Abstract
Background
Acute hydrocephalus is a common complication of aneurysmal subarachnoid hemorrhage (aSAH); however, attempts to predict shunt-dependent chronic hydrocephalus using clinical parameters have been equivocal.
Methods
Cohort study of aSAH is treated with external ventricular drainage (EVD) placement at our institution, 2001–2016, via logistic regression. EVD-related parameters included mean/total EVD output (days 0–2), EVD days, EVD days ≤ 5 mmHg, and wean/clamp fails. aSAH outcomes assessed included ventriculoperitoneal shunt (VPS) placement, delayed cerebral ischemia (DCI), radiographic infarction (RI), symptomatic vasospasm (SV), age, and aSAH grades.
Results
Two hundred and ten aSAH patients underwent EVD treatment for a median 12 days (range 1–54); 85 required VPS (40%). On univariate analysis, EVD output, total EVD days, EVD days ≤ 5 mmHg, and wean/clamp trial failures were significantly associated with VPS placement (p < 0.01 for all parameters). No EVD output parameter demonstrated a significant association with DCI, RI, or SV. On multivariate analysis, EVD output was a significant predictor of VPS placement, after adjusting for age and clinical and radiological grades; the optimal threshold for predicting VPS placement was mean daily output > 204 ml on days 0–2 (OR 2.59, 95% CI 1.31–5.07). Multiple wean failures were associated with unfavorable functional outcome, after adjusting for age, grade, and VPS placement (OR 1.65, 95% CI 1.10–2.47). We developed a score incorporating age, grade and EVD parameters (MAGE) for predicting VPS placement after aSAH.
Conclusions
EVD output parameters and wean/clamp trial failures predicted shunt dependence in an age- and grade-adjusted multivariable model. Early VPS placement may be warranted in patients with MAGE score ≥ 4, particularly following 2 failed wean trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is an uncommon neurosurgical emergency, with an approximate annual incidence of 9/100,000 person-years, and at least 2% of the general population harboring unruptured intracranial aneurysms. Morbidity and mortality following aneurysm rupture are considerable, with at least 20% of patients dying before hospital presentation, an additional 10% in-hospital mortalities, and 10–20% sustaining major permanent neurologic deficits [6]. Acute hydrocephalus is a major contributor to early neurologic injury and frequently requires cerebrospinal fluid (CSF) diversion via external ventricular drain (EVD) placement. A substantial proportion of aSAH patients who survive the initial injury are at risk of late complications, including chronic hydrocephalus requiring permanent CSF diversion via ventriculoperitoneal shunt (VPS) placement, delayed cerebral ischemia (DCI), radiographic infarct (RI), and symptomatic vasospasm (SV).
Ideal management of persistent hydrocephalus following the early acute phase of SAH is controversial, with conflicting recommendations in the literature regarding the relative benefits of early VPS placement versus serial attempts at EVD weaning [2, 12, 19]. Several factors have been associated with shunt dependence after aSAH, including hemorrhage grade, intraventricular hemorrhage (IVH), patient age, and treatment modality. In spite of this, only scant information has been reported regarding the predictive value of objective EVD parameters, such as daily outputs or pressure thresholds, in determining which patients are most likely to be shunt dependent. Moreover, there is insufficient data characterizing the relationship between EVD parameter and the risk of delayed ischemic complications (e.g., DCI, SV, RI). We hypothesized that EVD-related parameters influence the risk of shunt dependence and delayed ischemic complications. In order to test this hypothesis, we conducted a large, single-institution cohort study of aSAH patients treated with EVD, with the primary endpoint of VPS placement, and secondary endpoints of DCI, SV, and RI. In parallel, we carried out a systematic review of the literature and compared our predictive model with those previously reported [2, 12, 15, 19].
Methods
Study Cohort: Data Source, Data Collection, and Variable Definitions
A prospectively maintained institutional registry was queried for patients who underwent EVD placement in our neurosciences intensive care unit (ICU) for treatment of acute hydrocephalus secondary to aSAH. Patients with prior history of aSAH, existing VPS, prior CSF diversion procedure, < 72 h of quantitative EVD data collection, or early in-hospital mortality were excluded; all other adult patients with imaging-confirmed aSAH and adequate EVD data were included. Patient demographics were used for model adjustment, including age, sex, and major medical comorbidities, such as hypertension or nicotine use. Baseline disease parameters were similarly abstracted, including World Federation of Neurological Surgeons (WFNS) at both presentation and nadir, and modified Fisher (mFisher) score. During modeling, mFisher was tested in 3 ways: as a continuous variable (1–4), as a binary predictor stratified by risk of vasospasm (e.g., 1 or 2 vs. 3 or 4), and as a binary predictor stratified by IVH status (e.g., 1 or 3 vs. 2 or 4).
EVD parameters were captured for all patients during days 0–2 (e.g., initial 72 h following placement). This preceded the earliest VPS placements or EVD wean trials, preserving the maximum sample size for modeling, and was also determined to be optimally balanced between the competing demands of ideal data collection (e.g., modeled at a later time point), and the generation of a clinically useful prediction (e.g., modeled at an earlier time point). CSF output was modeled as both a continuous and categorical variable, stratified using 100 ml intervals of mean daily output. Other EVD variables included total days of EVD drainage, total days of low pressure drainage (defined as ≤ 5 mmHg), and number of wean/clamp trials, which were all modeled as continuous variables. The routine weaning protocol at our institution is to increase the EVD pressure threshold by 5 mmHg daily, with attention to the clinical neurologic status. A wean trial is considered a failure if the patient has a persistent neurologic change requiring reversal of the increase in pressure threshold. Once the threshold has been increased to 20 mmHg, the drain is clamped for 24 h, after which a final head computed tomography (CT) is acquired for a post-wean baseline, and the EVD is removed.
Endpoints
Late aSAH complications abstracted from the registry included chronic hydrocephalus, DCI, RI, and SV, which were confirmed as required via secondary retrospective chart review. Chronic hydrocephalus was assessed using VPS placement as a surrogate endpoint. DCI was defined in accordance with the most recent consensus recommendations, best summarized as focal neurological impairment or a decrease in Glasgow Coma Scale (GCS) ≥ 2, lasting ≥ 1 h, not apparent immediately after aneurysm occlusion, and not attributed to other causes [29]. SV was defined as DCI in association with neurologically correlated > 25% decrease in arterial diameter on digital subtraction angiography or magnetic resonance angiography or transcranial doppler ultrasonography finding of mean arterial velocity > 120 cm/s in the anterior, middle, or posterior cerebral artery. RI was defined as either: (1) new hypodensity located in a vascular distribution on non-enhanced CT imaging, or (2) new magnetic resonance imaging hyperintensity on diffusion-weighted imaging with associated hypo-intensity on apparent diffusion coefficient map; and (3) not attributable to periprocedural complications [24, 29]. Functional outcome at 3–6 months was categorized using the modified Rankin score (mRS).
Systematic Literature Review: Search Strategy and Data Abstraction
Medline and Embase were queried for English language articles reporting predictive models of late complications after aSAH. Inclusion criteria were observational studies or clinical trials of at least 10 patients with aSAH, reporting multivariate logistic regression analyses of either VPS placement in aSAH patients, 1986–2018. Publications that did not report final VPS status were excluded, as were articles that did not describe the model parameters in their Methods, or predictor effect sizes in their Results.
Primary screen identified 447 candidate records after de-duplication; all abstracts were reviewed in detail, and articles thought to meet inclusions underwent full-text assessment (n = 95; Fig. 1). Full-text reviews included bibliographic analyses to screen for additional candidate records (n = 36). Excluded manuscripts (n = 127) did not include quantitative EVD output data, did not report VPS outcome, included non-aSAH diagnoses without parsed data, or had inadequate cohort size. Four publications met criteria and were independently assessed for level-of-evidence using the Oxford Centre for Evidence-based Medicine (CEBM) 2009 guidelines [10, 17, 22, 28, 35]. Data points abstracted from all included publications were study design and CEBM grade; sample size; EVD output threshold defined as predictive of VPS placement (transformed to ml/24 h); and any other significant primary outcomes, regression parameters, or details pertinent to variable modeling and effect size. Odds ratios (OR) with 95% CI were calculated for our study cohort at each of these previously published thresholds, which were then assessed for relative predictive strength using area-under-the-curve (AUC) analysis of receiver operating characteristic (ROC) curves.
Statistical Analysis
Descriptive statistics were reported as frequency/proportion for categorical and median/range for continuous variables. Univariate statistical testing was carried out by VPS status using student’s t test for continuous and Chi-square or Fisher’s exact test for categorical data, with graphical evaluations used to confirm normal data distributions and suitability for parametric analysis. Logistic regression analysis was used for multivariable modeling of the 4 core study outcomes (VPS, DCI, RI, SV), adjusting for age and grade (WFNS [nadir] and mFisher), and with backward stepwise regression used for final modeling. AUC analysis of ROC curves was used for model optimization. Statistical assessments were carried out using SPSS Statistics 25.0 (IBM Corporation, Armonk, NY, 2018) or JMP Pro 14 (SAS Institute, Cary, NC, 2018), all tests were 2-sided, and significance was defined as p < 0.05.
Results
Baseline Patient Characteristics, Clinical Outcomes, and Univariate Analysis
From a total population of 656 aSAH patients treated at our institution during the study period, we identified 210 (32%) aSAH patients who underwent EVD placement for acute hydrocephalus and met all other study criteria. Eighty-five (40%) went on to VPS placement. There were no significant differences by VPS outcome along the following variables: age, sex, body mass index ≥ 30, active nicotine use, hypertension, type 2 diabetes mellitus, GCS at presentation and nadir, WFNS at presentation and nadir, aneurysm size and location, primary aneurysm treatment modality, or the presence of intraparenchymal hemorrhage (Table 1). mFisher was significantly different only when dichotomized, with patients having a score of 2 or 4 (versus 1 or 3), or 4 alone (vs. 1–3) having a higher rate of VPS (p = 0.01; p = 0.01). VPS was significantly associated with lower risk of DCI or death, as well as longer ICU and hospital lengths-of-stay (Table 1). VPS was not associated with aneurysm re-bleeding, RI, SV, out-of-home placement, total follow-up, and mRS at 3–6 months.
Quantitative EVD Output Parameters and Multivariate Analysis
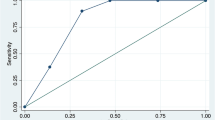
All EVD metrics assessed were significantly associated with VPS placement, including mean daily EVD output (days 0–2), total EVD output (days 0–2), total EVD days, EVD days at ≤ 5 mmHg, wean failures, and any clamp failure (Table 1). Mean daily EVD output 204 mL over days 0–2 was identified as the optimal threshold to predict shunt dependence, after adjusting for age, WFNS (nadir), and mFisher (Fig. 2a; AUC = 0.6). Multivariable modeling demonstrated that all EVD output parameters remained independently associated with VPS placement (Table 2), including mean daily output > 204 ml/24 h on days 0–2 (OR 2.58,95% CI 1.37–4.68), and 100 ml increase in mean daily output over days 0–2 (OR 1.85, 95% CI 1.26–2.74). No EVD output parameter demonstrated a significant association with DCI, RI, or SV.
a ROC curve for mean daily EVD output as a predictor of VPS status (adjusted for age, WNFS (nadir), and mFisher); b ROC for final iteration of MAGE score (AUC optimization resulted in equal weighting of all included variables); c Sampling distribution of patients in the study cohort by score, confirming relative normality with minimal skew toward lower values
Multivariable logistic regression was used to determine the final model (Table 3). Parameters that retained independence in the final model included mean daily EVD output > 204 ml on days 0–2 (OR 2.59, 95% CI 1.32–5.07), wean failures (OR 2.19, 95% CI 1.11–4.32), any clamp failure (OR 2.87, 95% CI 1.29–6.44), and total EVD days (OR 1.07, 95% CI 1.01–1.13). Total EVD days were associated with unfavorable functional outcomes (mRS > 2) at 3–6 months. Wean failures were significantly associated with an unfavorable outcome, but only after controlling for VPS status. Statistical testing for interaction between VPS status and either total EVD days or wean failures was non-significant (p = 0.38; p = 0.10).
Mayo Age, Grades, EVD (MAGE) Score for Predicting Shunt Dependence in aSAH
In order to provide a more clinically useful application of the final model, 6 variables were incorporated into a scoring system for predicting shunt dependence. In order to optimize the scoring system, we tested all possible weighting permutations of these 6 parameters, with each parameter assigned possible weights from 0 to 5. Each permutation was tested using ROC analysis, and the permutation achieving the highest overall AUC was selected as the Mayo Age, Grades, EVD (MAGE) score, with the EVD output threshold rounded from 204 to 200 ml, for simplicity of utilization (Fig. 2b, c). The optimal permutation of the scoring system was the iteration that set all 6 variables to equally weighted 0- or 1-point values (Table 4; AUC = 0.76). Of note, scores of 6 and 0 were exclusively observed in patients with and without VPS placement, whereas scores of 5 and 1 were, respectively, associated with odd ratios for VPS placement of 5.49 (95% CI 1.46–20.62) and 0.06 (95% CI 0.02–0.28), and positive/negative predictive values of 0.82 and 0.94.
Systematic Review and Comparative Assessment of Quantitative VPS Prediction Models
Four publications reporting multivariable models of VPS placement incorporating EVD output parameters as a primary predictor were identified (Table 5) [10, 17, 28, 35]. Two studies were cohort studies or retrospective subgroup analyses of prospective clinical trials (level 2B evidence); 2 were case series (level 4 evidence). EVD output thresholds were reported in all 4 studies and transformed to ml/24 h for comparison to the optimal threshold observed in our study (200 ml/24 h). All previously reported thresholds were significant predictors when applied to our study cohort, save for the model reported by Zolal et al.—an anomalous finding most likely attributable to their model containing a minority of aSAH patients (n = 32-of-86). ROC analysis demonstrated that all reported thresholds were comparable to our own when assessed via our study cohort, with our threshold demonstrating minimal superiority (AUCs = 0.69, 0.67, 0.64, 0.64). Other significant predictors reported in preceding analyses included age, Hunt and Hess grade (HHG) ≥ 4, acute hydrocephalus, intraparenchymal hemorrhage, posterior circulation aneurysm location, aneurysm coiling, and sonographic vasospasm.
Discussion
aSAH is a critical neurosurgical disease, characterized by high mortality and numerous in-hospital complications that frequently result in marked neurologic morbidity [5, 9, 16, 25, 26, 29]. Chronic, shunt-dependent hydrocephalus has been the focus of numerous studies attempting at predictive modeling, yet few models incorporate quantitative EVD parameters, and many have been clinically impractical, or yielded equivocal results. Correspondingly, our study objectives were to develop a useful model for predicting VPS placement after aSAH, and to compare its performance with previous reported models using the study sample as a validation cohort.
Overview of Predictive Models for VPS Placement in aSAH
Patient selection and timing for VPS placement in aSAH are a long-standing source of controversy, given the parallel risks of morbidity from under-treatment of chronic hydrocephalus or failed EVD wean/clamp trials, versus the placement of an unnecessary VPS. More than 40 models for predicting VPS placement in aSAH have been proposed, with at least 3 formal meta-analyses have reporting various summary findings [7, 31, 33]. In spite of these efforts, there remains little definitive understanding of how to interpret risk factors for shunt dependence after aSAH.
Wilson et al. synthesized data from 21 publications in 2017, restricting inclusion to level A and B evidence. In their analysis, they identified overall statistical significance in 8 of the 9 predictor variables tested, including Fisher grade, acute hydrocephalus, in-hospital complications, IVH, HHG ≥ 4, aneurysm re-bleeding, posterior circulation aneurysm location, and age ≥ 60 [31]. Female sex did not reach significance, in contrast to most prior reports, but in alignment with our own negative observation [3, 20, 33].
Xie et al. reviewed 25 studies—including case–control analyses and other lower quality publications—and performed both an overall analysis and subgroup analyses by evidence grade (e.g., cohort-or-better versus case–control) [33]. Twelve-of-fourteen predictors were significant in the overall model, including age ≥ 50, female sex, HHG ≥ 4, GCS ≤ 8, Fisher ≥ 3, acute hydrocephalus, EVD placement, IVH, posterior circulation or anterior communicating artery locations, in-hospital meningitis, and aneurysm re-bleeding. Parallel findings were reproduced in the subgroup analyses by level-of-evidence; however, while primary endovascular treatment was noted to be significantly associated with VPS among the cohort studies (RR 1.16, 95% CI 1.05–1.29), it remained non-significant as compared to open aneurysm clipping among the case–control analyses (OR 1.27, 95% CI 0.95–1.71).
Patient and Variable Selection in Quantitative EVD Output Modeling
Exceedingly few aSAH patients will develop shunt-dependent chronic hydrocephalus without having undergone in-hospital CSF diversion, with the largest population-based study of delayed hydrocephalus in aSAH reporting only 31 such cases in a sample of 8773 (0.4%) [30]. In spite of this, the vast majority of prior studies included all aSAH patients in their model, rather than restricting to those who had undergone EVD placement. The result is a weighting of those cohort toward individuals who are at the lowest risk of eventual VPS placement, and a critical source of confounding. More specifically, with a large number of data points included from individuals at low risk of chronic hydrocephalus, the predictive power of many parameters pertinent only to those patients with acute hydrocephalus would likely be compromised to a degree that would not be adequately addressed by simple adjustment in the multivariable model. Thus, a core characteristic distinguishing our study from most preceding works is the sole inclusion of patients with EVD placement for treatment of acute hydrocephalus.
Similarly, although several predictors in previous models overlapped—age, and the major SAH severity scores—the other covariates included were highly inconsistent, and frequently quite numerous [33]. Not only did many of these variables demonstrate considerable heterogeneity (e.g., I2 ≥ 50%), but the underlying models did not assess for covariance, over-fitting, or effect modification, potentially resulting in another source of residual confounding.
The limitations of preceding analyses influenced our approach to model development, for example in our decision to restrict consideration to unambiguous variables such as age, WFNS, and mFisher [1, 4, 11, 13, 18, 21, 27]. Further, in selecting potential predictors we limited consideration to parameters that are readily accessible in clinical practice, such as mean daily EVD output (days 0–2). The decision to set the EVD output parameter at this timepoint was twofold. First, by reviewing mean daily output over the first 3 days, we established a relative baseline for each patient, after initial aneurysm treatment, but prior to any major changes in EVD pressure thresholds (e.g., those associated with wean trials), as well as before the onset of DCI or any other major clinical sequela that may have influenced EVD management or output. Although this restricts the data to a more modest sample than capturing mean daily output for all EVD days would have, it also significantly limits sources of residual confounding and improves the interpretability of the data. Second, the 72-h threshold has practical value, as it is an early time point prior to the so-called vasospasm window, at which it may be particularly useful to earmark patients that may eventually go on to require VPS placement.
Finally, we formally assessed covariance and effect modification, and we took a parsimonious approach to covariate inclusion and validation, both in the final model and the MAGE score. This included deliberate, iterative assessment of various wean failure thresholds, with the final ≥ 2 dichotomization selected as both the threshold whose statistical association with VPS placement was the strongest, and the one that most aligned with our stated study goals of generating a practical scoring system, and reducing the patient risk associated with avoidable wean failures.
Comparative Assessment of Predictive Scoring Systems
The MAGE score is the first shunt-dependence prediction tool that incorporates EVD output. Notwithstanding, several publications have assessed VPS placement as a function of EVD output without an associated scoring system, while others have described scoring systems without incorporation of EVD output. With respect to both groups of preceding publications, we identified key preceding studies via systematic review, graded their levels-of-evidence using validated criteria, and applied reported thresholds and/or scoring systems to the current cohort where possible, to better inform a comparative analysis.
Four analyses identified CSF output thresholds as potential predictors of VPS placement, of which 3 maintained statistical significance when applied to the current study sample as a validation cohort. Unsurprisingly, higher thresholds were slightly better predictors of shunt dependence in patients who were over the line, but most publications reported thresholds that were close to the MAGE threshold of 200 ml. Although VPS decision making is ultimately an individualized practice, this lends additional robustness to the recommendation that shunt placement is likely to be necessary beyond that threshold.
The 4 most robust and well-validated scoring systems for VPS placement are Chronic Hydrocephalus Ensuing from SAH Score (CHESS), Barrow Neurological Institute (BNI), Shunt Dependency in Subarachnoid Hemorrhage (SDASH), and Post-subarachnoid Shunt Scoring (PS3)—each of which has been assessed in at least 1 validation cohort beyond the index study [8, 14, 20, 32]. While the BNI model is a radiographic score—essentially, mFisher with more strata and without IVH status—CHESS, SDASH, and PS3 recombine HHG, mFisher/IVH, and EVD/acute hydrocephalus status via differential weighting schemes (CHESS also includes binary variables for posterior circulation location and infarct status; Table 6).
Two additional scoring systems—the Failure Risk Index (FRI), reported by Chan et al, and the Discriminant Function (DF), described by Yamada et al.—also incorporated third ventricular width, a promising surrogate for third ventricular volume [3, 23, 34]. Unfortunately, both FRI and DF were reported as complex algebraic functions that require collection of at least 8 variable coefficients (reported to 3 significant figures each), rendering them impractical for routine clinical implementation [23].
Functional Outcomes and VPS Status
Two critical questions underlie our motivation to continue refining VPS models in aSAH: is there harm associated with repeated attempts to wean or clamp an EVD, and is VPS placement associated with a significant difference in long-term outcome? The impact of serial failed wean/clamp trials has received relatively little attention, with most neurosurgeons and neurointensivists operating under the assumption that intervals of supranormal intracranial pressure can be well tolerated, provided that the exposure is brief, and the clinical examination is used to quickly terminate an unsuccessful attempt. Ascanio et al. examined a sample of 114 non-traumatic SAH patients (80% aSAH), assessing for association between number of clamp trials and mRS at last follow-up [2]. In their study, VPS was avoided in 60% of patients who had 2 clamp trials, and 39% of patients who had 3 clamp trials, with no significant association noted between increased clamp trials and functional outcome, ICU length-of-stay, or meningitis.
In our final model, overall results were equivocal with respect to functional status: total EVD days were significantly associated with mRS > 2, even when adjusted for VPS status, while EVD output and clamp failure were non-significant predictors of functional outcome. By contrast, wean failures demonstrated significance only after adjusting for VPS status. This unexpected finding has two probable explanations: either excessive wean trials are potentially dangerous, but only among those patients who will ultimately need a VPS (and are therefore in a more vulnerable neurologic state), or that the observation is simply statistical noise.
Limitations
Our study has several key limitations, most prominently the small sample size (n = 210), and the risk of residual confounding or bias inherent to the observational design. The MAGE score, although novel and potentially useful, requires validation in an independent cohort prior to widespread clinical implementation. We excluded patients without EVD placement, and although doing so yielded a more robust model within the population-of-interest (e.g., aSAH patients who had an EVD placed), it also limited the study generalizability. Perhaps most importantly, the MAGE score performs best at its poles, but is less reliable over intermediate risk groups—a shortcoming that is also observed in its predecessors, including CHESS, BNI, SDASH, and PS3. Unfortunately, patients in the more equivocal range of scores are also those for whom such a scoring system would be most helpful, as the highest and lowest risk patients are rarely those for whom prediction of VPS outcome is challenging without the aid of a scoring system.
As detailed above, several important clinical considerations informed the decision to restrict EVD output modeling to the first 72 h, which directly limited the data available for assessment, and therefore potentially reduced the robustness of the model. In future studies, we hope to similarly evaluate a more nuanced parameter, such as ‘mean daily output prior to initial wean trial,’ which we hypothesize will also closely approximate 200 ml/24 h, but which we can only speculate about, based on the current study. Correspondingly, although we anticipate that the 200 ml/24 h threshold will retain significant predictive value at time points after 72 h, at present we can only make guarded recommendations in that regard.
Another important limitation is the handling of patient deaths in the cohort. As a number of patients died in both groups, several of whom underwent VPS placement, it was determined that excluding all mortalities would potentially bias the cohort. Although the possibility of residual confounding in association with patients who had life-sustaining therapy withdrawn is noted, this was addressed in part by adjusting for WFNS, which is widely validated as a reliable predictor of unfavorable outcomes, including death.
A final limitation that warrants specific discussion is the inclusion of non-significant parameters in the MAGE scoring system. In order to appropriately address not only the results of the study cohort itself, but also of the systematic review and comparative analysis, age and WFNS parameters were incorporated into the MAGE system in spite of their having been non-significant in the study cohort. Although this methodological decision draws support from the vast majority of the preceding literature, as well as the significant predictive value of the MAGE system as applied to the study cohort, it also emphasizes the need for validation of the MAGE system via an independent patient cohort.
In spite of these important limitations, we hold that our findings represent a valuable step forward in our understanding of this challenging disease process and its optimal management. Further, and perhaps more importantly, we highlight the way forward for future iterations of a more individualized scoring system, as the first such instrument that incorporates quantitative EVD output data, relies on validated and reproducible metrics, and adjusts for age, as well as clinical and radiological grades.
Conclusions
Our analysis shows that EVD parameters are useful to predict future shunt dependence. Specifically, EVD output during the first 72 h after placement, duration of EVD drainage (particularly at low levels of drainage), and wean/clamp trial failures were associated with VPS placement. Using these variables, we developed and optimized the first scoring system based on quantitative EVD output for the prediction of VPS outcome in aSAH. Taken together with the multiple increased risks and costs associated with prolonged hospitalization and extended EVD drainage, VPS placement should be strongly considered without further EVD clamping attempts in patients with a MAGE score ≥ 5, and in most patients with a MAGE score of 4 (particularly following 2 failed wean trials).
References
Aggarwal A, Dhandapani S, Praneeth K, Sodhi HBS, Pal SS, Gaudihalli S, et al. Comparative evaluation of H&H and WFNS grading scales with modified H&H (sans systemic disease): a study on 1000 patients with subarachnoid hemorrhage. Neurosurg Rev. 2018;41:241–7.
Ascanio LC, Gupta R, Adeeb N, Moore JM, Griessenauer CJ, Mayeku J, et al: Relationship between external ventricular drain clamp trials and ventriculoperitoneal shunt insertion following nontraumatic subarachnoid hemorrhage: a single-center study. J Neurosurg. 2018;130:956–962. https://doi.org/10.3171/2017.10.JNS171644.
Chan M, Alaraj A, Calderon M, Herrera SR, Gao W, Ruland S, et al. Prediction of ventriculoperitoneal shunt dependency in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2009;110:44–9.
Chiang VL, Claus EB, Awad IA. Toward more rational prediction of outcome in patients with high-grade subarachnoid hemorrhage. Neurosurgery. 2000;46:28–35 (discussion 35–6, 2000).
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–37.
De la Garza RR, Haranhalli N, Kobets AJ, Nakhla J, Brook AL, Yassari R, et al. The effect of July admission on inpatient morbidity, mortality, and discharge disposition after endovascular coiling in subarachnoid hemorrhage. World Neurosurg. 2018;109:e170–4.
de Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, Seifert V, et al. Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single-institution series and meta-analysis. Neurosurgery. 2007;61:924–33 (discussion 933–4, 2007).
Diesing D, Wolf S, Sommerfeld J, Sarrafzadeh A, Vajkoczy P, Dengler NF. A novel score to predict shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2018;128:1273–9.
Diringer MN, Bleck TP, Hemphill JC, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15:211.
Erixon HO, Sorteberg A, Sorteberg W, Eide PK. Predictors of shunt dependency after aneurysmal subarachnoid hemorrhage: results of a single-center clinical trial. Acta Neurochir. 2014;156:2059–69.
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery. 2006;59:21–7.
Gupta R, Ascanio LC, Enriquez-Marulanda A, Griessenauer CJ, Chinnadurai A, Jhun R, et al. Validation of a predictive scoring system for ventriculoperitoneal shunt insertion after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2018;109:e210–6.
Inagawa T. Risk factors for cerebral vasospasm following aneurysmal subarachnoid hemorrhage: a review of the literature. World Neurosurg. 2016;85:56–76.
Jabbarli R, Bohrer A-M, Pierscianek D, Müller D, Wrede KH, Dammann P, et al. The CHESS score: a simple tool for early prediction of shunt dependency after aneurysmal subarachnoid hemorrhage. Eur J Neurol. 2016;23:912–8.
Lai L, Morgan MK. Predictors of in-hospital shunt-dependent hydrocephalus following rupture of cerebral aneurysms. J Clin Neurosci. 2013;20:1134–8.
Lawton MT, Vates GE. Subarachnoid Hemorrhage. N Engl J Med. 2017;377:257–66.
Lewis A, Kimberly WT. A retrospective analysis of cerebrospinal fluid drainage volume in subarachnoid hemorrhage and the need for early or late ventriculoperitoneal shunt placement. J Neurosurg Sci. 2016;60:289–95.
Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389:655–66.
Maragkos GA, Enriquez-Marulanda A, Salem MM, Ascanio LC, Chida K, Gupta R, et al. Proposal of a grading system for predicting discharge mortality and functional outcome in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg. 2018. https://doi.org/10.1016/j.wneu.2018.09.148.
Motiei-Langroudi R, Adeeb N, Foreman PM, Harrigan MR, Fisher WS, Vyas NA, et al. Predictors of shunt insertion in aneurysmal subarachnoid hemorrhage. https://www.sciencedirect.com/science/article/pii/S1878875016312402/pdfft?md5=aed7a7c763beeabb5ebae1de2180d842&pid=1-s2.0-S1878875016312402-main.pdf&isDTMRedir=true&download=true. Accessed 22 Dec 2018.
Oshiro EM, Walter KA, Piantadosi S, Witham TF, Tamargo RJ. A new subarachnoid hemorrhage grading system based on the glasgow coma scale: a comparison with the Hunt and Hess and world federation of neurological surgeons scales in a clinical series. Neurosurgery. 1997;41:140–8.
Phillips B, Ball C, Sackett D, Badenoch D, Straus S, Haynes B, et al. Oxford Centre for evidence-based medicine levels of evidence. Updated by Jeremy Howick March 2009; 2016.
Pinggera D, Kerschbaumer J, Petr O, Ortler M, Thome C, Freyschlag CF. The volume of the third ventricle as a prognostic marker for shunt dependency after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2017;108:107–11.
Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, et al. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:1862–6.
Rabinstein AA, Lanzino G. Aneurysmal subarachnoid hemorrhage: unanswered questions. Neurosurg Clin N Am. 2018;29:255–62.
Rabinstein AA, Wijdicks EFM. Cerebral vasospasm in subarachnoid hemorrhage. Curr Treat Options Neurol. 2005;7:99–107.
Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2005;2:110–8.
Tso MK, Ibrahim GM, Macdonald RL. Predictors of shunt-dependent hydrocephalus following aneurysmal subarachnoid hemorrhage. World Neurosurg. 2016;86:226–32.
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Paul Muizelaar J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies. Stroke; 2010. https://www.ahajournals.org/doi/abs/10.1161/strokeaha.110.589275. Accessed 17 Dec 2018.
Walcott BP, Iorgulescu JB, Stapleton CJ, Kamel H. Incidence, timing, and predictors of delayed shunting for hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2015;23:54–8.
Wilson CD, Safavi-Abbasi S, Sun H, Kalani MYS, Zhao YD, Levitt MR, et al. Meta-analysis and systematic review of risk factors for shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;126:586–95.
Wilson DA, Nakaji P, Abla AA, Uschold TD, Fusco DJ, Oppenlander ME, et al. A simple and quantitative method to predict symptomatic vasospasm after subarachnoid hemorrhage based on computed tomography: beyond the Fisher scale. Neurosurgery. 2012;71:869–75.
Xie Z, Hu X, Zan X, Lin S, Li H, You C. Predictors of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage? A systematic review and meta-analysis. World Neurosurg. 2017;106:844.e6.
Yamada S, Nakase H, Park Y-S, Nishimura F, Nakagawa I. Discriminant analysis prediction of the need for ventriculoperitoneal shunt after subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2012;21:493–7.
Zolal A, Juratli T, Dengl M, Ficici KH, Schackert G, Sobottka SB. Daily drained CSF volume is a predictor for shunt dependence—a retrospective study. Clin Neurol Neurosurg. 2015;138:147–50.
Funding
None.
Author information
Authors and Affiliations
Contributions
AP, CSG, GK, LPC, MJL, and AAR contributed to the study design. MJL and AAR contributed to the study supervision and approved of manuscript on behalf of study authors. AP and CSG contributed to the first draft of manuscript. GK, LPC, MJL, and AAR contributed to the critical revision of manuscript. CSG and GK contributed to the statistical support.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical Approval/Informed Consent
All pertinent aspects of this study were reviewed and approved by our Institutional Review Board, including a waiver of informed consent for a minimal risk study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perry, A., Graffeo, C.S., Kleinstern, G. et al. Quantitative Modeling of External Ventricular Drain Output to Predict Shunt Dependency in Aneurysmal Subarachnoid Hemorrhage: Cohort Study. Neurocrit Care 33, 218–229 (2020). https://doi.org/10.1007/s12028-019-00886-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00886-2