Abstract
Background
Biomarkers indicative of intracerebral hemorrhage (ICH) may help triage acute stroke patients in the pre-hospital phase. We hypothesized that serum concentration of glial fibrillary acidic protein (GFAP) in combination with ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1), measured by a rapid bio-assay, could be used to distinguish ICH from ischemic stroke.
Methods
This prospective two-center study recruited patients with a clinical diagnosis of acute stroke both in the pre-hospital phase and at hospital admission (within 4 and 6 h after symptom onset, respectively). Blood samples were analyzed for concentrations of GFAP and UCH-L1 using ELISA techniques. The reference standard was the diagnosis of ICH, ischemic stroke, or stroke mimicking condition achieved after clinical workup including brain imaging.
Results
A total of 251 patients were included (mean age [± SD] 72 ± 15 years; 5 ICH, 23 ischemic strokes and 14 stroke mimics in the pre-hospital part; and 59 ICH, 148 ischemic strokes and 2 stroke mimics in the in-hospital part). Mean delay (± SD) from symptom onset to blood withdrawal was 130 ± 79 min for the pre-hospital patients and 136 ± 86 min for the in-hospital patients. Both GFAP and UCH-L1 serum concentrations were higher in patients having ICH as compared to other diagnoses (GFAP: median 330 ng/L [interquartile range 64–7060, range 8–56,100] vs. 27.5 ng/L [14–57.25, 0–781], p < 0.001; UCH-L1: 401 ng/L [265–764, 133–1812] vs. 338 ng/L [213–549.5, 0–2950], p = 0.025). Area-under-the-curve values were 0.866 (95% CI 0.809–0.924, p < 0.001) for GFAP, and 0.590 (0.511–0.670, p = 0.033) for UCH-L1. Regarding overall diagnostic accuracy, UCH-L1 did not add significantly to the performance of GFAP.
Conclusions
GFAP may differentiate ICH from ischemic stroke and stroke mimics. A point-of-care test to distinguish between ischemic and hemorrhagic strokes might facilitate triage to different treatment pathways or locations, or be used to select patients for trials of ultra-early interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In acute stroke, biomarkers able to differentiate “true” stroke patients from stroke mimicking conditions (such as migraine and epileptic seizures) and biomarkers differentiating between ischemic stroke and intracerebral hemorrhage (ICH) in the pre-hospital phase would speed up triage and could allow diagnosis-specific treatment [1, 2].
The US Food and Drug Administration (FDA) recently approved a combined biomarker test including glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1) to forgo computed tomography (CT) imaging in patients with mild traumatic brain injury (Banyan BTI™) [3]. GFAP is a cytoskeletal protein predominantly expressed in astrocytes. It is not released under physiological conditions. Thus, blood levels in healthy individuals are very low [4, 5]. UCH-L1 is an enzyme found in neurons and neuroendocrine cells in high quantity [6]. It is maintaining synaptic plasticity and self-repair mechanisms.
The value of GFAP as a biomarker in acute stroke has been studied extensively in recent years. Several multicenter studies demonstrated a rapid increase of GFAP in blood samples of ICH patients, compared to slow release in ischemic stroke [7,8,9,10]. Within the first hours after symptom onset, elevated GFAP serum concentrations indicate ICH with a moderate sensitivity of about 75–90% and a specificity of > 95% [7,8,9,10]. GFAP levels strongly correlate with ICH volume [7, 9, 11]. Fewer data are available for UCH-L1 in stroke. In an animal model, UCH-L1 concentrations were found elevated in rats subjected to ischemic stroke while remaining normal in rats with ICH [12]. UCH-L1 has also been tested in combination with GFAP in a pilot study performed in stroke patients with various time intervals between symptom onset and blood withdrawal [13]. Here, these results indicated no usefulness of UCH-L1 regarding the differentiation of ischemic stroke and ICH.
The aim of this study was to explore the diagnostic value of the combined GFAP/UCH-L1 biomarker test in the context of acute stroke (i.e., for differentiating ICH from ischemic stroke and stroke mimics). We were particularly interested to evaluate the markers in the very early time window. We therefore included blood samples collected both in the pre-hospital phase (in a mobile stroke unit) and at hospital admission.
Methods
Study Design
The “Biomarker for Rapid Diagnosis of Stroke III” (BE FAST III) study was designed as a two-center investigation including acute stroke patients both in a pre-hospital (conducted at Østfold county, Norway, as a part of the Norwegian Acute Stroke Prehospital Project [NASPP]) [14] and an in-hospital (conducted at the Department of Neurology, Goethe University, Frankfurt, Germany) setting. By means of a mobile stroke unit (MSU), patients were recruited for the pre-hospital phase from October 2014 to February 2016. The in-hospital inclusion of patients was conducted from October 2013 to March 2017. Based on the findings of previous studies [7, 9] we anticipated a mean serum GFAP concentration of 200 ng/L (± 350) for ICH patients and of 10 ng/L for ischemic stroke patients. We expected an enrollment ratio between ICH and ischemic stroke of 1:7. For a power of 80% to detect a two-sided p value < 0.05, the minimum targeted number of patients was calculated to be n = 240 (30 ICH patients and 210 ischemic stroke patients) using an online sample size calculator (www.clincalc.com). The study protocol was approved by the institutional review board of both the Goethe-University Frankfurt am Main and the regional committees for medical and health research ethics in Norway. The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. It followed the guidelines of the Standards for Reporting of Diagnostic Accuracy initiative [15]. The standard of stroke care in the participating facilities (MSU or emergency room) includes the drawing of a blood sample upon initial patient contact for routinely diagnostic reasons. An aliquot of this sample was preserved for study purposes (prior to study inclusion). During hospital stay, written informed consent was obtained from patients and legal representatives, respectively. Withdrawal of consent led to the proper discard of the stored aliquot.
Inclusion of Patients
All eligible patients were screened for neurological deficits suggestive of acute stroke. Inclusion criteria were: (I) clinical symptoms compatible with acute stroke, (II) age ≥ 18 years, (III) time from symptom onset (or last seen well) to ambulance ≤ 4 h for the pre-hospital setting, and time from symptom onset (or last seen well) to hospital admission ≤ 6 h for the in-hospital setting. For the in-hospital inclusion, the patients needed to have (IV) a National Institute of Health Stroke Scale (NIHSS) score at hospital admission of ≥ 4 points. There was no NIHSS limit for pre-hospital inclusion of patients. Exclusion criteria were: (I) stroke (including ischemic stroke and ICH) and transient ischemic attack in the past 3 months, (II) traumatic brain injury in the past 3 months (including known head concussion at symptom onset of the current stroke), and (III) brain tumor at any time in the past medical history. It is known that traumatic brain injury and malignant glioma increase serum GFAP and UCH-L1 concentrations, respectively [16, 17]. Pregnant females or females < 50 years with uncertainty of pregnancy were not included.
The following clinical baseline variables were recorded: age, sex, admission NIHSS, presence of a hemiparesis at hospital admission, presence of clinical signs indicating hemispheric involvement (aphasia, neglect, homonymous hemianopia, forced gaze deviation, impairment of consciousness), history of arterial hypertension, diabetes or hyperlipidemia (defined according to current guidelines) [18], and time interval between symptom onset and hospital admission. All patients received brain imaging in the acute phase (i.e., either in the MSU or after hospital admission. However, the primary endpoint of the study was the final diagnosis at hospital discharge, categorized as ischemic stroke (including transient ischemic attack), ICH, or stroke mimic. Stroke mimics were defined as conditions clinically imitating the symptoms of acute stroke such as postictal hemiparesis, otogenic vertigo, or transient focal neurological deficits secondary to systemic metabolic alterations. The diagnosis was established according to the International Classification of Diseases (revision 10) based on all available clinical data, brain imaging, and laboratory results.
Blood Sampling
Venous blood was drawn in the MSU and at hospital admission on a routine basis using 10-ml serum tubes with clot activator (BD Vacutainer). Blood samples were delivered to the laboratory at the respective hospitals for centrifugation within 2 h after collection. Serum was aliquoted in 0.5 mL aliquots and frozen down, first at − 20 °C and then moved to − 80 °C for long-term storage until analysis. A temperature-controlled transport on dry ice was later used for shipment. It has been shown previously that GFAP is stable for several days at 4 °C. Up to four freezing/thawing cycles do not influence GFAP concentrations [19].
GFAP and UCH-L1 Measurements
Determination of GFAP and UCH-L1 serum concentrations was performed at Banyan Biomarkers (San Diego, USA) by means of enzyme-linked immunosorbent assays (ELISA). For detection of UCH-L1 and GFAP, each assay used different proprietary mouse monoclonal antibodies for both solid-phase immobilization and for detection. Standard curves using native GFAP and recombinant UCH-L1 were generated for each assay, and quantitative determination of the biomarker levels in the de-identified samples was based on a quadratic curve fit formula. Banyan has successfully used these sensitive biomarker assays in a series of previously published studies in adults with traumatic brain injury and neurodegenerative diseases [16, 20]. Serum samples were processed and assayed by laboratory technicians who were blinded to all clinical information.
ICH Volume Quantification
According to the study protocol at least one brain scan, irrespective of modality (magnetic resonance imaging, CT), was required either at hospital admission or already in the mobile stroke unit. Location of ICH was determined on the first available brain imaging and classified into “lobar” or “deep”. Intraventricular hemorrhage expansion, if present, was also documented. ICH volumes were quantified by means of the (a × b × c)/2 method [21]. In patients with ischemic strokes, infarct sizes were not quantified.
Statistical Analysis
Statistical analyses were performed using IBM® SPSS® Statistics, Version 22 (Statistical Package for the Social Sciences, Armonk, NY, USA). Because GFAP and UCH-L1 serum concentrations were not normally distributed between individuals, statistical comparisons were made using the nonparametric Mann–Whitney U test. Correlation analyses were performed by means of the nonparametric Spearman rank test. The optimal serum GFAP and UCH-L1 cutoff level to distinguish ICH from ischemic stroke and stroke mimicking conditions were calculated using receiver operating characteristic (ROC) curve analysis. Area-under-the-curve (AUC) values were also calculated. Sensitivity and specificity measures and the positive predictive value (PPV) and the negative predictive value (NPV) were derived from cross-tabulations. For the combined analysis, a logistic regression model with diagnosis as the dependent variable and GFAP and UCH-L1 as the independent variables was used. ROC analysis and diagnostic accuracy measures were then calculated based on the predicted probability values. A significance level of alpha = 0.05 was chosen for all tests.
Results
A total of 251 patients were included in this study. Mean age (± SD) was 72 ± 15 years, and 56% were female. Forty-two patients were recruited in the pre-hospital part of the study, and 209 patients were recruited at hospital admission. The diagnostic workup revealed 5 ICH, 23 ischemic strokes and 14 stroke mimics in the pre-hospital part, and 59 ICH, 148 ischemic strokes and 2 stroke mimics in the in-hospital part. Mean delay (± SD) from symptom onset to blood withdrawal was 130 ± 79 min for the pre-hospital patients and 136 ± 86 min for the in-hospital patients. The baseline variables of the two collectives are presented in Table 1a, b.
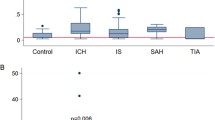
Serum GFAP was higher in ICH patients as compared to ischemic stroke patients and mimics (median 330 ng/L [interquartile range 64–7060, range 8–56,100] vs. 27.5 ng/L [14–57.25, 0–781], p < 0.001). Serum UCH-L1 was also higher in ICH patients as compared to ischemic stroke patients and mimics, but the relative difference was markedly smaller than for GFAP (401 ng/L [265–764, 133–1812] vs. 338 ng/L [213–549.5, 0–2950], p = 0.025; Fig. 1a, b).
a Boxplots displaying the distribution of GFAP values for patients with ischemic stroke, intracerebral hemorrhage (ICH) and stroke mimics. The y-axis of the main figure is log-scaled. The inset graph has an arithmetic y-axis. The box boundaries mark the 25th and 75th percentile, the line within the box indicates the median. Whiskers above and below the box mark the 90th and 10th percentiles. Outliers (1.5–3 × interquartile range) are marked with circles, extreme values (> 3 × interquartile range) are marked with asterisks. b Boxplots displaying the distribution of UCH-L1 values for patients with ischemic stroke, intracerebral hemorrhage (ICH) and stroke mimics. For boxplot features, see a. c ROC analyses of GFAP and UCH-L1 for the differentiation of intracerebral hemorrhage from ischemic stroke and stroke mimics
ROC analysis revealed AUC values for GFAP of 0.866 (95% CI 0.809–0.924, p < 0.001) and for UCH-L1 of 0.590 (0.511–0.670, p = 0.033; Fig. 1c). A GFAP serum concentration of 72 ng/L was identified as optimal cutoff point for differentiating ICH from ischemic stroke and stroke mimics (sensitivity 0.75, specificity 0.84; for PPV and PNV see Table 2). Specificity was 0.95 at a cutoff point of 149 ng/L (sensitivity 0.62) and 1.0 at a cutoff point of 789 ng/L (sensitivity 0.46). The optimal cutoff point for UCH-L1 was 291 ng/L (sensitivity 0.73, specificity 0.45). Evaluating both biomarkers together in a combined model only marginally improved the AUC compared to GFAP alone (0.873 [0.817–0.929], p < 0.001). Values for optimal sensitivity and specificity were 0.86 and 0.73, respectively (Table 2).
Diagnostic accuracy was comparable for GFAP in both younger and older patients, in both patients having a less severe and more severe neurological deficit at inclusion, and in both patients included at earlier or later time points (Fig. 2).
ROC analyses of GFAP (black lines) and UCH-L1 (red lines) for the differentiation of intracerebral hemorrhage from ischemic stroke and stroke mimics in different strata (age below or above the mean age of 75 years, NIHSS below or above the median value of 10, time delay to study inclusion below or above the median of 120 min) (Color figure online)
We obtained significant correlations between GFAP and UCH-L1 serum concentrations for patients with ICH (Spearman rho 0.246, p = 0.001) as well as for patients with ischemic stroke (0.296, p = 0.018). GFAP was strongly correlated with ICH volume (0.679, p < 0.001). For UCH-L1, a significant correlation with ICH volume was not observed (0.161, p = 0.207). Table 3 depicts GFAP levels of ICH patients by decile and the corresponding ICH volumes. Neither for GFAP nor for UCH-L1 we found significant associations between serum concentrations and ICH location (mean GFAP [± SD]: lobar: 8898 ± 16,928 ng/L, deep: 6780 ± 14,131 ng/L, p = 0.406; mean UCH-L1 [± SD]: lobar: 530 ± 380 ng/L, deep: 626 ± 449 ng/L, p = 0.376).
GFAP levels were correlated to NIHSS values both for ICH and ischemic stroke patients (see Fig. 3a). A relationship between GFAP levels and age, time to blood sampling, and creatinine values was not observed for ICH patients (see Fig. 3a). UCH-L1 values correlated with age, particularly for ischemic stroke patients. In ICH, UCH-L1 values were associated with NIHSS values. Again, no clear association was obtained for time to blood sampling and creatinine values (see Fig. 3b).
We noticed seven patients with ischemic stroke having very high UCH-L1 concentrations (1905–2950 ng/L; classified as extreme values by nonparametric statistics; see Fig. 1b). A closer look into these cases revealed that all of them were patients older than 80 years (mean age [± SD] 86 ± 5 years). NIHSS values at admission ranged between 4 and 24. Mean delay (± SD) to blood sampling was 146 ± 93 min. Mean creatinine values were within normal ranges (1.1 ± 0.4 mmol/L), but mean CRP values were elevated (4.0 ± 3.8 mg/dL).
Discussion
This study prospectively evaluated the diagnostic value of a combined GFAP/UCH-L1 biomarker test in the context of acute stroke. We confirmed that diagnostic accuracy of GFAP serum concentrations to differentiate ICH from ischemic stroke and stroke mimics was high through all age categories, time to blood sampling strata and degrees of clinical severity. UCH-L1 values also differed between ICH and ischemic stroke patients, but had much less discriminatory potential.
Besides a number of exploratory studies, two larger multicenter trials have been published investigating GFAP in the context of acute stroke [7, 9]. Despite using different prototype GFAP assays, those results are well comparable with our current findings. Consistently, all studies reported a moderate sensitivity (75–80%) for diagnosing ICH at high specificity levels. Looking closer into our dataset, 25% of our ICH patients revealed low GFAP values (below the cutoff). Considering that the discriminatory potential overall did not correlate with time to blood sampling and the severity of clinical symptoms, the reason for this is still not entirely clear. A strong correlation exists between ICH volume and GFAP levels which was reconfirmed in our study. This suggests that very small bleedings may be missed due to little brain tissue destruction and limited GFAP release. The same might be true for “pure” intraventricular hemorrhage (without parenchymal affection), which did not occur in our dataset. Thus, a certain “natural lower limit” of ICH volume may exist for this test. On the other side, with the development of better detection techniques, there is a good chance for improving sensitivity in the future. Regarding specificity, all ischemic stroke patients had low to slightly elevated values. Setting the cutoff point to 789 ng/L led to both a specificity and a positive predictive value (PPV) of 100%, meaning that all patients with GFAP values above had ICH. The NPV however, remained at 84%. Therefore, this test may be better employed to identify patients with ICH than to rule out ICH.
The UCH-L1 results are more difficult to interpret in the context of stroke. Overall, we found higher UCH-L1 values in the ICH group as compared to ischemic stroke and stroke mimics. However, the discriminatory potential was low in comparison to GFAP, and a few patients having ischemic stroke provided strongly elevated serum concentrations. Interestingly, a similar finding was reported in a smaller single-center study on UCH-L1 in acute stroke [13]. In our dataset, all ischemic stroke patients classified as “outliers” in terms of UCH-L1 concentrations aged above 80 years. Remarkably, other clinical variables did not differ significantly from ischemic stroke patients with low UCH-L1 values. As mentioned above, UCH-L1 has been evaluated as a marker of traumatic brain injury [16, 20]. Thus, one possibility explaining these outlier UCH-L1 values is that elderly patients fell during the occurrence of the stroke. In light of the literature, other preexisting or concomitant conditions leading to blood–brain barrier damage might be responsible for this finding (e.g., epileptic seizures) [22]. Since the molecular mass of UCH-L1 is small (24 kDa), it may permeate the blood–brain barrier easily [22].
GFAP is expressed in high quantity in astroglial cells to maintain the cytoskeleton. The release of GFAP in acute ICH is assumed to be a consequence of the “passive” destruction of astroglial cells by means of the expanding hematoma rather than of an active release due to astrogliosis [4]. The strong correlation between ICH volume and GFAP serum levels supports this assumption [7, 9]. As a consequence of this “passive” release in parallel with the evolving hematoma, GFAP levels might be lower in the very early time window after ICH onset [4]. Indeed, one study suggested that diagnostic accuracy is best between 2 and 6 h after symptom onset [23]. However, this has not been confirmed in larger studies, where a similar discriminatory potential was found in patients included within 60 min compared to the remaining patients [7, 9]. More data on the release kinetics of GFAP and the diagnostic accuracy in the early phase of stroke are warranted. In ischemic stroke, a delayed release of GFAP is observed, due to the gradual occurrence of astroglial necrosis after vessel occlusion (usually not before 6 to 12 h after stroke onset) [4]. UCH-L1 is abundantly present in neurons and neuroendocrine cells [6]. Given that this protein accounts for 1–2% of total brain protein, it is comprehensible that the “crude” destruction of neurons induced by the expanding hematoma leads to the release of the protein from the brain into the blood in ICH patients. On the other side, a strong correlation between ICH volume and UCH-L1 values was not observed in our study, suggesting a more complex interaction between UCH-L1 serum levels and brain hemorrhage. Early cerebral ischemia may also be a trigger for UCH-L1 release [24], particularly in elderly patients. In contrast, GFAP levels do not increase in the acute phase of ischemic stroke (even in case of severe perfusion deficits) if cellular necrosis has not yet occurred (i.e., the growing of the “infarct core”) [4, 25]. For acute traumatic brain injury patients, different patterns of biomarker release depending on the predominant injury mechanism have been suggested. Diffuse injury was associated with primarily neuronal cell death with higher levels of UCH-L1 release compared to GFAP (“glial neuronal ratio” < 1). In contrast, focal mass lesions resulted in pan-necrosis. Here, both glial and neuronal proteins are released, with an increased level of GFAP compared to UCH-L1 (“glial neuronal ratio” > 1) [26, 27].
Shortcomings in sensitivity make it impossible to base thrombolysis treatment decisions on such a test, as ICH cannot be ruled out with certainty [28]. However, elevated GFAP values increase the probability of ICH from 10 to 20% (based on epidemiology) to > 95%, depending on the cutoff value chosen. This may help to triage patients and to initiate simple therapeutic measures including blood pressure lowering and anticoagulation reversal. In fact, several large ICH trials (e.g., on blood pressure reduction and coagulation activation) failed to show a benefit in terms of clinical outcome, but showed some tendency for the respective measure to reduce hematoma growth [29,30,31]. Thus, a test able to identify ICH patients rapidly may allow the application of therapeutic strategies at much earlier time points and earlier transport to a comprehensive stroke center with additional neurosurgical capacity.
Our study has limitations. Our intention when designing the study protocol was to cover a wide range of the stroke treatment time window. In particular, we aimed at including a relevant number of patients with very short symptom onset to blood sampling times (in order to potentially establish this test on a point-of-care platform in the pre-hospital setting in the future). Therefore, both pre-hospital and in-hospital sample collection was performed. However, rather unexpectedly, the mean time difference between the samples collected at hospital admission and in the MSU was small. The reason for this is that stroke patients recruited within the metropolitan area of Frankfurt have rapid access to hospital stroke care whereas recruitment in the geographically more widespread area covered by NASPP [14] is associated with delays compared to MSUs operating in large cities. Thus, the “true” diagnostic accuracy for patients included in a MSU at shorter time windows cannot be assessed from this dataset. On the other side, our analysis did not reveal signals that GFAP concentrations and diagnostic accuracy, respectively, change over time. Interestingly, the in-hospital patients (Frankfurt cohort) were older, had larger bleedings and more frequently a cortical ICH location (see Table 1a, b) compared to the pre-hospital patients. These imbalances likely result from the fact that the University hospital is served by several primary and secondary care hospitals, leading to a higher proportion of elderly and more severely affected patients. There could be other independent pathophysiological confounders influencing the serum concentrations of the respective biomarker, such as systemic infection, cerebral small vessel disease, hypercholesterolemia or smoking. The strengths of our study are the application of an advanced biochemical test for GFAP and UCH-L1 that was recently approved for use in patients with traumatic brain injury by the FDA [3]. Furthermore, the study concept included a pre-hospital and an in-hospital part, followed a clear pathophysiological rationale, and used well-established inclusion criteria for biomarker sampling in acute stroke.
Conclusion
In summary, the prospective evaluation of a newly FDA-approved traumatic brain injury biomarker test in the setting of stroke confirms a high diagnostic accuracy for GFAP to differentiate ICH and ischemic stroke. For UCH-L1, we postulate more complex release mechanisms, which are—in ICH cases—less related to hematoma volume alone. The current study is still a proof-of-concept trial, since the biomarker analysis has been performed after the sample collection phase and not directly “at the scene” in the emergency room or in the ambulance car. Whether the future application of GFAP on a point-of-care system can provide the opportunity to rapidly establish the diagnosis of ICH and to initiate diagnosis-specific measures in the pre-hospital phase needs to be explored in future trials.
Abbreviations
- GFAP:
-
Glial fibrillary acidic protein
- UCH-L1:
-
Ubiquitin carboxy-terminal hydrolase-L1
- ICH:
-
Intracerebral hemorrhage
- NIHSS:
-
National Institute of Health Stroke Scale
- AUC:
-
Area under the curve
- IQR:
-
Interquartile range
References
Foerch C, Montaner J, Furie KL, Ning MM, Lo EH. Invited article: searching for oracles? Blood biomarkers in acute stroke. Neurology. 2009;73:393–9.
Ng GJL, Quek AML, Cheung C, Arumugam TV, Seet RCS. Stroke biomarkers in clinical practice: a critical appraisal. Neurochem Int. 2017;107:11–22.
https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm596531.htm. Accessed 3 Jan 2019.
Brunkhorst R, Pfeilschifter W, Foerch C. Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage-pathophysiological background and clinical findings. Transl Stroke Res. 2010;1:246–51.
Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res. 2000;25:1439–51.
Bishop P, Rocca D, Henley JM. Ubiquitin C-terminal hydrolase L1 (UCH-L1): structure, distribution and roles in brain function and dysfunction. Biochem J. 2016;473:2453–62.
Foerch C, Niessner M, Back T, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem. 2012;58:237–45.
Katsanos AH, Makris K, Stefani D, et al. Plasma glial fibrillary acidic protein in the differential diagnosis of intracerebral hemorrhage. Stroke. 2017;48:2586–8.
Luger S, Witsch J, Dietz A, et al. Glial fibrillary acidic protein serum levels distinguish between intracerebral hemorrhage and cerebral ischemia in the early phase of stroke. Clin Chem. 2017;63:377–85.
Perry LA, Lucarelli T, Penny-Dimri JC, et al. Glial fibrillary acidic protein for the early diagnosis of intracerebral hemorrhage: systematic review and meta-analysis of diagnostic test accuracy. Int J Stroke. 2019;14:390–9.
Foerch C, Curdt I, Yan B, et al. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J Neurol Neurosurg Psychiatry. 2006;77:181–4.
Ren C, Zoltewicz S, Guingab-Cagmat J, et al. Different expression of ubiquitin C-terminal hydrolase-L1 and alphaII-spectrin in ischemic and hemorrhagic stroke: potential biomarkers in diagnosis. Brain Res. 2013;1540:84–91.
Ren C, Kobeissy F, Alawieh A, et al. Assessment of serum UCH-L1 and GFAP in acute stroke patients. Sci Rep. 2016;6:24588.
Hov MR, Zakariassen E, Lindner T, et al. Interpretation of brain CT scans in the field by critical care physicians in a mobile stroke unit. J Neuroimaging. 2018;28:106–11.
Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem. 2003;49:1–6.
Bazarian JJ, Biberthaler P, Welch RD, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol. 2018;17:782–9.
Jung CS, Foerch C, Schanzer A, et al. Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain. 2007;130:3336–41.
Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947.
Missler U, Wiesmann M, Wittmann G, Magerkurth O, Hagenstrom H. Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin Chem. 1999;45:138–41.
Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73:551–60.
Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5.
Blyth BJ, Farahvar A, He H, et al. Elevated serum ubiquitin carboxy-terminal hydrolase L1 is associated with abnormal blood-brain barrier function after traumatic brain injury. J Neurotrauma. 2011;28:2453–62.
Dvorak F, Haberer I, Sitzer M, Foerch C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc Dis. 2009;27:37–41.
Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–56.
Wunderlich MT, Wallesch CW, Goertler M. Release of glial fibrillary acidic protein is related to the neurovascular status in acute ischemic stroke. Eur J Neurol. 2006;13:1118–23.
Mondello S, Jeromin A, Buki A, et al. Glial neuronal ratio: a novel index for differentiating injury type in patients with severe traumatic brain injury. J Neurotrauma. 2012;29:1096–104.
Mondello S, Papa L, Buki A, et al. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care. 2011;15:R156.
Lorenz MW, Lauer A, Foerch C. Quantifying the benefit of prehospital rapid treatment in acute stroke: benchmark for future innovative clinical trials. Stroke. 2015;46:3168–76.
Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–65.
Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37.
Qureshi AI, Palesch YY, Barsan WG, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033–43.
Funding
The Norwegian Air Ambulance Foundation (non-profit organization) sponsored the prehospital part of the study. For the inhospital part of the study there was no special funding. Banyan Biomarkers provided the GFAP and UCH-L1 measurements free of charge.
Author information
Authors and Affiliations
Consortia
Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following three requirements: (a) substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article critically for important intellectual content; and (c) final approval of the published article.
Corresponding author
Ethics declarations
Conflict of interest
FOB reports personal fees from Medtronic, non-financial support from Boehringer Ingelheim, grants from Stryker, outside the submitted work. JHS reports non-financial support from Boehringer Ingelheim and from Biogen (travel expenses), outside the submitted work. SPR is an employee of Banyan Biomarkers, and Banyan Biomarkers has submitted patents relative to the use of GFAP and UCH-L1. CF reports non-financial support from Banyan Biomarkers during the conduct of the study (Banyan Biomarkers provided the GFAP and UCH-L1 measurements free of charge), personal fees from Boehringer Ingelheim (advisory board honoraria), personal fees from Bristol Myers Squibb (speaker honoraria), personal fees from Boehringer Ingelheim (lecturer—stroke school), outside the submitted work. CF has a patent GFAP for the identification of intracerebral hemorrhage (EP1519194A1). All other authors report no conflicts of interest.
Ethical Approval
The study protocol was approved by the institutional review board of both the Goethe-University Frankfurt am Main and the regional committees for medical and health research ethics in Norway. The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
Informed consent was obtained from patients and legal representatives, respectively.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luger, S., Jæger, H.S., Dixon, J. et al. Diagnostic Accuracy of Glial Fibrillary Acidic Protein and Ubiquitin Carboxy-Terminal Hydrolase-L1 Serum Concentrations for Differentiating Acute Intracerebral Hemorrhage from Ischemic Stroke. Neurocrit Care 33, 39–48 (2020). https://doi.org/10.1007/s12028-020-00931-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-00931-5