Abstract
Purpose
Gallbladder and pancreas share common embryological origin, and malignancies of these organs may share common tumor antigens. CA 242 is a tumor marker for pancreatic cancer, but has not been studied in gallbladder cancer (GBC). We measured serum CA 242 levels in patients with GBC and compared it with those in patients with gallstones (GS) and healthy volunteers.
Methods
We enrolled consecutive patients with GBC (cases), GS (disease controls), and healthy volunteers (healthy controls). Serum CA 242, CEA, and CA 19–9 levels were measured using ELISA. Receiver operator curve was plotted for all the three markers.
Results
We studied 117 patients with GBC, 58 with GS, and 10 healthy volunteers. Among patients with GBC, 81 (69%) also had GB calculi. Patients with GBC more often had elevated CA 242 levels (64%) compared to those with GS (17%; p < 0.001) and healthy controls (0%; p < 0.001). The median levels of CA 242 was higher in the GBC group (59 [199] U/ml) compared to the GS group (10 [13] U/ml; p < 0.001) and the control group (3 [14.5] U/ml; p < 0.001). The sensitivity, specificity, positive predictive value (PPV), and negative predictive values of CA 242 for diagnosis of GBC were 64%, 83%, 88%, and 53%, respectively. At a cutoff of 45 U/ml, the specificity and PPV increased to 100%. CA 242 had higher AOC (0.759) compared to CEA (0.528) and CA 19–9 (0.430).
Conclusions
CA 242 is a promising tumor marker for GBC and performs better than CEA and CA 19-9.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gallbladder cancer (GBC) occurs with varying frequency in different parts of the world [1, 2]. In Northern India, it has an incidence as high as 13.5 per 100,000 population, and it is the third common cause of cancer deaths in women [3, 4]. Unlike other malignancies, there is no tumor marker available for the detection and management of patients with GBC. A tumor marker could aid in diagnosing GBC when cytological confirmation is not possible, in assessing tumor burden, and in assessing response to therapy.
CA 242 is a sialiated carbohydrate antigen that has been used as a tumor marker for pancreatic cancer with promising results [5–8]. GB and pancreas share a common embryological origin, and thus, tumors arising from them may share some common antigens. For instance, CA 19–9 was first used in pancreatic cancer and was then evaluated and found useful in the diagnosis of GBC [9]. However, CA 19–9 is elevated even in patients with gallstones, especially if there is associated cholestasis [10]. In contrast, serum levels of CA 242 have been found to be usually normal in those with benign conditions [5, 11]. The role of CA 242 as a tumor marker in differentiating benign and malignant biliary disease needs to be elucidated.
The aim of the present study was to determine the serum levels of CA 242 in patients with GBC and to compare those levels with those in patients with gallstones (GS), and with those in healthy volunteers.
Patients and Methods
Patient Selection
All consecutive patients with suspected GBC or GS who attended the gastroenterology or surgical services of our tertiary care center from June 2002 to May 2004 were invited to participate in the study. All patients enrolled in the study provided written informed consent. The study was approved by the ethics committee of our hospital and was conducted in accordance with the Helsinki guidelines.
The diagnosis of GBC was based on clinical, radiological, and cytological evidence of gallbladder malignancy. An ultrasound-guided aspiration cytology was obtained from the gallbladder mass using standard precautions and examined by an experienced cytologist (RS) for evidence of malignant cells. An ultrasound and contrast-enhanced computerized tomography were used to stage each case of GBC, according to Henson's staging criteria [12]. All patients with GBC were also assessed for ultrasound evidence of GS and categorized as having no GB calculi, a solitary GB calculus, or multiple GB calculi.
All patients with symptomatic GS disease underwent an ultrasound examination to rule out incident GBC. They then underwent cholecystectomy, and resected GB specimens were examined for the presence of dysplasia or carcinoma in situ. Those with no histological evidence of GBC were classified as disease controls. Healthy controls were volunteers who underwent an ultrasound examination and had no evidence of GB pathology. All the participants underwent a detailed clinical evaluation to assess disease symptomatology and characteristics.
Assessment of CA 242 Levels
A 5-ml blood sample was obtained from each participant, from which sera was extracted after centrifugation. The sera samples were stored at −20°C. We used an enzyme-linked immunosorbent assay (CAN Ag Diagnostics, Sweden), as per manufacturer's instructions, to determine the serum levels of CA 242 in all the samples. We considered CA 242 to be elevated if the value was >20 U/ml, as recommended by the manufacturers. The inter-assay and intra-assay variations were 4.1% and 4%, respectively.
Assessment of CEA and CA 19–9
Serum CEA and serum CA 19–9 levels were also studied in 42 patients with GBC and 9 patients with GS. CEA levels were assessed using ELISA (United Biotech Inc, CA) and cutoff of 5 U/ml as considered to be elevated as per manufacturer's recommendations. CA 19–9 levels were assessed using ELISA (BioCheck Inc, CA) and cutoff of 35 U/ml levels as considered to be elevated as per manufacturer's recommendations. The inter-assay and intra-assay variations were <5%.
Statistical Analysis
Statistical analysis was performed using SPSS Inc., Chicago, IL (version 13). Student's t test was used to compare the age between quantitative variables across the three groups. Chi-square tests were used to compare the gender distribution as well as levels of tumor markers positivity between the three groups. Mann–Whitney U test was used to compare the CA 242 levels as CA 242 had a nonparametric distribution. p value of <0.05 was considered to be significant. Sensitivity, specificity, positive predictive value (PPV), and negative predictive values (NPV) with their 95% CI were also determined. Receiver operator curve was plotted for CA 242, CA 19–9, and CEA levels as a diagnostic test for GBC group in comparison to GS group.
Results
We studied 117 patients with GBC, 58 patients with GS, and 10 healthy controls (Table 1). The mean ages of patients with GBC was higher (54 [11] years) than that of patients with GS (46 [14] years; p = 0.001) and that of controls (40 [7.5] years; p = 0.001). In the above three groups, the proportion of women was 64%, 81%, and 60%, respectively, the proportion being higher in the GS group than the others.
The patients with GBC reported having symptoms for a mean duration of 4.5 months at presentation. The disease characteristics at presentation were history of pain in 102 (87%), history of weight loss in 101 (87%), presence of a palpable GB lump in 85 (73%), icterus in 71 (61%), and ascites in 11 (10%) patients. Ultrasound and computerized tomographic examination of the abdomen showed presence of either a GB mass or GB wall thickening. Associated GB calculi were present in 81 cases, 91% of which were multiple and 9% were solitary. There were lymph node metastasis in 67%, hepatic metastasis in 59%, and ascites in 10% of the patients. Most patients (82%; 96/117) had advanced stage of the disease (Henson's stage III or IV) and thus were unresectable. All patients had evidence of adenocarcinoma on aspiration cytology.
All the patients with GS were symptomatic with biliary colic or postprandial dyspepsia. Three patients had evidence of cholestasis due to biliary obstruction secondary to stones. Ultrasound showed presence of multiple calculi in 76% and solitary calculus in 24% of patients. None had any radiological features suggestive of GBC. All underwent cholecystectomy, and none of the resected GB specimens had any foci of cancer, carcinoma in situ, or dysplasia.
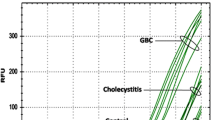
Patients with GBC more often had elevated CA 242 levels (64%) compared to those with GS (17%; p < 0.001) and healthy controls (0%; p < 0.001) (Fig. 1). The median levels of CA 242 were higher in the GBC group (59 [199]) compared to the GS group (10 [13]; p < 0.001) and the control group (3 [14.5]; p < 0.001). All patients in the GS group had CA 242 levels ≤42 U/ml. For diagnosis of GBC among patients with GB disease, the sensitivity, specificity, PPV, and NPV of CA 242 were found to be 64%, 84%, 88%, and 53%, respectively. A ROC curve was plotted for CA 242, and the area under the curve was 0.759 (p < 0.001) (Fig. 2). At a cutoff of 45 U/ml, the specificity and positive predictive value increased to 100% each, but the sensitivity and NPV were 53% and 51%, respectively.
A scatter plot showing the values of CA 242 on the y-axis in the three different groups: patients with gallbladder cancer (group 1), patients with gallstones (group 2), and normal controls (group 3). Each dot represents a patient, and y-axis is on a logarithmic scale. The continuous horizontal line from the y-axis is at 20 U/ml (the manufacturer's cutoff), and the broken line is at 45 U/ml (our proposed cutoff)
There was no relationship of CA 242 levels with age, gender, stage of the disease, bilirubin level, or presence of ascites. However, patients with GBC who did not have GS (n = 36) more often had an elevated CA 242 levels than those with GS (n = 81) (72% vs. 44%; p = 0.005). Among patients with GBC, those without gallstones had the higher median levels of CA 242 (195 [IQR 1,766]) (Table 2) compared to those with gallstones (31 [IQR 164]; p = 0.004).
The serum CEA and CA 19–9 levels were also studied in 41 of the patients with GBC and 9 of the patients with GS. CEA levels were elevated in 61% (25/41) in patients with GBC in contrast to 55.6% (5/9) patients with GS. The median CEA level was 9.5 (IQR 28) U/ml in patients with GBC in contrast to 6 (IQR 11.5) U/ml in patient with GS (p = 0.791). The sensitivity, specificity, PPV, and NPV of CEA were found to be 61%, 44%, 83%, and 25%, respectively. A ROC curve was plotted for CEA, and the area under the curve was 0.528 (p = NS). CA 19–9 levels were elevated in 17% (7/41) in patients with GBC in contrast to 33% (3/9) patients with GS. The mean CA 19–9 level was 5 (IQR 19) U/ml in patients with GBC in contrast to 4.5 (IQR 11) U/ml in patient with GS (p = 0.486). The sensitivity, specificity, PPV, and NPV of CA 19–9 were found to be 17%, 67%, 70%, and 15%, respectively. A ROC curve was plotted for CA 19–9, and the area under the curve was 0.430 (p = NS).
Discussion
In our study, we have shown that CA 242 has a high specificity and high positive predictive value in the diagnosis of GBC and in differentiating malignant biliary disease from benign biliary disease. Our study, on a fairly large number of patients with GBC, has validated the role of CA-242 in the diagnosis of GBC. We have also compared its role in patients with GBC vis-à-vis gallstones instead of healthy controls alone. This has importance for a clinical standpoint as in clinical situations we have to differentiate malignant from benign biliary diseases. We also found that CA 242 had better characteristics as a diagnostic test compared to CEA and CA 19–9.
There are no good established serum-based tumor markers for GBC. However, there has been a need for a good marker for various reasons. Establishing the diagnosis of GBC has been traditionally by performing fine needle aspiration cytology in patients suspected to have radiological evidence of malignancy. However, this may result in tumor seeding which may preclude curative radical resection, may be contraindicated, or consent may be refused for such a procedure. Also, if a tumor marker establishes the presence of malignancy preoperatively and estimates the tumor burden, it would help the surgeons in planning surgery. Radical surgery for GBC is being increasingly performed in order to give better long-term survival to patients [13]. After radical surgery, tumor marker would have a role in following up patients for presence of residual tumor or tumor recurrence as radical surgeries as radiological evaluation has poor predictability due to postoperative changes. Also, after administering chemotherapy or radiotherapy, tumor markers may have a role in assessing response to therapy. It would be useful in planning of management of patients with suspected malignant biliary obstruction before invasive procedures such as endoscopic cholangiography with brush cytology are attempted to palliate or diagnose their condition.
The scenario for GBC has rapidly changed with improving healthcare in high incidence areas for GBC, increasing number of incidental cancers detected due to wide availability of laparoscopic cholecystectomy, emerging chemotherapeutic options, and increasing interest in radical surgeries [14–16]. Thus, there is now a felt need for a good tumor marker which would aid in better patient management.
None of the patients in the healthy control group had values of CA 242 above the cutoff level (20 U/ml). The highest value in patients with GS disease was <42 U/ml. In a study by Rothlin et al., the mean CA 242 values among 10 patients with GS were 13.1 ± 13.1 U/ml [6]. In that study, the mean values of CA 242 of patients with pancreatic cancer were as high as 4,360 ± 16,300 U/ml. Thus, raising the existing cutoff of 20 to 45 U/ml would increase the specificity further without significantly compromising the sensitivity. This principle of increasing the cutoff to increase the specificity at the cost of some loss of sensitivity has been used for various tumor markers like alpha-fetoprotein, CEA, CA 19–9, etc. [17].
Patients with GBC who had no associated GS were different from the group with GS in terms of higher positivity and higher levels of CA 242. The other parameters, which could affect positivity, like the stage of disease, age, gender, etc., were similar in both the groups. This may possibly indicate that the tumor in the setting of stones may be different from those without GS. In patients with gallstones, recurrent mucosal inflammation, low grade infection, and mucosal injury may play a key role in the pathogenesis of GBC which may alter the mucosal cells in a manner different than the cells in patients without gallstones, possibly resulting in different expression of the tumor antigens [18]. We did not find any correlation between the levels of the tumor marker and the stage of the disease. However, in pancreatic cancer and colorectal cancer, CA 242 levels have been found to correlate with the stage of the disease [5, 19]. This may be due to the fact that we had predominantly patients with advanced disease and thus comparison between early versus advanced disease was not possible. A larger study, which includes more patients with incidentally detected early cancer, would be able to provide the answer.
One of the limitations in our study was that we had limited number of patients with early stage of GBC. This is because patients with GBC in India tend to present late in the course of illness due to lack of awareness, nonspecific symptomatology at onset of disease, difficulty in accessing medical care, and lack of any screening program for this disease. We thus cannot comment on the relationship of tumor marker levels and stage of the disease. Our study was not age- and gender-matched as it was not a part of the design. We did not find any influence of age or gender on the CA 242 levels in all the three groups. Our patients in the GBC group were older than the other two groups, as we had not specifically matched for age the patients as per design. Patients with GBC are usually older than patients with GS at the time of presentation, which explains the difference across the three groups [20]. The number of healthy volunteers was few which is unlikely to affect the overall results. Previous large studies on healthy volunteers have already established that they have low levels of CA 242. The serum CEA and CA 19–9 could be performed only in 42 patients with GBC and 9 patients with GS due to certain technical problems. However, both these markers performed poorly in contrast to CA 242, though the number of patients evaluated was small.
To conclude, CA 242 is a promising tumor marker in diagnosing GBC. We achieved a sensitivity of 64% and specificity of 83% in detecting presence of GB cancer. As there is no well-established tumor marker for this disease, this marker holds promise. Raising the cutoff to 45 U/ml may further increase the specificity of this test to 100%. CA 242 is useful in detecting presence of malignancy in patients presenting with biliary symptoms. Further studies are needed to validate our results. It would also be worthwhile to study the role of CA 242 as a marker to assess response to therapy.
References
Zatonski WA, Lowenfields AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case controlled study of the Search Programme of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132–8.
Parkin DM, Muir CS, Whelan SL, GAo YT, Ferlay J, Powells J (eds). (1992) Cancer incidence in 5 continents. Vol VI. IARC Scientific publications NO 120. Lyon: IARC
ICMR National Cancer Registry Program, Biennial Report 1988–89. An epidemiological study. New Delhi: ICMR; 1992. p. 14–5.
Kapoor VK, McMichael AJ. Gallbladder cancer: an Indian disease. Natl Med J India. 2003;16:209–13.
Rothlin MA, Joller H, Largiader F. CA 242 is a new tumor marker for pancreatic cancer. Cancer. 1993;71:701–7.
Haglund C, Lundin J, Kuusela P, Roberts PJ. CA 242, a new tumour marker for pancreatic cancer: a comparison with CA 19–9, CA 50 and CEA. Br J Cancer. 1994;70:487–92.
Pasanen PA, Eskelinen M, Partanen K, Pikkarainen P, Penttila I, Alhava E. Clinical evaluation of a new serum tumour marker CA 242 in pancreatic carcinoma. Br J Cancer. 1992;65:731–4.
Ozkan H, Kaya M, Cengiz A. Comparison of tumor marker CA 242 with CA 19–9 and carcinoembryonic antigen (CEA) in pancreatic cancer. Hepatogastroenterology. 2003;50:1669–74.
Strom BL, Maislin G, West SL, et al. Serum CEA and CA 19–9: potential future diagnostic or screening tests for gallbladder cancer? Int J Cancer. 1990;45:821–4.
Del Favero G, Fabris C, Panucci A, Basso D, Plebani M, Baccaglini U, et al. Carbohydrate antigen 19–9 (CA 19–9) and carcinoembryonic antigen (CEA) in pancreatic cancer. Role of age and liver dysfunction. Bull Cancer. 1986;73:251–5.
Engaras B, Hafstrom L, Kewenter J, Nilsson O, Wedel H. Standard serum concentrations and normal fluctuations of CEA, CA 50 and CA 242 during twelve months in men and women aged 60–64 years without malignant disease. Eur J Surg. 1999;165:110–6.
Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of the disease, grade and survival rates. Cancer. 1992;70:1493–7.
Dixon E, Vollmer Jr CM, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg. 2005;241:385–94.
Batra Y, Pal S, Dutta U, et al. Gallbladder cancer in India: a dismal picture. J Gastroenterol Hepatol. 2005;20:309–14.
Park JS, Oh SY, Kim SH, Kwon HC, Kim JS, Jin-Kim H, et al. Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a phase II study. Jpn J Clin Oncol. 2005;35:68–73.
Thongprasert S, Napapan S, Charoentum C, Moonprakan S. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol. 2005;16:279–81.
Pasanen PA, Eskelinen M, Partanen K, Pikkarainen P, Penttila I, Alhava E. Receiver operating characteristic (ROC) curve analysis of the tumour markers CEA, CA 50 and CA 242 in pancreatic cancer: results from a prospective study. Br J Cancer. 1993;67:852–5.
Dutta U, Garg PK, Kumar R, Tandon RK. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol. 2000;95:784–7.
Kim SB, Fernandes LC, Saad SS, Matos D. Assessment of the value of preoperative serum levels of CA 242 and CEA in the staging and postoperative survival of colorectal adenocarcinoma patients. Int J Biol Markers. 2003;18:182–7.
Dutta U, Nagi B, Garg PK, Sinha SK, Singh K, Tandon RK. Patients with gallstones develop gallbladder cancer at an earlier age. Eur J Cancer Prev. 2005;14:381–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rana, S., Dutta, U., Kochhar, R. et al. Evaluation of CA 242 as a Tumor Marker in Gallbladder Cancer. J Gastrointest Canc 43, 267–271 (2012). https://doi.org/10.1007/s12029-011-9288-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-011-9288-7