Abstract
Purpose
The study was designed to investigate the clinicopathological correlations, relationship to apoptotic index, and prognostic significance of estrogen receptor beta expression in colorectal carcinoma.
Methods
The study was carried out on 40 patients with newly diagnosed colorectal cancer. The patients’ data were collected prospectively and the 2 years overall survival was the endpoint. Estrogen receptor beta expression was assessed by immunohistochemistry. Apoptotic body index was calculated by counting apoptotic cells using the modified TUNEL assay.
Results
Estrogen receptor beta positivity was detected in 65% of colorectal cancer cases, while estrogen receptor alpha positivity was found in only 7% of cases. The rate of estrogen receptor beta immunoreactivity was significantly higher in low-grade colorectal tumors. The median apoptotic index in estrogen receptor beta positive cases was significantly higher than in estrogen receptor beta negative cases (6% versus 3%; p = 0.01). The median overall survival was higher in estrogen receptor beta positive cases (22 versus 18 months); however, the difference was not statistically significant.
Conclusions
The study results reinforce the importance of the estrogen receptor beta rather than the estrogen receptor alpha in colorectal cancer. Lack of estrogen receptor beta expression is associated with loss of differentiation and decreased apoptosis. Future studies should include validation of estrogen receptor beta as a prognostic marker and exploration of its role as a target in the management of colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oncogenic effects of estrogens have been investigated extensively in breast cancer where hormone receptor modulators are now an integral part of targeted treatment. Little is known about estrogen signaling in colorectal cancer although women are less susceptible to this cancer than men [1, 2]. A large-scale population analysis found that hormone replacement therapy has an additive protective effect for post-menopausal women at all concentrations and durations of exposure [3].
It is not surprising that estrogens are implicated in the development or progression of diseases, which include various types of cancer, osteoporosis, cardiovascular diseases, and obesity [4, 5]. However, recent epidemiological, clinical, and experimental evidence shows that estrogens also confer protection against cell proliferation and malignant transformation [6–9].
Since the identification of two types of estrogen receptor (ERα and ERβ), the complex effects of estrogen on various tissue types have become apparent. Indeed the discovery of ERβ changed the concept of estrogen signaling, opening a new chapter in estrogenic effects and in the design of estrogenic pharmaceuticals [4, 5, 10].
A mouse model bearing germ line mutations in the adenomatosis polyposis coli gene developed multiple intestinal tumors. In this model, the prevention of this tumor formation by estrogen correlated with an increase in ERβ expression and a decrease in ERα expression [6, 11]. In addition to these experimental models, estrogenic stimulation of colon cancer cells has also been found to express ERβ consistently, inducing apoptosis in a dose-dependent manner [7, 9, 12, 13]. ERα seems to mediate the proliferative effects of estrogens [14, 15]; on the contrary, ERβ seems to direct the anti-proliferative effects of estrogen [7, 9, 12, 16].
Expression of estrogen receptors differ with tissue type [8]. ERβ expression was first characterized in prostatic and ovarian tissues [17]. Further investigation of tissue expression in colonic mucosal epithelium found the over-expression of ERβ coupled with negligible expression of ERα. These findings suggest that ERβ is involved in the protective effect of hormone replacement therapy on colonic carcinogenesis [13, 17, 18].
The reduction in the number of ERβ receptors in colon cancer might relate to worsening stage and grade of the tumor [19]. A subsequent work indicated that lower expression of wild-type ERβ was associated with poorer differentiation and higher pathological tumor stage [20]. Similar associations between the low expression of ERβ and advanced disease have been shown in gastric carcinoma [21]. Although these findings suggest a role for ERβ signaling in colon carcinogenesis, the work was done on paraffin-embedded histology samples with no follow-up of patients. Prospective data on ERβ expression and outcome measures such as TNM staging, formal grading, or overall survival in patients with colon cancer are needed before conclusions can be made on ERβ as a prognostic indicator [2].
Aim of Work
We conducted this prospective study to investigate the clinicopathological correlations, relationship to apoptotic body index, and prognostic significance of ERβ expression in colorectal carcinoma.
Patients and Methods
The study was carried out on 40 patients with newly diagnosed colorectal cancer and who were admitted to Mansoura University Hospital and Oncology Centre during the period December 2006 to May 2007. All cases were eligible for the study unless there is an associated significant medical co-morbidity or other malignancy.
The patients were subjected to:
-
Thorough history taking and clinical examination
-
Laboratory investigations including liver, kidney functions, blood picture, tumor markers (CEA and CA19.9)
-
Radiological investigations including barium enema, abdomino-pelvic CT scan, X-ray or CT scan of the chest, and bone scan when needed
-
Colonoscopy of the entire colon and colonoscopic biopsy of suspected lesions
The patients were adequately managed according to our institutional protocols. Cases presenting with metastatic disease (12/40) were managed by systemic chemotherapy only, except two patients who presented with limited liver metastasis, one of whom was managed by surgical excision of both the primary and the liver metastasis and the other one by surgical excision of the primary plus radiofrequency ablation of the liver metastases. Cases presenting with non-metastatic disease (stages I–III; 28/40) were managed by surgical resection and post-operative adjuvant chemotherapy (chemo-radiotherapy in cases of rectal carcinoma) as follows: wide surgical resection and anastomosis; in addition, surgical staging includes an assessment of liver metastases, nodal spread of disease, and extension of tumor through the bowel wall and liver metastases. For proper pathological nodal staging, at least 12 nodes should be removed. Adjuvant chemotherapy was given to patients with stage III and patients with stage II at high risk. Stage II is defined as high risk if they present at least one of the following characteristics: lymph nodes sampling <12; poorly differentiated tumor; vascular, lymphatic or perineural invasion; tumor presentation with obstruction or tumor perforation, and pathological T4 stage.
The patients were followed up by clinical routine laboratory evaluation and CEA determination every 3 months, ultrasonography of the liver every 6 months and, alternatively, CT scans of the chest and abdomen for patients who are at higher risk for recurrence, and colonoscopy at year 1 and thereafter every 3–5 years. Other laboratory and radiological examinations were restricted to patients with suspicious symptoms. For advanced disease history, physical examination, CEA if initially elevated, and a CT scan of the involved regions were performed every 2–3 months of palliative chemotherapy. Two years overall survival was the study endpoint.
Apoptotic body index calculation entails: (1) detection of the apoptotic nuclei: the terminal deoxynulceotidyl transferase-mediated dUTP-biotin nick end-labeling method is used after MEB stain (chemical kit for in situ detection of apoptosis MEB stain apoptosis direct part IM 3/17- IMMUNOTECH, a coulter company BP, 177–13276 Marseille [Cedex 9-France]) and visualized by immunofluorescence as +ve and −ve; and (2) the index is expressed as the number of apoptotic tumor cells divided by the total number of non-apoptotic tumor cells in the same field with evaluation of 1,000 nuclei in randomly selected areas in each specimen [22].
Immunohistochemical staining for ERβ expression is performed: formalin-fixed, paraffin-embedded tissue sections were obtained from 40 colorectal cancer specimens (all samples submitted for the assays were obtained from the primary lesions). After routine deparaffinization in xylene, the sections were hydrated through a series of graded alcohols, distilled water, and phosphate-buffered saline (PBS) at pH 7.2–7.4. Antigen retrieval was performed using Tris–EDTA (pH 9). The slides were put in DAKO autostainer S 3,400 which performed the following steps:
-
Incubation in 3% H2O2 for 5 min
-
Washing the slides with PBS at pH 7.2–7.4
-
Incubation with monoclonal rabbit anti-human estrogen receptor ß diluted 1:50 in DAKO antibody diluent S3022 for 30 min at room temperature
-
Washing the slides with phosphate-buffered saline at pH 7.2–7.4
-
Applying the EnVision TM dual link kit (K5007) optimized for DAKO cytomation automated systems for 30 min
-
Washing the slides with phosphate-buffered saline at ph 7.2–7.4
-
Applying 3,3′-di-amino-benzidine tetrahydrochloride as chromogen for 5 min
-
Rinsing well in distilled water for 5 min
The slides in the autostainer were removed and hematoxylin counterstaining was performed. Slides were dehydrated in ascending grades of alcohol and were cleared in xylene for three changes and cover slips were applied. Sections from normal prostatic tissue were used as positive controls. Negative controls were processed by substituting the primary antibody with non-immune mouse serum.
The intensity of the immunohistochemical staining was scored on a four-point scale as follows: – = negative (<10% of cells with nuclear staining), + = weak (weak nuclear staining intensity or 10–50% of cells with nuclear staining), ++ = moderate (moderate nuclear staining intensity and >50% of cells with nuclear staining), and +++ = strong expression (strong nuclear staining intensity and >50% of cells with nuclear staining) [23]. Two independent pathologists evaluated and scored all sections using the scale. When interobserver disagreement was observed, specimens were reassessed by simultaneous examination by two pathologists.
As there is no consensus about a cutoff value to distinguish positive from negative cases, we used the 10% cutoff used in cancer breast, which has been also adapted in other studies of ERβ in colorectal cancer [11, 23, 24]. In addition, 15 randomly selected cases were also stained for classic estrogen receptors (ERα) by immunohistochemistry.
Statistical Analysis
For descriptive statistics of qualitative variables, the frequency distribution procedure was run with calculation of the number of cases and percentages. For descriptive statistics of quantitative variables, the mean, range, and standard deviation were used to describe central tendency and dispersion. For analysis of the differences in proportions, chi-square test was used; Fisher’s exact test was used if the assumptions of chi-square were violated. Mann–Whitney U test was used to compare the level of quantitative variables between ERβ +ve and −ve cases. Survival analysis was calculated by the Kaplan–Meier product-limit estimator. A comparison of survival was performed by the log-rank test. Data were analyzed on a personal computer running SPSS© for Windows (Statistical Package for Social Scientists) Release 15. All tests are considered significant if p ≤ 0.05; all statistical tests were two-sided.
Results
The study included 40 patients with newly diagnosed colorectal carcinoma: 23 males (57.5%) and 17 females (42.5%). The mean age of studied cases was 47.7 years (range of 25–65 years). Descriptive statistics of studied cases are summarized in Table 1.
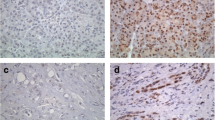
ERβ immunoreactivity at a 10% cutoff was detected in 65% of colorectal cancer cases; various grades of expression are illustrated in Fig. 1. Classic estrogen receptors (ERα) immunoreactivity was found in only 7% of studied cases.
There is no difference between ERβ +ve and −ve groups regarding the mean age of the studied cases (47.3 versus 46.7 years, respectively; p = 0.6); also, there is no significant difference between the sex, site of colorectal cancer, the presence of LN infiltration, perineural invasion, or distant metastasis in ERβ +ve and −ve groups. Fifty percent of ERβ-positive cases present with early stages I and II, while the majority of ERβ-negative cases (79%) present with stages III and IV. However the difference did not reach statistical significance (p = 0.08) (Table 2).
Univariate analysis showed that high-grade tumors and the presence of vascular invasion were significantly associated with lack of ERβ expression (p = 0.02 and 0.04, respectively) (Figs. 2 and 3). The median apoptotic index in ERβ-positive cases was 6% (range 1–14%), while in ERβ-negative cases the median apoptotic index was 3% (range 1–10). The difference was statistically significant (p = 0.01) as shown in Fig. 4. In multivariate analysis, only the apoptotic index and tumor grade were significantly associated with differential expression of ERβ (Table 3).
At the end of follow-up, seven cases suffered tumor recurrence, three cases developed liver metastases, three cases developed peritoneal involvement, and one case developed local recurrence. The expression of ERβ did not affect the rate of recurrence (Table 2).
The median overall survival of the studied cases was 22 months (95% CI 19–25 months). The median survival in ERβ-positive cases was 22 months (95% CI 13.4–30.5 months); in ERβ-negative cases, the median survival was 18 months (95% CI 8.9–27 months); however, the difference was not statistically significant (log rank 1.2, p = 0.27) as shown in Fig. 5.
Discussion
Colorectal cancer is the third most common cancer in both men and women. Despite advances in early diagnosis and treatment, colorectal cancer is still the third leading cause of cancer deaths [25].
Several epidemiologic studies have shown that colon cancer might be influenced by estrogens and estrogen use might be associated with a low risk of colon cancer [26–28].
In the current study, ERβ immunoreactivity was detected in 65% of colorectal cancer cases, while ERα positivity was found in only 7% of cases, which is consistent with the results shown by other studies [11, 23, 24]. These results confirm the assumptions that effects of estrogens on colon cancer susceptibility could be mediated by ERβ [6]. It has been previously reported that antiestrogens, especially tamoxifen, inhibit the growth of colon cancer cell lines [29, 30] and prevent liver metastases from colorectal cancers in animal models [31]. Whether ERβ-positive colorectal carcinomas respond to treatment with antiestrogens deserves further studies.
Hormone-dependent cancers have an inverse relation between tumor progression and ERβ expression. The same relation is seen in colorectal cancer in cell lines [13, 32]. These experimental findings are proven in human clinical specimens of our study where the rate of ERβ immunoreactivity was significantly higher in low-grade than high-grade colorectal tumors. This is in agreement with Konstantinopoulos et al. [23] who reported a decline in ERβ expression paralleling the loss of differentiation of malignant colon cells. These findings suggest a protective role for ERβ against colon carcinogenesis.
Cancers of the proximal and distal colon may have different underlying mechanisms; however, in the current work, ERβ expression is independent of the tumor’s localization. This is consonant with the data reported by other investigators [11, 24].
In this study, we found no significant relation between ERβ expression and the presence of LN or distant metastasis. However, the expression rate of ERβ was higher in cases with stages I and II compared to the stages II and IV, but the difference did not attain statistical significance. This is in agreement with Castiglione et al. [33] who reported that the expression mean of ERβ was higher in early stages, but the difference was of borderline significance (p = 0.068). A large sample size may be needed before we can draw conclusions about the relation between ERβ expression and staging in colorectal cancer.
Induction of apoptosis by ERβ has been described via differing mechanisms including increased DNA fragmentation [13], upregulation of the proapoptotic BAXα gene [34], or due to increased p53 signaling [35]. In the current work, the median apoptotic index in ERβ-positive cases was significantly higher than in ERβ-negative cases (6% versus 3%; p = 0.01). This proves the data reported in experimental models that loss of ERβ leads to decreased apoptosis in the cells of the colonic epithelium [36].
The median overall survival was higher in ERβ-positive cases (22 versus 18 months); however, this difference was statistically insignificant. Previous studies examining the relation between ERβ expression and overall survival in colorectal cancer did not report a significant difference [11, 24]. A larger-sample study with a longer follow-up period is necessary to further investigate the relationship between ERβ expression and survival of colorectal cancer patients.
Conclusion
Our results reinforce the importance of the ERβ rather than the ERα in colorectal cancer and may therefore help better understand how estrogens work in the etiology of colorectal cancer.
Lack of ERβ expression in colorectal cancer is associated with loss of differentiation and decreased apoptosis, independent of the tumor’s localization.
Future studies should include validation of ERβ as a prognostic marker and exploration of ERβ as a target for clinical decision-making in the management and chemoprevention of colorectal cancer.
References
Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88(10):2398–424.
Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9(4):385–91.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288(3):321–33.
Ascenzi P, Bocedi A, Marino M. Structure–function relationship of estrogen receptor alpha and beta: impact on human health. Mol Aspects Med. 2006;27(4):299–402.
Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–70.
Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11(3):537–51.
Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, et al. Survival versus apoptotic 17beta-estradiol effect: role of ER alpha and ER beta activated non-genomic signaling. J Cell Physiol. 2005;203(1):193–201.
Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26(3):465–78.
Galluzzo P, Caiazza F, Moreno S, Marino M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr Relat Cancer. 2007;14(1):153–67.
Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, Tsokos M, et al. Validation of a model of colon cancer progression. J Pathol. 2000;192(4):446–54.
Witte D, Chirala M, Younes A, Li Y, Younes M. Estrogen receptor beta is expressed in human colorectal adenocarcinoma. Hum Pathol. 2001;32(9):940–4.
Caiazza F, Galluzzo P, Lorenzetti S, Marino M. 17Beta-estradiol induces ERbeta up-regulation via p38/MAPK activation in colon cancer cells. Biochem Biophys Res Commun. 2007;359(1):102–7.
Qiu Y, Waters CE, Lewis AE, Langman MJ, Eggo MC. Oestrogen-induced apoptosis in colonocytes expressing oestrogen receptor beta. J Endocrinol. 2002;174(3):369–77.
Marino M, Acconcia F, Bresciani F, Weisz A, Trentalance A. Distinct nongenomic signal transduction pathways controlled by 17beta-estradiol regulate DNA synthesis and cyclin D(1) gene transcription in HepG2 cells. Mol Biol Cell. 2002;13(10):3720–9.
Fernando RI, Wimalasena J. Estradiol abrogates apoptosis in breast cancer cells through inactivation of BAD: Ras-dependent nongenomic pathways requiring signaling through ERK and Akt. Mol Biol Cell. 2004;15(7):3266–84.
Marino M, Galluzzo P, Leone S, Acconcia F, Ascenzi P. Nitric oxide impairs the 17beta-estradiol-induced apoptosis in human colon adenocarcinoma cells. Endocr Relat Cancer. 2006;13(2):559–69.
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–30.
Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82(12):4258–65.
Jassam N, Bell SM, Speirs V, Quirke P. Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes’ staging. Oncol Rep. 2005;14(1):17–21.
Wong NA, Malcomson RD, Jodrell DI, Groome NP, Harrison DJ, Saunders PT. ERbeta isoform expression in colorectal carcinoma: an in vivo and in vitro study of clinicopathological and molecular correlates. J Pathol. 2005;207(1):53–60.
Wang L, Guan X, Gong W, Yao J, Peng Z, Wei D, et al. Altered expression of transcription factor Sp1 critically impacts the angiogenic phenotype of human gastric cancer. Clin Exp Metastasis. 2005;22(3):205–13.
Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119(3):493–501.
Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, et al. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer. 2003;39(9):1251–8.
Xie LQ, Yu JP, Luo HS. Expression of estrogen receptor beta in human colorectal cancer. World J Gastroenterol. 2004;10(2):214–7.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300.
al-Azzawi F, Wahab M. Estrogen and colon cancer: current issues. Climacteric. 2002;5(1):3–14.
Genazzani AR, Gambacciani M, Simoncini T, Schneider HP. Hormone replacement therapy in climacteric and aging brain. International Menopause Society Expert Workshop, 15–18 March 2003, Pisa, Italy. Climacteric. 2003;6(3):188–203.
Giovannucci E. Obesity, gender, and colon cancer. Gut. 2002;51(2):147.
Ziv Y, Gupta MK, Milsom JW, Vladisavljevic A, Kitago K, Fazio VW. The effect of tamoxifen on established human colorectal cancer cell lines in vitro. Anticancer Res. 1996;16(6B):3767–71.
Arai N, Strom A, Rafter JJ, Gustafsson JA. Estrogen receptor beta mRNA in colon cancer cells: growth effects of estrogen and genistein. Biochem Biophys Res Commun. 2000;270(2):425–31.
Kuruppu D, Christophi C, Bertram JF, O’Brien PE. Tamoxifen inhibits colorectal cancer metastases in the liver: a study in a murine model. J Gastroenterol Hepatol. 1998;13(5):521–7.
Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61(2):632–40.
Castiglione F, Taddei A, Degl’Innocenti DR, Buccoliero AM, Bechi P, Garbini F, et al. Expression of estrogen receptor beta in colon cancer progression. Diagn Mol Pathol. 2008;17(4):231–6.
Linsalata M, Russo F, Notarnicola M, Guerra V, Cavallini A, Clemente C, et al. Effects of genistein on the polyamine metabolism and cell growth in DLD-1 human colon cancer cells. Nutr Cancer. 2005;52(1):84–93.
Hsu HH, Cheng SF, Wu CC, Chu CH, Weng YJ, Lin CS, et al. Apoptotic effects of over-expressed estrogen receptor-beta on LoVo colon cancer cell is mediated by p53 signalings in a ligand-dependent manner. Chin J Physiol. 2006;49(2):110–6.
Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, et al. Role of estrogen receptor beta in colonic epithelium. Proc Natl Acad Sci U S A. 2006;103(8):2959–64.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elbanna, H.G., Ebrahim, M.A., Abbas, A.M. et al. Potential Value of Estrogen Receptor Beta Expression in Colorectal Carcinoma: Interaction with Apoptotic Index. J Gastrointest Canc 43, 56–62 (2012). https://doi.org/10.1007/s12029-010-9214-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-010-9214-4