Abstract
Background
Acute encephalopathy (AE) is a common complication of critical illness and is associated with increased short and long-term mortality. In this study, we evaluated the role of cefepime in causing AE.
Methods
Retrospective case–control study involving consecutive patients enrolled in the intensive care units (ICUs) of Mayo Clinic Rochester, MN between July 1, 2004 and December 31, 2015. AE was defined by the presence of delirium or depressed level of consciousness in the absence of deep sedation. Controls were identified as patients not developing AE and were matched by propensity score for age, Charlson Comorbidity Index, 24-h Apache III score and invasive ventilation use.
Results
The total number of eligible ICU admissions during our study period was 152,999. AE was present in 57,726 (37.7%) with a median AE duration of 17 (interquartile range [IQR] 4.0–51.8) hours. We matched 14,645 cases with AE with the same number of controls. Cefepime was used in 1241 (4.2%) patients and its use was associated with greater incidence of AE [713 (4.9%) vs 528 (3.6%), p < 0.001] and duration [unit estimate 0.73; (95% CI 0.542–0.918)]. On multivariate analysis, cefepime was associated with an increased likelihood of AE after controlling for shock, midazolam infusion and acute kidney injury [OR 1.24 (95% CI 1.10–1.27)]. These associations were also present after controlling for prior chronic kidney disease.
Conclusion
The use of cefepime is associated with increased likelihood and duration of AE. These associations are stronger among patients with impaired renal function, but can also occur in patients without renal impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute brain failure (ABF) is a common complication of critical illness and is associated with increased short and long-term mortality [1]. While delirium only reflects alterations in the content of consciousness, ABF reflects change in both content and level of consciousness [1]. The risk of ABF in the ICU is greater in older patients with more severe acute disease and greater comorbidities [1]. An expert panel recently recommended using the term acute encephalopathy (AE) to refer to alterations of consciousness in critically ill patients [2]; the definition of AE that is consistent with our previous definition of ABF. Hence we decided to use the term AE instead of ABF.
Cefepime is a fourth generation cephalosporin, with an extended spectrum of antimicrobial activity that is usually reserved to treat suspected or confirmed severe nosocomial infections. Because cefepime is excreted predominantly unchanged, a reduction in renal function increases the elimination half-life, leading to drug accumulation in blood and CSF [3]. Supratherapeutic levels of cefepime can cause neurological symptoms that include encephalopathy, myoclonus, seizures, hallucinations, and coma [4].
Although cefepime-induced neurotoxicity (CIN) typically occurs in patients with renal impairment in whom the dose of the antibiotic is not appropriately adjusted, neurological symptoms have been reported despite dose adjustments [4,5,6,7]. The incidence of CIN has been reported to range from 1 to 15% [3, 4, 8]. Yet, the association of cefepime with delirium in the ICU has been questioned [9]. We conducted a case–control study to determine the association between cefepime use and AE in a large ICU population.
Methods
The study was approved by the Mayo Clinic institutional review board (IRBe number 14-001118). This study was conducted in accordance with the declaration of Helsinki.
Study Design and Setting
We enrolled consecutive patients who had been admitted to the ICUs in Mayo Clinic Rochester, Minnesota between July 1, 2004 and December 31, 2015. Patients admitted to the Neuroscience, Pediatric and Neonatal ICUs were excluded from the study population. We have previously defined the concept of ABF, electronic search strategy, derivation and validation of the search strategy and grading of ABF [1, 10]. However as per the recent guidelines, we decided to use the updated term AE instead of ABF. Cases were defined as patients who had developed AE during the ICU stay. AE was defined as having delirium (positive Confusion Assessment Method for the ICU [CAM-ICU]) or depressed level of consciousness (by two consecutive scores of Glasgow Coma Scale [GCS] < 15 and/or Full Outline of Unresponsiveness [FOUR] score < 16) in the absence of deep sedation (Richmond Agitation Sedation Scale [RASS] < −3). For ventilated patients, GCS score of < 11 and FOUR scores of < 13 were considered abnormal. Deeply sedated patients (defined by a RASS score -3 or lower) were excluded because a reliable neurological examination cannot be performed in these instances. To focus on cases which were more likely to develop delirium as part of their ICU course rather than as a consequence of their presenting disease, patients who developed AE in the first 6 h in the ICU were excluded. AE duration was also calculated for the cases (i.e. time from when AE was first noticed until when it resolved). We used a minimum duration of 4 h to exclude AE caused by sedation and to minimize errors, as including these patients could overestimate the incidence of AE.
After identifying patients with AE (57,726), we randomly selected 14,645 cases with AE and 14,645 controls from the general ICU population. The case: control ratio in our study was 1:1 and the controls were matched within 0.1 standard deviation of cases, which is more stringent than the typical caliper width considered optimal (i.e. within 0.2 standard deviation) [11].
Cefepime use in the ICU
Cefepime was used for a wide array of infections, including hospital-acquired pneumonia, febrile neutropenia, skin/soft tissue infections, blood stream infections, intracranial infections/meningitis, urinary tract infections etc. The use of cefepime for treating specific microorganisms and the dosing of the antibiotic (including adjustments for patients with acute or chronic kidney injury) was based on the dosing recommendations from the Mayo Clinic antimicrobial therapy guide, which is developed by experts in our Infectious Diseases division and undergoes timely updates to account for the changes in the prevalence of specific organisms/infections (and resistance rates) in our local environment. Cefepime serum levels are not checked in our practice; hence they were not available for this study. The dosing of cefepime is constantly monitored by an ICU pharmacist and the doses are adjusted based on frequent reassessment of the creatinine clearance. The dosing for cefepime used in our hospital is shown in Table 1.
Data Sources
We extracted the raw data from the electronic medical records using the ICU Data Mart. The steps of development of this database, data security and validation of the demographics have been previously reported [12, 13]. The recorded data included age, sex, comorbities including past medical history of hypertension, diabetes mellitus, diabetes with complications, myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular accident, hemiplegia/paraplegia, asthma or chronic obstructive pulmonary disease (COPD), peptic ulcer, moderate/severe liver disease, cirrhosis, renal failure, and malignancy. Acute kidney injury (AKI) was defined according to the Kidney Disease Improving Global Outcome (KDIGO) criteria and we have previously reported the development and validation of the electronic algorithm for its identification [14]. We used AKI separately and also combined AKI and chronic kidney disease (CKD) for analyses and referred to them as renal disease. A Charlson comorbidity index (CCI), 24-h Acute Physiology and Chronic Health Evaluation III (APACHE III) score and presence of shock were obtained using previously validated computerized algorithms at out institution [13, 15, 16]. ICU Data Mart was also used to extract the data on use of sedatives and the use of cefepime.
Statistical Methods and Analyses
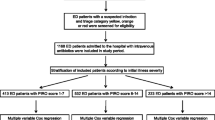
Descriptive summaries were presented as frequencies and percentages or median and interquartile range as appropriate. Propensity score matching was done using age, CCI, APACHE III score at 24 h, and invasive ventilator use as variables of interest (Fig. 1). The baseline and post propensity score matching comparisons between the cases and controls has been shown in supplementary table 1. These variables were chosen for propensity matching as these have been shown to be strongly associated with AE and would have confounded the results [1]. We decided not to include sepsis as part of the propensity score matching, as APACHE III score can be co-linear and thus produce redundancy. Comparisons between subjects with and without AE were performed using χ2 Chi square tests for categorical variables. Wilcoxon rank sum tests were used to compare means for continuous variable. In this study we used two types of outcomes: (1) a binary outcome (AE presence or absence) for which AE was analyzed using logistic regression and thus the associations were presented as odds ratios and corresponding 95% confidence intervals, whereas and (2) a continuous outcome (i.e. AE duration) that was analyzed using multivariable linear regression model and presented as parameter estimate, which is a slope estimate. The four variables that were used for propensity matching were not used for multivariable analyses. Due to large sample size, variables having a p value of ≤ 0.05 on univariate analysis were considered as candidates in the multivariable logistic regression model building. Further analysis was also performed in a similar manner using univariate and multivariable linear regression with AE days as a continuous outcome. The area under ROC curve (receiver operating characteristics curve) for the model used for matching was 0.733, for the multivariable model with AKI was 0.611 and for renal disease was 0.600. All tests were two sided and p values less than 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Inc, Cary, NC).
Results
The initial total numbers of ICU admissions during our study period were 152,999. AE was present in 57,726 (37.7%) ICU admissions and among them 4571 (7.9%) had received cefepime. The prevalence of AE among patients who received cefepime was higher in patients who developed AKI [3508 (64.4%) vs 1937 (35.6%), p < 0.001] and those who had renal disease (AKI and CKD) [3130 (65.8%) vs 1625 (34.2%), p < 0.001].
For the case–control analyses, we identified 14,645 cases that were diagnosed with AE and 14,645 propensity score matched controls. The details of the cases and controls are shown in Table 2. Cefepime was administered to 1241 (4.2%) patients and they were more likely to develop AE (4.9% vs 3.6%, OR 1.4 [95% CI 1.2–1.5], p < 0.001). The prevalence of AE among patients who received cefepime was higher in patients who developed AKI [488 (64.7%) vs 225 (46.2%), p < 0.001] and those with renal disease [522 (62.7%) vs 191 (46.8%), p < 0.001].
On multivariable analyses, the factors independently associated with AE included shock, midazolam infusion, AKI, renal disease (acute and chronic kidney injury), and cefepime use. Cefepime was associated with AE with an OR 1.24 (1.10–1.39) in patients with AKI and with an OR of 1.25 (1.11–1.40) in patients with renal disease as shown in Table 3.
The median duration of AE in our cohort was 17(IQR 4–52) hours. Patients who received cefepime had a longer AE duration [5 (IQR 4–41) vs 4 (IQR 4–17) hours, p < 0.001] (supplementary table 2). Among patients who received Cefepime, AE duration was longer in patients with AKI [7.2 (IQR 4–65) vs 4 (IQR 4–19) hours, p < 0.001] and those with renal disease [5 (IQR 4–53) vs 4 (IQR 4–21) hours, p < 0.001].
The factors associated with AE duration on the multivariable analyses included shock, use of midazolam, use of cefepime, and the presence of AKI renal disease. Cefepime was associated with prolonged AE duration in patients with AKI [parameter estimate 0.73 (95% CI 0.54–0.92), p < 0.0001] and those with renal disease [parameter estimate 0.74 (95% CI 0.55–0.93), p < 0.0001] (supplementary table 3).
To further validate our findings, we performed sensitivity analyses after excluding patients with dementia (n = 505) and excluding the patients who did not receive any antibiotics (n = 13,881) during the ICU admission. The association of Cefepime with AE remained present. The results of these analyses are presented in the supplementary tables 4–7.
Discussion
Our large retrospective case–control study shows that cefepime use is associated with AE and longer AE duration in critically ill patients. Although it is more common in patients with acute and chronic renal disease, cefepime is also associated with AE in patients with normal renal function. In our study, the associations of cefepime with AE occurrence and AE duration were independent of other factors also associated with higher risk of AE, such as age, comorbidities, acute illness severity, shock, AKI, renal disease and infusion of midazolam. These findings were also present on sensitivity analyses after excluding patients with dementia and after excluding patients who did not receive antibiotics.
Cefepime has been known to cause neurotoxicity, especially patients with renal dysfunction in whom the dose of cefepime is not adequately adjusted, though nearly 15% of patients may still develop CIN despite adequate dosing [3,4,5, 7]. CIN has been proposed to be caused by competitive binding of cefepime to GABA class receptors, thus interfering with inhibitory GABA neurotransmission and leading to central excitation [17]. Furthermore the presence of inflammatory conditions, organ acid accumulations and renal dysfunction may predispose patients to disruptions in the blood brain barrier allowing increased CNS penetration of cefepime. [18]
The true prevalence of CIN is unclear. Ranges have varied between 1 and 15% across various studies [3, 4, 8]. In our initial cohort of nearly 160,000 patients who were admitted to the ICU, we found AE in 7.9% of patients exposed to cefepime and this rate was nearly twice as high in patients with renal failure. Yet, our study design can only describe associations and cannot establish causation.
The average time from the administration of cefepime to the development of signs of neurotoxicity was 4 days (IQR 1–6 days) and there was a gap of several days between the development of neurological signs and symptoms and cefepime discontinuation [3]. Delayed recognition of neurotoxicity may be more common in patients who develop AE without positive neurological signs. Reported interventions for cefepime neurotoxicity include discontinuation of the drug or reduction in dose, treatment with one or more antiepileptic drugs and hemodialysis cases [3, 19].
A study by Grahl et al. found no association between cefepime use and the occurrence of delirium in the ICU [9]. There were some key differences between the study by Grahl et al. and ours, although both of these studies excluded patients with primarily neurological disease. First, our study had a much larger size and therefore it may have identified associations that could have been missed on a smaller cohort. In fact, Grahl et al. found associations with delirium with first, second, and third-generation cephalosporins (likely more commonly used), but not with cefepime, suggesting that a methodological rather than a biological reason could explain the findings. Second, our study benefited from a case–control design, which increases the solidity of the analyses [20]. Third, our population had more comorbidities [Charlson score of 5.0 (IQR 3.0–7.0) vs 2 (IQR 1–4) in the cohort examined by Grahl et al.]. Lastly, our finding that cefepime use was not only associated with the presence of AE, but also with its duration, lends further credence to the validity of the association.
The main limitations of our study are inherent to its retrospective design. While we tried to control for the most pertinent variables, there is a possibility that there might have potentially confounding factors, both known and unknown that might have influenced the occurrence of AE, especially in the ICU setting. Notably, we did not evaluate the effect of the concurrent use of other antimicrobials. Therefore, causality cannot be concluded from our results and the association of cefepime with AE requires further evaluation. Our hospital is a large referral center and hence the findings of this study might be affected by referral bias and might not be representative of the experience in other settings. Serum cefepime levels were not checked in our hospital practice and electroencephalogram (EEG) was not done routinely among these patients; hence we did not include that data in our analyses. Ideally, risk factors for AE should be examined starting from a large cohort of critically patients and comparing different exposures (such as antibiotics) in patients who develop AE vs those who do not; yet, we think that the clear differences in incidence and duration of AE in relation to cefepime exposure observed in our cohort are convincing. Despite these limitations, this is the largest case control study to our knowledge looking at the role of cefepime in the prevalence and duration of AE. Further prospective studies are needed to confirm these findings.
Conclusion
Cefepime use is associated with a greater likelihood of AE and longer AE duration in critically ill patients. These associations are stronger among patients with impaired renal function, but can also occur in patients without renal impairment. While our study cannot determine cefepime neurotoxicity as a cause of AE, the strength and independence of the associations identified in our analyses suggest that cefepime neurotoxicity should be considered as a potential modifiable factor for the development and persistence of AE in the ICU.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Singh TD, O’Horo JC, Gajic O, et al. Risk factors and outcomes of critically ill patients with acute brain failure: a novel end point. J Crit Care. 2018;43:42–7.
Slooter AJC, Otte WM, Devlin JW, et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 2020;46(5):1020–2.
Payne LE, Gagnon DJ, Riker RR, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017;21(1):276.
Fugate JE, Kalimullah EA, Hocker SE, et al. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit Care. 2013;17(6):R264.
Capparelli FJ, Diaz MF, Hlavnika A, et al. Cefepime- and cefixime-induced encephalopathy in a patient with normal renal function. Neurology. 2005;65(11):1840.
Gangireddy VG, Mitchell LC, Coleman T. Cefepime neurotoxicity despite renal adjusted dosing. Scand J Infect Dis. 2011;43(10):827–9.
Lindsay H, Gruner S, Brackett J. Cefepime-induced neurotoxicity despite dose adjustment for renal disease: a brief report and review of the literature. J Pediatric Infect Dis Soc. 2017;6(2):199–201.
Huwyler T, Lenggenhager L, Abbas M, et al. Cefepime plasma concentrations and clinical toxicity: a retrospective cohort study. Clin Microbiol Infect. 2017;23(7):454–9.
Grahl JJ, Stollings JL, Rakhit S, et al. Antimicrobial exposure and the risk of delirium in critically ill patients. Crit Care. 2018;22(1):337.
Reddy DR, Singh TD, Guru PK, et al. Identification of acute brain failure using electronic medical records. J Crit Care. 2016;34:12–6.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–61.
Herasevich, V., Kor, D.J., Li, M.Pickering, B.W. ICU data mart: a non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform 2011;28(11):42, 4–5.
Herasevich V, Pickering BW, Dong Y, Peters SG, Gajic O. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85(3):247–54.
Ahmed A, Vairavan S, Akhoundi A, et al. Development and validation of electronic surveillance tool for acute kidney injury: a retrospective analysis. J Crit Care. 2015;30(5):988–93.
Keegan MT, Whalen FX, Brown DR, Roy TK, Afessa B. Acute physiology and chronic health evaluation (APACHE) III outcome prediction after major vascular surgery. J Cardiothorac Vasc Anesth. 2008;22(5):713–8.
Harrison AM, Yadav H, Pickering BW, Cartin-Ceba R, Herasevich V. Validation of computerized automatic calculation of the sequential organ failure assessment score. Crit Care Res Pract. 2013;2013:975672.
Durand-Maugard C, Lemaire-Hurtel AS, Gras-Champel V, et al. Blood and CSF monitoring of cefepime-induced neurotoxicity: nine case reports. J Antimicrob Chemother. 2012;67(5):1297–9.
Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–96.
Li, H.T., Lee, C.H., Wu, T., et al. Clinical, electroencephalographic features and prognostic factors of cefepime-induced neurotoxicity: a retrospective study. Neurocrit Care 2019.
Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234–42.
Funding
No funding was needed for this study.
Author information
Authors and Affiliations
Contributions
AAR takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis. AAR had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. TDS, JOH, CND and JM contributed substantially to the study design, data analysis and interpretation, and the writing and final editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Heikinki. The protocol was approved by the institutional review boards of Mayo Clinic, Rochester for the use of patients’ medical records. The manuscript has not been published elsewhere and is not under consideration by another journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, T.D., O’Horo, J.C., Day, C.N. et al. Cefepime is Associated with Acute Encephalopathy in Critically Ill Patients: A Retrospective Case–Control Study. Neurocrit Care 33, 695–700 (2020). https://doi.org/10.1007/s12028-020-01035-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01035-w