival, this has been questioned in a recent meta-analysis [27, 28]. Two recent randomized controlled trials have also concluded that there is no overall survival benefit with intensive surveillance even if it achieved a greater proportion of patients operated with curative intent [29, 30].
There are several limitations to our study. Firstly, the study was conducted on patients treated from the period of 1999–2007 and thus advancements in surgical technique and adjuvant treatment over the past 10 years may have influenced the applicability of these findings in the current era. We were limited by the need to include patients with at least 10 years of follow-up in our study and thus could not include patients who underwent surgery beyond 2007. In mitigation, TME was already the standard of care during the study period. While the neoadjuvant population among our study cohort was less than 5%, our loco-regional recurrence rate of 7.7% was comparable to that of the 10-year local relapse rates in the German Rectal Cancer Study [31]. The study also lacked details on the chemotherapy regimens among patients who received adjuvant therapy, in particular whether fluoropyrmidine-based therapy was used in isolation or in combination with oxaliplation as a doublet regimen. This is a significant detail as oxaliplatin in addition to fluoropyrimidine-based adjuvant chemotherapy has been demonstrated to significantly reduce recurrence risk [32]. Another limitation of our study lies in our failure to include elements of economic analysis due to the retrospective nature of our study. The implications and economic burden to the country of any surveillance recommendation are beyond the scope of this study. This is an important factor to consider during the formulation of surveillance protocols. Two studies focusing on economic analysis have found value in more intensive surveillance protocols with an estimated cost of 5000USD for every life year gained [33, 34]. However, health care economics vary between countries and it remains unknown if these findings are applicable to our population. A detailed economic analysis to determine if an intensive surveillance protocol is cost-effective and clinically viable should be considered.
Conclusion
The risk of rectal cancer recurrence is highest within the first 3 years but can occur up to 10 years after curative resection. Metastatic burden appears to be predominantly distant in nature and surveillance regimes may need to be altered to increase early detection of distant metastases.
References
(2015) American Cancer Society Cancer Statistics.
Trends in Cancer Incidence in Singapore 2010–2014. http://www.nrdo.gov.sg/.
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26.
Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829–36. discussion 36-8
Hansen MH, Balteskard L, Dorum LM, Eriksen MT, Vonen B, Norwegian Colorectal Cancer G. Locally recurrent rectal cancer in Norway. Br J Surg. 2009;96:1176–82.
MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–60.
Ortholan C, Francois E, Thomas O, Benchimol D, Baulieux J, Bosset JF, et al. Role of radiotherapy with surgery for T3 and resectable T4 rectal cancer: evidence from randomized trials. Dis Colon Rectum. 2006;49:302–10.
Pahlman L, Bohe M, Cedermark B, Dahlberg M, Lindmark G, Sjodahl R, et al. The Swedish rectal cancer registry. Br J Surg. 2007;94:1285–92.
Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783–99.
Kobayashi H, Mochizuki H, Morita T, Kotake K, Teramoto T, Kameoka S, et al. Timing of relapse and outcome after curative resection for colorectal cancer: a Japanese multicenter study. Dig Surg. 2009;26:249–55.
Chauvenet M, Lepage C, Jooste V, Cottet V, Faivre J, Bouvier AM. Prevalence of patients with colorectal cancer requiring follow-up or active treatment. Eur J Cancer. 2009;45:1460–5.
Scheer A, Auer RA. Surveillance after curative resection of colorectal cancer. Clin Colon Rectal Surg. 2009;22:242–50.
Edge SBBD, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed; 2010.
Desch CE, Benson AB 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–9.
Scholefield JH, Steele RJ, British Society For G, Association of Coloproctology for Great B, Ireland. Guidelines for follow up after resection of colorectal cancer. Gut 2002; 51 Suppl 5: V3–5.
Van Cutsem EJ, Oliveira J, Group EGW. Colon cancer: ESMO clinical recommendations for diagnosis, adjuvant treatment and follow-up. Ann Oncol 2008; 19 Suppl 2: ii29–30.
National Comprehensive Cancer Network (NCCN) Guidelines in Clinical Oncology. 2016.
Sargent DJ, Patiyil S, Yothers G, Haller DG, Gray R, Benedetti J, et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25:4569–74.
Bouvier AM, Launoy G, Bouvier V, Rollot F, Manfredi S, Faivre J, et al. Incidence and patterns of late recurrences in colon cancer patients. Int J Cancer. 2015;137:2133–8.
Broadbridge VT, Karapetis CS, Beeke C, Woodman RJ, Padbury R, Maddern G, et al. Do metastatic colorectal cancer patients who present with late relapse after curative surgery have a better survival? Br J Cancer. 2013;109:1338–43.
Cottet V, Bouvier V, Rollot F, Jooste V, Bedenne L, Faivre J, et al. Incidence and patterns of late recurrences in rectal cancer patients. Ann Surg Oncol. 2015;22:520–7.
Chiang JM, Hsieh PS, Chen JS, Tang R, You JF, Yeh CY. Rectal cancer level significantly affects rates and patterns of distant metastases among rectal cancer patients post curative-intent surgery without neoadjuvant therapy. World J Surg Oncol. 2014;12:197.
Pilipshen SJ, Heilweil M, Quan SH, Sternberg SS, Enker WE. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer. 1984;53:1354–62.
Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317–29.
Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon rectum. 2015;58:713–25.
Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2007: CD002200.
Jeffery M, Hickey BE, Hider PN, See AM. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:CD002200.
Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263–70.
Rosati G, Ambrosini G, Barni S, Andreoni B, Corradini G, Luchena G, et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol. 2016;27:274–80.
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33.
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ, Park YS, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15:1245–53.
Borie F, Combescure C, Daures JP, Tretarre B, Millat B. Cost-effectiveness of two follow-up strategies for curative resection of colorectal cancer: comparative study using a Markov model. World J Surg. 2004;28:563–9.
Renehan AG, O'Dwyer ST, Whynes DK. Cost effectiveness analysis of intensive versus conventional follow up after curative resection for colorectal cancer. BMJ. 2004;328:81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
What does this paper add to the literature:
This paper studies a cohort of rectal cancer patients who have been followed up beyond 10 years after curative surgery. It provides insight on the incidence of recurrences beyond the usual 5-year follow-up duration and the recurrence patterns of rectal cancer to allow modifications of current surveillance regimes.
Rights and permissions
About this article
Cite this article
Tan, W.J., Tan, H.J., Dorajoo, S.R. et al. Rectal Cancer Surveillance—Recurrence Patterns and Survival Outcomes from a Cohort Followed up Beyond 10 Years. J Gastrointest Canc 49, 422–428 (2018). https://doi.org/10.1007/s12029-017-9984-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-017-9984-z