Abstract
Background
Acute myocardial infarction (AMI) is the rarest and least studied cardiac complication of aneurysmal subarachnoid hemorrhage (aSAH). Precise estimates of the incidence of AMI after aSAH are unavailable. Our goal was to estimate the incidence of registry-based AMI (rb-AMI) after aSAH and determine its association with clinical outcomes.
Methods
Adult patients with aSAH in the National Inpatient Samples from 2002 to 2014 were included in the study. We evaluated risk factors for rb-AMI using univariate and multivariate regression models. Clinical outcomes that were assessed included functional status at discharge, in-patient mortality, length of stay, and total hospitalization cost, adjusting for patient demographics and cardiovascular risk factors through an inverse probability weighted analysis. Subgroup analyses were further performed stratified by rb-AMI type (ST-segment elevation myocardial infarction [STEMI] vs. non-STEMI [NSTEMI]).
Results
A total of 139,734 patients with aSAH were identified, 3.6% of whom had rb-AMI. NSTEMI was the most common type of rb-AMI occurring after aSAH (71% vs. 29% for NSTEMI vs. STEMI, respectively). Patient characteristics associated with higher odds of rb-AMI included age, female sex, poor aSAH grade, and various cardiovascular risk factors. Rb-AMI was also associated with poor functional status at discharge, higher in-hospital mortality, and a longer and more costly hospital stay.
Conclusions
Rb-AMI occurs in 3.6% of patients with aSAH and is associated with poor functional status at discharge, higher in-patient mortality, and a longer and more costly hospitalization. Differentiating between different types of rb-AMI would be important in optimizing the management of patients with aSAH. Our definition of rb-AMI likely includes patients with neurogenic stress cardiomyopathy, which may confound the results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial injury (i.e., cardiac troponin concentration > 99th percentile) occurs in approximately 40% of all cases of subarachnoid hemorrhages (SAH) [1, 2]. The occurrence of myocardial injury after SAH correlates directly with hemorrhage severity and is strongly associated with poor discharge disposition and high mortality [2]. The management of patients with myocardial injury often conflicts with the management of aneurysmal SAH (aSAH), complicating the care of patients with aSAH [3]. Adequate management of myocardial injury after aSAH, however, is of paramount importance, as acute myocardial infarction (AMI) after aSAH can lead to sudden death, cardiogenic shock, acute pulmonary edema, or cardioembolic stroke and is associated with poor outcomes after aSAH [4,5,6,7].
Myocardial injury following aSAH can be due to ischemic or nonischemic causes [8]. The management of nonischemic causes of myocardial injury is aimed at optimizing care of the underlying diagnosis [8]. In contrast, the management of ischemic causes of myocardial injury is aimed at the preservation of myocardial tissue through prompt reperfusion in AMI caused by a coronary thrombus (type 1 AMI) or through physiologic parameter optimization in AMI caused by an imbalance of oxygen supply and demand (type 2 AMI) [8]. Estimates of the incidence of AMI after aSAH are scarce, and the association between AMI and outcomes after aSAH has not been studied. We used the National Inpatient Sample (NIS) in this study to estimate the incidence of AMI after aSAH and its association with outcomes. By providing data from 20% of all discharges from US hospitals, the NIS provides a uniquely large sample size for studying rare events, such as AMI after SAH, and can be used to produce national-level estimates by taking the NIS’s complex survey design into account. Of note, given the inherent design of the NIS, our definition of AMI based on the registry likely includes patients with neurogenic stress cardiomyopathy, which may confound the results.
Methods
Data Source
We used data from the NIS (Healthcare Cost and Utilization Project [HCUP], Agency for Healthcare Research and Quality) [9]. Our institutional review board exempts studies using the NIS from individual review. Since 2012, the NIS provides a sample of discharges from all HCUP hospitals [10]. To provide national-level estimates, analyses of the NIS must be weighted accounting for its complex survey design [10]. Trend weights, provided by HCUP, can be used in place of the former discharge weights for years prior to 2012 to provide national estimates [10]. We obtained trend weights for years 2002–2011 from HCUP [11]. In October of 2015, the codes of the International Classification of Diseases (ICD) Version 10 were implemented, leading to a shift in the reporting of diseases [12]. Therefore, we limited our analysis of the NIS to years 2002–2014.
Inclusion and Exclusion Criteria
We included individuals who (1) had an ICD, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis of SAH (430) or intracerebral hemorrhage (431, 432.9) and (2) underwent microsurgical clipping (ICD-9-CM procedure code: 39.51), coil embolization (39.72, 39.75, 39.76, 39.79), or another repair of aneurysm (39.52). Included patients fulfilling any of the following criteria were excluded: (1) age < 18 years old, (2) diagnosis of traumatic brain injury (800.0–801.9, 803.0–804.9, 850.0–854.1, 873.0–873.9), (3) diagnosis (747.81) or management (39.53, 92.30) of an arteriovenous malformation and/or fistula, or (4) a length of stay < 1 day with an associated discharge to home.
SAH Grading
We estimated SAH severity using the NIS-SAH severity scale (NIS-SSS), a validated severity adjustment score for patients with aSAH in the NIS [13]. The NIS-SSS is obtained by assessing the presence of findings suggestive of poor neurological status: coma (780.01, 780.03), hydrocephalus (331.3, 331.4), treatment of hydrocephalus (02.2, 02.31–02.39), paresis/plegia (438.2–438.53), cranial nerve deficits (378.5–378.56, 379.4–379.43), aphasia (438.1–438.89), and mechanical ventilation (96.04, 96.7–96.72). Each finding was then weighted by its contribution to a poor functional outcome at discharge. We classified patients into poor grade on admission if they had an NIS-SSS value > 7, which is suggestive of a Hunt and Hess grade in the III–V range.
Demographics and Cardiovascular Risk Factors
We obtained data on patient age, sex, payment type and hospital location, and teaching status. We also obtained data on cardiovascular risk factors using codes described by Elixhauser et al. [14]. Specifically, we obtained information on history of congestive heart failure (398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4–425.9, 428.x), cardiac arrythmias (426.0, 426.13, 426.7, 426.9, 426.10, 426.12, 427.0–427.4, 427.6–427.9, 785.0, 996.01, 996.04, V45.0, V53.3), valvular disease (093.2, 394.x–397.x, 424.x, 746.3–746.6, V42.2, V43.3), pulmonary circulation disorders (415.0, 415.1, 416.x, 417.0, 417.8, 417.9), uncomplicated (401.x) and complicated (402.x–405.x) hypertension, chronic pulmonary disease (416.8, 416.9, 490.x–505.x, 506.4, 508.1, 508.8), uncomplicated (250.0–250.3) and complicated (250.4–250.9) diabetes mellitus, renal failure (403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 585.x, 586.x, 588.0, V42.0, V45.1, V56.x), coagulopathy (286.x, 287.1, 287.3–287.5), alcohol abuse (265.2, 291.1–291.3, 291.5–291.9, 303.0, 303.9, 305.0, 357.5, 425.5, 535.3, 571.0–571.3, 980.x, V11.3), and drug abuse (292.x, 304.x, 305.2–305.9, V65.42).
Myocardial Infarction and Outcomes
We defined registry-based AMI (rb-AMI) as a diagnosis of ST-segment elevation myocardial infarction (STEMI) (410.0–410.02 [acute myocardial infarction of anterolateral wall], 410.1–410.12 [acute myocardial infarction of other anterior wall], 410.2–410.22 [acute myocardial infarction of inferolateral wall], 410.3–410.32 [acute myocardial infarction of inferoposterior wall], 410.4–410.42 [acute myocardial infarction of other inferior wall], 410.5–410.52 [acute myocardial infarction of other lateral wall], 410.6–410.62 [true posterior wall infarction], 410.8–410.82 [acute myocardial infarction of other specified sites], and 410.9–410.92 [acute myocardial infarction of unspecified sites]) or a diagnosis of non-STEMI (NSTEMI) (410.7–410.72 [subendocardial infarction]). To select for rb-AMI occurring during the same hospital admission as the SAH, codes reflecting old myocardial infarction (412), other acute and subacute forms of ischemic heart disease (411.x), angina pectoris (413.x), or other forms of chronic ischemic heart disease (414.x) were not part of our definition of rb-AMI. Our primary outcome was poor functional status at discharge, defined by using the NIS severity outcome measure (NIS-SOM) [13]. The NIS-SOM defines poor functional status at discharge as the presence of any of the following: in-hospital mortality; discharge to a skilled nursing facility, extended care facility, or hospice; placement of a tracheostomy tube (31.1, 31.2.31.21, 31.29); and/or placement of a gastrostomy tube (43.1, 43.11, 43.19, 44.32, 44.38, 44.39). The NIS-SOM has been validated to have a strong correlation with a modified Rankin scale > 3. Outcomes of secondary interest were in-hospital mortality, length of stay, and total monetary charges, adjusted for inflation to December of 2014, using Consumer Price Indexes obtained from the US Bureau of Labor Statistics [15]. Of note, total charges reported in the NIS that include all charges made to the primary payer generally do not include professional fees or noncovered charges, and may include emergency department charges incurred prior to admission to the hospital.
Statistical Analysis
We used R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) [16]. We estimated summary statistics for demographics, cardiovascular risk factors, SAH characteristics, rb-AMI characteristics, and outcomes by performing univariate analyses. We screened for independent predictors of rb-AMI after aSAH by performing a multivariate logistic regression by using patient characteristics as predictors. To further explore the effect of continuous variables on rb-AMI after aSAH we fitted polynomial splines and incorporated them into a generalized additive model. Using the summary statistics of the entire population, we performed χ2 tests and Student’s t-tests to evaluate associations between patient characteristics and rb-AMI. We calculated stabilized inverse probability weights using independent predictors of rb-AMI after aSAH for our inverse probability weighted analysis. We obtained the product of these stabilized inverse probability weights and the original trend weights and used these products as new weights to perform an adjusted survey analysis [17, 18]. Finally, as a sensitivity analysis, we tested if our inverse probability weighted approach was robust by performing a multivariate logistic regression adjusting for the same covariates that we used to estimate the inverse probability weights.
Results
Incidence of rb-AMI After aSAH
A total of 139,734 individuals were identified in this study with aSAH, of whom 4993 (3.6%) developed rb-AMI (Fig. 1, Table 1, Supplemental Table 1). Of the patients with rb-AMI, 29% had STEMI. The incidence of rb-AMI after aSAH was highest in 2008 (4.3%, 95% confidence interval [CI] 3.9–4.9%), but overall, the incidence of rb-AMI after aSAH remained stable across years with no evident temporal trends observed over time (Fig. 2).
Characteristics of Patients with rb-AMI After aSAH
When compared with patients without AMI, patients who had rb-AMI after aSAH were older (60.9 years vs. 54.4 years) and more likely to be women (74% vs. 68%) (Table 1). Patients with rb-AMI were also more likely to have a history of congestive heart failure, arrythmia, valvular disease, pulmonary circulation disorders, complicated hypertension, chronic pulmonary disease, uncomplicated diabetes, renal failure, or coagulopathy, but were less likely to have uncomplicated hypertension or alcohol abuse (Table 1). Patients with rb-AMI after aSAH were more likely to have a high grade aSAH (69% vs. 38%), were more commonly treated with endovascular coiling (61% vs. 53%), and were more commonly insured by Medicare or Medicaid (52% vs. 39%) (Table 1). Poor functional status (83% vs. 54%) and in-hospital mortality (27% vs. 12%) were more common in patients with rb-AMI after aSAH (Table 1). Similarly, hospitalization was longer (23 days vs. 18.5 days) and more costly ($364,766 vs. $264,581) (Table 1).
Independent Predictors of rb-AMI After aSAH
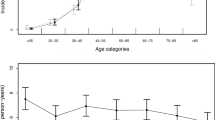
Patient characteristics independently associated with rb-AMI after SAH in multivariate analysis included age, female sex, poor grade SAH, aneurysm clipping (vs. coiling), history of congestive heart failure, cardiac arrythmia, pulmonary circulation disorders, hypertension, and coagulopathy (Table 2). Through a polynomial spline incorporated into a generalized additive model, we were able to observe that the effect of age on the odds of developing rb-AMI after aSAH increased sharply between the third and fifth decade of life and continued to increase gradually until plateauing after the eighth decade of life (Fig. 3).
Adjusted association between age and rb-AMI after aSAH. Polynomial spline for the relationship between age and rb-AMI after aSAH, adjusted for female sex, poor aSAH grade, aneurysm clipping, congestive heart failure, arrhythmia, valvular disease, pulmonary circulation disorders, uncomplicated hypertension, complicated hypertension, renal failure, coagulopathy, and alcohol abuse. aSAH, aneurysmal subarachnoid hemorrhage, rb-AMI, registry-based acute myocardial infarction
Association Between rb-AMI and Outcomes
In unadjusted analyses, rb-AMI was associated with higher odds of poor functional status at discharge (odds ratio [OR] 4, 95% CI 3.4–4.8, p < 0.001), higher odds of in-hospital mortality (OR, 2.6, 95% CI, 2.3–3, p < 0.001), a longer length of stay (mean difference [MD] 4.5 days, 95% CI 3.2–5.7, p < 0.001), and a more costly hospitalization (MD $100,185, 95% CI $74,690–125,6789, p < 0.001) (Supplemental Table 2). STEMI and NSTEMI were also associated with higher odds of poor functional status at discharge, higher odds of in-hospital mortality, a longer length of stay, and a more costly hospitalization in unadjusted analyses (Supplemental Table 2).
After adjusting for its independent predictors, rb-AMI after aSAH remained associated with higher odds of poor functional status at discharge (OR 2.9, 95% CI 2.8–3.1, p < 0.001) and in-hospital mortality (OR 2.1, 95% CI 2–2.2, p < 0.001) (Fig. 4). Rb-AMI after aSAH also remained associated with a longer (MD 4.3 days, 95% CI 3.5–5.1, p < 0.001) and more costly hospitalization (MD $102,797, 95% CI $91,014–114,580, p < 0.001) (Fig. 4). STEMI also remained associated with higher odds of poor functional status at discharge (OR 4.8, 95% CI 3.9–4.9, p < 0.001), in-hospital mortality (OR 2.9, 95% CI, 2.7–3.2, p < 0.001), and a longer (MD 4 days, 95% CI 2.7–5.3, p < 0.001) and more costly hospitalization (MD $95,774, 95% CI $75,901–115,647, p < 0.001) (Fig. 4). Similarly, the association between NSTEMI and higher odds of poor functional status at discharge (OR 2.5, 95% CI 2.3–2.6, p < 0.001) and in-hospital mortality (OR 1.8, 95% CI 1.7–1.9, p < 0.001) and a longer (MD: 4.6 days, 95% CI: 3.7–5.5, p < 0.001) and more costly hospitalization (MD $105,384, 95% CI $91,510–119,257, p < 0.001) remained after covariate adjustment (Fig. 4). The associations observed in our analysis were similar to the sensitivity analyses using multivariate logistic regressions, varying only in magnitude but not in direction or level of significance (Supplemental Tables 3–6).
Adjusted association between rb-AMI after aSAH and (a) poor functional outcome at hospital discharge, (b) in-hospital mortality, (c) length of stay, and (d) hospitalization total charges. Stabilized inverse probability weights were used to adjust for age, female sex, poor aSAH grade, aneurysm coiling (vs. clipping), congestive heart failure, arrhythmia, pulmonary circulation disorders, uncomplicated hypertension, complicated hypertension, and coagulopathy. aSAH, aneurysmal subarachnoid hemorrhage, CI, confidence interval, NSTEMI, non–ST-segment elevation myocardial infarction, OR, odds ratio, rb-AMI registry-based acute myocardial infarction, STEMI, ST-segment elevation myocardial infarction
Discussion
In this study, we found that the incidence of rb-AMI after aSAH is 3.6%, with NSTEMI being the most common type of rb-AMI occurring after aSAH (71% vs. 29% for NSTEMI vs. STEMI, respectively). Patient characteristics that were independently associated with higher odds of rb-AMI after aSAH included age, female sex, poor aSAH grade, and a history of congestive heart failure, cardiac arrhythmias, pulmonary circulation disorders, and coagulopathy. The patient characteristics that were associated with lower odds of rb-AMI after aSAH included clipping of aneurysm and arterial hypertension. Finally, patients with rb-AMI after aSAH had higher odds of poor functional status at discharge and in-hospital mortality and a longer and more costly hospital stay, after adjusting for independent predictors of rb-AMI after aSAH.
Death or disability occur in approximately two thirds of aSAH cases [19]. Because of advances in management, aSAH mortality has dropped sizably in recent years [19, 20]. These reductions in aSAH mortality, however, have come at the expense of increased rates of disability after SAH, with poor cognitive and functional outcomes being common after aSAH [21]. In addition to delayed ischemic neurological deficits, medical complications are important drivers of disability beyond the initial effects of an aSAH [22]. The role of medical complications in patient disability makes their study essential for continued improvement of outcomes after aSAH.
Cardiac complications of aSAH include sudden death, transient left ventricular dysfunction, and AMI [23,24,25]. One in four patients with aSAH dies before reaching hospital admission [23]. The exact mechanisms of aSAH-related sudden death are unknown, but risk factors for aSAH-related sudden death are similar to those of sudden cardiac death, suggesting that altered cardiovascular function is a likely culprit [23]. In patients with aSAH for whom serum samples are available, myocardial injury occurs in 40% of all patients and heralds AMI (types 1 or 2) or transient left ventricular dysfunction [26]. In 30% of patients with myocardial injury after aSAH, transient left ventricular dysfunction occurs and is commonly known as neurogenic stress cardiomyopathy [24, 27, 28]. The presence of stress cardiomyopathy is suggested by wall motion abnormalities spanning across more than one coronary artery territory but requires coronary angiography or ventriculography for definitive diagnosis [29].
AMI is the rarest and least studied cardiac complication of aSAH. In a previous study of the NIS, the incidence of AMI after aSAH was estimated at 0.28% [30]. The incidence of AMI after acute ischemic stroke, however, is higher (1.6%) [30, 31]. Given that the incidence of myocardial injury is higher in patients with SAH than in patients with ischemic stroke, we expected the incidence of AMI after aSAH to be higher [2]. In our analysis, we found that the incidence of rb-AMI after aSAH is 3.6% [30]. Moreover, we found that STEMI comprises 29% of all rb-AMI after aSAH, making its incidence 1.0% in our study, which closely matches the one previously reported in the International Cooperative Study on the Timing of Aneurysm Surgery [22].
Patient characteristics that were associated with rb-AMI after aSAH included increased age, female sex, poor SAH grade, and diverse cardiovascular risk factors. Female sex has been consistently reported as a risk factor for neurogenic stress cardiomyopathy [32]. Differences in sensitivity to the effect of adrenergic agonists across genders have been reported and may explain the higher incidence of cardiac complications of aSAH observed in women [33]. A poor aSAH grade was also associated with rb-AMI after aSAH. Indeed, troponin levels after aSAH directly correlate with aSAH grade and have been proposed as an objective biomarker of SAH severity for outcome prediction [34]. Interestingly, hypertension appeared to be protective of rb-AMI after aSAH. Hypertension is commonly managed with adrenergic receptor blockers or antagonists of the renin angiotensin aldosterone pathway, and pretreatment with either one of these drug classes has been associated with improved functional outcomes after stroke [35, 36]. In line with our current findings, we previously reported an inverse association between hypertension and myocardial injury after aSAH [37].
Limitations
As previously mentioned, cardiac complications after aSAH include sudden death, transient left ventricular dysfunction or stress cardiomyopathy, and AMI [23,24,25]. Although a standardized definition of rb-AMI existed in the NIS before the start of our study’s sample, this definition did not distinguish between AMI and stress cardiomyopathy [38]. It was not until 2018 that the universal definition of myocardial infarction included a distinction between AMI and stress cardiomyopathy [29]. The distinction between rb-AMI and stress cardiomyopathy, particularly NSTEMI, is therefore limited in our study population, and overlap between these two clinical entities is highly likely in our sample. The definitive diagnosis of stress cardiomyopathy requires coronary angiography or ventriculography [29]. However, the role these invasive tests play in the management of patients with aSAH has yet to be determined and requires further study, as patients with stress cardiomyopathy appear to have unique complications for which directed interventions may be beneficial [3]. In addition, our assessment of poor functional outcome was limited to the time of discharge and does not reflect long-term functional outcome, as follow-up data are not available in the NIS. Additional limitations to our study inherent to the NIS’s design include the potential for additional coding inaccuracies, retrospective data analysis, a lack of data to further classify the severity of aSAH, such as the Hunt-Hess grade, and a lack of imaging data.
Conclusions
The incidence of rb-AMI after aSAH is 3.6%, with NSTEMI being the most common type of rb-AMI occurring after aSAH. Patients developing rb-AMI after aSAH were older, were more commonly women, had a distinct profile of cardiovascular risk factors, and were more likely to have a poor SAH grade. Patients with rb-AMI after aSAH had higher odds of poor functional status at discharge and in-hospital mortality and a longer and more costly hospital stay, after adjusting for baseline characteristics.
References
Morris NA, Chatterjee A, Adejumo OL, et al. The risk of takotsubo cardiomyopathy in acute neurological disease. Neurocrit Care. 2019;30(1):171–6.
Alkhachroum AM, Miller B, Chami T, Tatsuoka C, Sila C. A troponin study on patients with ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage: type II myocardial infarction is significantly associated with stroke severity, discharge disposition and mortality. J Clin Neurosci. 2019;64:83–8.
Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39(22):2047–62.
Fujita K, Fukuhara T, Munemasa M, Numba Y, Kuyama H. Ampulla cardiomyopathy associated with aneurysmal subarachnoid hemorrhage: report of 6 patients. Surg Neurol. 2007;68(5):556–61 (Discussion 561).
Ridwan S, Kristof R. Cardiac arrest in patients with poor-grade aneurysmal subarachnoid hemorrhage: a single-center experience. J Neurol Surg A Cent Eur Neurosurg. 2019;80(6):409–12.
Kimura T, Kamide T, Onodera K, et al. Clinical features of neurogenic pulmonary edema in patients with subarachnoid hemorrhage. World Neurosurg. 2020;135:e505–9.
Cho SM, Geocadin RG, Caturegli G, et al. Understanding characteristics of acute brain injury in adult extracorporeal membrane oxygenation: an autopsy study. Crit Care Med. 2020;48(6):e532–6.
DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661–78.
Healthcare Cost and Utilization Project. HCUP Overview Course (Accessible Version). http://www.hcup-us.ahrq.gov/overviewcourse.jsp. Accessed 3 Feb 2020.
Healthcare Cost and Utilization Project. Producing National HCUP Estimates (Accessible Version). http://www.hcup-us.ahrq.gov/tech_assist/nationalestimates/508_course/508course_2018.jsp. Accessed 3 Feb 2020.
Healthcare Cost and Utilization Project. Trend Weights for HCUP NIS Data. http://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Accessed 3 Feb 2020.
Healthcare Cost and Utilization Project. Release of the 2015 National Inpatient Sample (NIS) (November 2017). http://www.hcup-us.ahrq.gov/news/announcements/nis_111517.jsp. Accessed 3 Feb 2020.
Washington CW, Derdeyn CP, Dacey RG Jr, Dhar R, Zipfel GJ. Analysis of subarachnoid hemorrhage using the nationwide inpatient sample: the NIS-SAH severity score and outcome measure. J Neurosurg. 2014;121(2):482–9.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
U.S. Bureau of Labor Statistics. Consumer Price Index. http://www.bls.gov/cpi/. Accessed 3 Feb 2020.
Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2019.
Rubin DB. Matched sampling for causal effects. Cambridge: Cambridge University Press; 2006.
Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. 2014;49(1):284–303.
Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology. 2010;74(19):1494–501.
Lantigua H, Ortega-Gutierrez S, Schmidt JM, et al. Subarachnoid hemorrhage: who dies, and why? Crit Care. 2015;19:309.
Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–36.
Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg. 1990;73(1):18–36.
Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Risk factors of sudden death from subarachnoid hemorrhage. Stroke. 2017;48(9):2399–404.
van der Bilt I, Hasan D, van den Brink R, et al. Cardiac dysfunction after aneurysmal subarachnoid hemorrhage: relationship with outcome. Neurology. 2014;82(4):351–8.
van der Velden LB, Otterspoor LC, Schultze Kool LJ, Biessels GJ, Verheugt FW. Acute myocardial infarction complicating subarachnoid haemorrhage. Neth Heart J. 2009;17(7–8):284–7.
Samuels MA. The brain-heart connection. Circulation. 2007;116(1):77–84.
Kilbourn KJ, Levy S, Staff I, Kureshi I, McCullough L. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 2013;115(7):909–14.
Banki N, Kopelnik A, Tung P, et al. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. J Neurosurg. 2006;105(1):15–20.
Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618–51.
Kim YW, Neal D, Hoh BL. Risk factors, incidence, and effect of cardiac failure and myocardial infarction in aneurysmal subarachnoid hemorrhage patients. Neurosurgery. 2013;73(3):450–7 (quiz 457).
Alqahtani F, Aljohani S, Tarabishy A, Busu T, Adcock A, Alkhouli M. Incidence and outcomes of myocardial infarction in patients admitted with acute ischemic stroke. Stroke. 2017;48(11):2931–8.
Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118(4):397–409.
Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36(4):1233–8.
Guette P, Launey Y, Arnouat M, et al. Prognostic value of high-sensitivity troponin T in aneurysmal subarachnoid hemorrhage: a prospective observational study. Brain Inj. 2019;33(10):1372–8.
Neil-Dwyer G, Walter P, Cruickshank JM. Beta-blockade benefits patients following a subarachnoid haemorrhage. Eur J Clin Pharmacol. 1985;28(Suppl):25–9.
Hassan Y, Al-Jabi SW, Aziz NA, Looi I, Zyoud SH. Effect of prestroke use of angiotensin-converting enzyme inhibitors alone versus combination with antiplatelets and statin on ischemic stroke outcome. Clin Neuropharmacol. 2011;34(6):234–40.
Malik AN, Gross BA, Rosalind Lai PM, Moses ZB, Du R. Neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2015;83(6):880–5.
The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. Myocardial infarction redefined--a consensus document of the Joint European Society of Cardiology/American college of cardiology committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21(18):1502–13.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
CC: data acquisition, analysis, and writing of the article. IN: data acquisition. HN: data acquisition. PMR: critical review. WG: critical review. NP: critical review. KF: critical review. MA: critical review. RD: study supervision, interpretation, and critical review. All authors have read and approved of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MAS: Proctor for Covidien and Codman. WBG: Codman. The remaining authors have no conflicts to disclose.
Ethical Approval
We adhered to all applicable ethical guidelines. Our institutional review board waives individual review of studies using data from the National Inpatient Sample.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cerecedo-Lopez, C.D., Ng, I., Nguyen, H.B. et al. Incidence and Outcomes of Registry-Based Acute Myocardial Infarction After Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 36, 772–780 (2022). https://doi.org/10.1007/s12028-021-01365-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01365-3