Abstract
Background
Sepsis-induced brain dysfunction (SIBD) is often encountered in sepsis patients and is related to increased morbidity. No specific tests are available for SIBD, and neuroimaging findings are often normal. In this study, our aim was to analyze the diagnostic value of volumetric analysis of the brain structures and to find out its significance as a prognostic measure.
Methods
In this prospective observational study, brain magnetic resonance imaging (MRI) sections of 25 consecutively enrolled SIBD patients (17 with encephalopathy and 8 with coma) and 22 healthy controls underwent volumetric evaluation by an automated segmentation method.
Results
Ten SIBD patients had normal MRI, and 15 patients showed brain lesions or atrophy. The most prominent volume reduction was found in cerebral and cerebellar white matter, cerebral cortex, hippocampus, and amygdala, whereas deep gray matter regions and cerebellar cortex were relatively less affected. SIBD patients with normal MRI showed significantly reduced volumes in hippocampus and cerebral white matter. Caudate nuclei, putamen, and thalamus showed lower volume values in non-survivor SIBD patients, and left putamen and right thalamus showed a more pronounced volume reduction in coma patients.
Conclusions
Volumetric analysis of the brain appears to be a sensitive measure of volumetric changes in SIBD. Volume reduction in specific deep gray matter regions might be an indicator of unfavorable outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances in the management of critically ill patients suffering from organ dysfunction have demonstrated that brain is one of the most prominently effected organs during critical illness. Acute brain dysfunction is commonly seen due to sepsis-induced neuroinflammatory processes and is related to morbidity and mortality in the intensive care unit (ICU). Survivors of sepsis may undergo long-term cognitive dysfunction that affects the quality of life [1,2,3,4,5]. However, there are no specific diagnostic tests and treatment methods for sepsis-induced brain dysfunction (SIBD), and the exact mechanisms of brain involvement in sepsis is not as yet clear [6].
Unlike other neurologic disorders, the development of neurologic dysfunction in patients with sepsis and its relationship to the development of neuroanatomic abnormalities on computed tomography or magnetic resonance imaging (MRI) has not been adequately studied. Neuroimaging findings of sepsis patients with cognitive dysfunction are often unremarkable. A variety of findings may be observed in the acute setting in SIBD patients. These include ischemic lesions, diffuse leukoencephalopathy, severe vasogenic edema with predominant posterior (parietal/occipital) distribution abnormalities, and brain atrophy [7,8,9].
We recently found an association between proinflammatory cytokine and complement breakdown products levels of SIBD patients and occurrence of septic shock, coma, and mortality [10]. Moreover, in our previous prospective imaging trial of SIBD patients, we reported brain atrophy in 16.1% of the cases by the visual rating of the images and voxel-based morphometric analysis showed loss of volume particularly in certain cortical areas and deep gray matter regions [11]. Decreased volume of the hippocampus was also reported in sepsis patients [12]. Thus, we hypothesized that neuroinflammation is induced in SIBD patients as a response to endogenous or exogenous danger signals, thus leading to disruption of blood–brain barrier and local release of proinflammatory mediators, enhanced oxidative stress and mitochondrial dysfunction, and ultimately neuronal death particularly in widespread cortical areas as well as in subcortical nuclei [13].
In this study, our primary aim was to analyze the individual volumes of potentially vulnerable structures including limbic and subcortical gray nuclei which have dense connections with cortex as well as volumes of the cerebral and cerebellar white matter in sepsis patients. Our secondary aim was to investigate the relevance of volumetric changes with disease severity and clinical outcome scores.
Methods
Study Design and Population
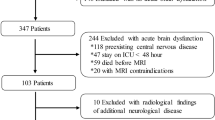
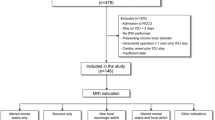
This prospective observational study was performed in the adult medical and surgical ICU of the Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey, and was approved by the Institutional Review Board (approval number: 2013/98), and the written informed consent was obtained from all individual surrogates of the patients. Patients were enrolled at our institution between February 2013 and June 2017. Critically ill adults consecutively admitted to the ICU with severe sepsis and septic shock as defined by 2012 international sepsis criteria [14] who were spending not less than 48 h in the ICU and had an acute onset of brain dysfunction were considered for inclusion in this study. Acute brain dysfunction was defined as acute (occurring over a period of hours or days) onset of altered mental status (encephalopathy or coma), clinically or electroencephalographically detectable seizures, and focal neurologic deficits [7, 15]. Exclusion criteria included any preexisting central nervous disease (brain lesions, neurodegenerative, inflammatory, cerebrovascular disease, central nervous system infection, and traumatic brain injury), or any contraindication for MRI. Twenty-two healthy controls were also enrolled in this study to calculate the qualitative volume studies. The control group consisted of healthy individuals matched for age and gender. Participants in the control group had no history of any systemic, psychiatric, and neurologic disorder.
Baseline clinical and demographic data were collected by ICU staff at enrollment in the present study. Patients’ demographic characteristics including age, gender, comorbidities, the reason for ICU admission, admission category (medical or surgery), neurologic history, days in the ICU, duration of hospitalization, and clinical outcome were recorded. The severity of illness was measured at enrollment using the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and Simplified Acute Physiology Score 2 (SAPS2), and severity of sepsis was evaluated on a daily basis during the ICU stay using the Sequential Organ Failure Assessment (SOFA) score. Neurologic recovery was assessed using the Glasgow Coma Scale—Extended (GOSE) during ICU discharge. From admission to the day of discharge vital signs, microbiological data, standard laboratory tests, use of steroids, duration of septic shock, days of mechanical ventilation, and days of sedation were recorded daily.
Neurologic Examination
Components of our neurologic assessment in the ICU included the level of consciousness and encephalopathy assessment. Using the Confusion Assessment Method for the ICU [16], trained research nurses assessed patients for encephalopathy twice daily from ICU admission until death or ICU discharge. Daily sedation discontinuation protocol was applied in the ICU and sedation interruption made daily at 7:30 a.m. Level of consciousness was measured by the Richmond Agitation-Sedation Scale [17]. Patients with GCS ≤ 8 were diagnosed as coma. Coma was diagnosed if patients showed a state of unarousable unresponsiveness in non-sedated patients or after discontinuation of sedation in previously sedated patients [18].
Brain MRI
MRI was performed on a 1.5-T scanner (Achieva; Philips, Eindhoven, The Netherlands). Imaging protocol included T2-weighted (axial and coronal), T1-weighted (axial and sagittal), FLAIR (fluid-attenuated inversion recovery; axial), and diffusion-weighted images with b 0.500 and 1000 m s/mm2) and ADC (apparent diffusion coefficient) map images. A macrocyclic chelate (Dotarem®; Guerbet Laboratory, Roissy, France) was administered for contrast-enhanced imaging. MRI features were investigated by two neuroradiologists (SS and MB). To determine the presence of atrophy, subjective visual simple scale was used. There was no disagreement for MRIs classified as normal. In rare cases of disagreement for brain MRIs showing atrophy and/or lesions, a consensus decision was made in a meeting attended by all authors.
Image Acquisition and Processing
Since our aim was to analyze the volumes of the certain limbic structures and deep gray nuclei as well as cortical and white matter volumes of the cerebrum and cerebellum, we utilized an automated segmentation method (FreeSurfer), which automatically analyzes the individual volumes of our region of interest areas. High-resolution, three-dimensional T1-weighted images were acquired for 25 SIBD patients and 22 control participant using a 1.5-T scanner with circular polarized eight-channel head coil. The pulse sequence parameters were: repetition time/echo time = 25/4.6 s, flip angle = 30, field of view = 240 mm, acquired voxel size = 1.00/1.00/1.00 mm (reconstructed = 0.86/0.86/1.00 mm), 150 coronal slices without gap, and scan duration = 5.01 min (per volume). The acquired images were analyzed with FreeSurfer 4.05 using the same workstation. This procedure, described previously [19], automatically segmented ≤ 40 unique structures based on their voxel densities and assigned a neuroanatomic label to each voxel. This assignment is performed automatically by the algorithm, using the probabilistic information gathered from a manually labeled training set of brain volumes. Each segmentation was then visually inspected for accuracy, and manual editing was carried out when necessary. Investigators who assessed brain volumes were blind to patient identity and all medical information.
Statistical Analysis
Sample size of study groups was calculated based on previous [12] and our own volumetry studies, which showed 9–15% volume reduction in sepsis patients as compared to healthy controls in the most frequently affected brain regions, hippocampus, and cerebral cortex [12, 20]. Sample size required to detect the 9% volume decrease with a power of 90% and a significance level of 5% was determined to be 22 patients/study group using an online calculator (http://biomath.info/power/). Descriptive statistics were applied to demographic and clinical variables. Quantitative data are presented as mean ± standard deviation or median and interquartile range. Qualitative data are presented as frequencies and percentages. The analysis of covariance was used to compare brain volumes, controlling for age at scan and total intracranial volume. Tukey’s post hoc test was used for two-group comparisons. The Spearman’s correlation test was used to examine the relationship between volumetric data and disease severity scores. p < 0.05 was considered statistically significant. All statistical analyses were performed using XLSTAT, version 2014.5.03 (AddinSoft, Inc., NY, USA).
Results
Clinical Features
Twenty-five SIBD patients (14 men and 11 women; 54.6 ± 9.02 year old) and twenty-two age- and sex-matched healthy controls (11 men and 11 women; 55.7 ± 7.65 year old) were included in this study. Reasons for brain imaging were encephalopathy (n = 17) or coma (n = 8). None of the patients had focal neurologic deficits. All patients underwent mechanical ventilation and sedation. During their follow-up, 21 (84.0%) patients developed septic shock, and bacteremia was shown in 6 (24.0%) patients. Steroid treatment was administered to 12 (48.0%) patients, and hemofiltration was performed to 12 (48.0%) patients. Source of infection was pneumonia in 14, intraabdominal infection in 8, urinary tract infection in 2, and catheter-related infection in 1 patient. Culture obtained from the infection source yielded gram-negative bacteria (n = 21), gram-positive bacteria (n = 1), and mixed bacteria (n = 3). Baseline APACHE II, SOFA, and SAPS2 scores were 22.3 ± 4.4, 8.2 ± 3.6, and 44.3 ± 15.9, respectively. SIBD patients were followed up for an average duration of 49.2 (± 25.3) days. The average duration in the ICU was 26.0 (± 13.8) days. Eleven patients died during their hospitalization. Demographic and clinical characteristics of the study population are presented in Table 1. All imaging procedures were performed during the clinically active stage of SIBD, and the mean time delay from neurologic signs to the brain scan was 6.7 ± 6.0 days. SIBD patients were divided according to the visual evaluation of their brain MRI as patients with normal MRI (n = 10, 40.0%) and abnormal MRI (n = 15, 60.0%). The second group consisted of patients with brain lesions (n = 8, 32.0%) and patients with brain atrophy as the predominant pathological finding (n = 7, 28%). Brain lesions included acute cerebrovascular infarct (n = 2), non-specific white matter lesions (n = 4), and posterior reversible leukoencephalopathy syndrome (n = 2).
Volumetric Analysis of Brain Regions
Comparison of volumes of several different brain regions among SIBD patients and healthy controls showed reduced volume of total cerebral cortex volume, bilaterally reduced volumes of cerebral and cerebellar white matter, cerebral cortex, hippocampus, and amygdala in SIBD patients. Volumes of brainstem, cerebellar cortex, and deep gray matter structures had been relatively preserved in SIBD patients with the exceptions of left putamen and right thalamus which showed more moderate and unilateral volume reductions. The most prominent volume reductions were observed in bilateral white matter and hippocampi (Table 2). When volumes were compared among healthy controls, SIBD patients with normal MRI, and those with abnormal (lesion or atrophy) MRI, healthy controls showed significantly higher volume values in most regions. Notably, SIBD patients with normal MRI displayed moderately increased volume values in both pallidum nuclei and left caudate nucleus. However, two-group comparisons did not yield a significant difference between patients with normal MRI and healthy controls in these three regions (Table 3). When compared to healthy controls, SIBD patients with pathological MRI features showed significantly reduced volumes of total cerebral cortex volume, total white matter volume, bilateral hippocampus, cerebral white matter and cerebral cortex, left putamen, right cerebellar white matter, and right thalamus. Notably, SIBD patients with visually normal MRIs showed significantly reduced total white matter, hippocampus, and cerebral white matter volumes as compared to healthy controls. No significant volume differences could be found among SIBD patients with and without pathological MRI features (Table 3).
Association of Brain Volumes with SIBD Outcome
Volumetric results of SIBD patients who survived (survivors, n = 14) and died during their hospital stay (non-survivors, n = 11) were compared with each other and healthy controls. Both survivor and non-survivor SIBD groups showed significantly reduced right and left hippocampus and right, left, and total cerebral white matter volumes as compared to healthy controls. Mean right hippocampus volume of non-survivors was lower than those of healthy controls and survivor SIBD patients. Non-survivor SIBD patients also displayed reduced volume values in both putamen nuclei, right, left, and total cerebral cortex regions, left amygdala, right thalamus, and right cerebellar white matter compared to healthy controls. Notably, volumes of both caudate nuclei of survivor SIBD patients were higher than those of non-survivor SIBD patients and healthy controls. Although non-survivor SIBD patients showed trends toward displaying lower volumes than survivor patients in all examined brain regions, in this two-group comparison, statistical significance could be attained only in left caudate, right caudate, and right hippocampus regions (Table 4). Volumes of both hippocampi; right, left, and total cerebral white matter regions; and right, left, and total cerebral cortex were reduced in SIBD patients with coma or encephalopathy as compared to healthy controls. Although SIBD patients with coma showed trends toward exhibiting lower volumes than patients with encephalopathy for all brain regions, these differences did not reach statistical significance. However, when compared to healthy controls, SIBD patients showed reduced volumes in more diverse brain regions than patients with encephalopathy. SIBD patients with coma had the lowest volume values in both cerebellar white matter, left putamen, left amygdala, and right thalamus regions, whereas SIBD patients with encephalopathy showed the lowest volume values in right cerebral cortex only (Table 5). No significant correlation was found among volumes of different brain regions versus APACHE II, SAPS2, SOFA, and GOSE (at discharge) scores (p values ranging between 0.111 and 0.982 and R coefficient values ranging between − 0.303 and 0.326). Significant differences were observed between discharge GOSE scores of patients with normal (5.60 ± 2.55) and abnormal (3.30 ± 2.61) MRI (p = 0.022) and survivors (6.14 ± 1.66) versus non-survivor (1.82 ± 1.83) patients (p = 0.000), but no difference was observed in patients with encephalopathy (4.18 ± 2.67) versus coma (4.38 ± 3.16) (p = 0.440).
Discussion
In this study, we investigated volume alterations of different brain regions of SIBD patients. Most prominent volume differences were found in hippocampus, amygdala, and cerebral white matter regions, whereas cerebellar white matter and cerebral cortex regions were also affected to a lesser degree in SIBD patients. By contrast, deep gray matter regions and cerebellar cortex were relatively preserved. Moreover, a more pronounced volume loss in deep gray matter was associated with coma and death during the hospital stay. Thus, the present study confirmed our assertion regarding association between neurologic involvement and volume loss in cortical and subcortical areas as well as in white matter of the cerebrum and cerebellum. In regard to etiologies underlying brain volume alterations, neuroinflammation by-products of cytokines and complement breakdown products, that are associated with clinical outcome in SIBD, could be likely responsible factors [10]. However, endothelial dysfunction and neurovascular uncoupling might accompany SIBD and lead to alterations in brain volume, as well [13]. Therefore, association of volumetric study results with endothelial activation markers is recommended in future studies.
In our previous voxel-based morphometry analysis the most prominent volume loss was detected in limbic structures including medial temporal lobe and insula. Likewise, in a previous study conducted in sepsis survivors, volume reduction was found in the hippocampus. However, gray matter and white matter volumes were comparable to those of healthy controls [12]. A potential reason for this discrepancy could be that the present study was conducted on patients, who were at the active stage of SIBD and had major neurologic symptoms, whereas the prior study was performed in sepsis patients with or without neurologic involvement 6–24 months after discharge from the hospital. To our knowledge, our study is the first comprehensive volumetric evaluation of SIBD patients during the clinically active stage.
A notable finding was that there were no significant regional volume differences between MRIs with pathological findings and MRIs that were independently reported to be normal by two radiologists. Moreover, the volumetric evaluation could detect atrophy in hippocampus and cerebral white matter in normal-appearing MRIs, further emphasizing the higher sensitivity of this method. Another outstanding finding was minimal volume increase in caudate and pallidum nuclei in normal-appearing MRIs. A likely explanation of this could be increased expansion of extracellular fluid and microscopic blood–brain barrier disruption, which is another measure of neuronal disintegration, as shown in autoimmune encephalitis patients [21]. The validity of this assertion needs to be further confirmed by methods assessing microstructural integrity.
Hippocampal volumes appear to be primarily affected in other inflammatory brain disorders, as well. In autoimmune encephalitis patients with leucine-rich glioma-inactivated protein 1 (LGI1) or N-methyl-d-aspartate receptor (NMDAR) antibodies, hippocampal volumes are reduced in association with the severity of cognitive decline [21, 22]. Intriguingly, in LGI1 encephalitis gray matter regions are relatively preserved [22]. Hippocampal volume reduction is conceivable in inflammatory (e.g., autoimmune encephalitis) and infectious (e.g., herpes simplex virus encephalitis) disorders mainly targeting limbic structures [23]. However, hippocampal volumes are also reduced in both progressive and non-progressive forms of multiple sclerosis which does not show a specific predilection to the limbic system [24]. Moreover, in great likeness to SIBD patients, intraperitoneal administration of bacterial lipopolysaccharide induces a volume change in hippocampal and cortical regions, while preserving caudate nuclei [25].
The reason behind increased susceptibility of certain brain regions to volume change in SIBD could be increased activation of neuroinflammation and microglial cells in these regions. As a matter of fact, increased expression of inflammasome complex and apoptosis factors, and enhanced microglial activity and oxidative stress have been associated with hippocampal destruction in different experimental sepsis models [25,26,27,28]. Microglial activation has also been related to hippocampal volume decrease and concomitant cognitive dysfunction in several disorders including Alzheimer’s disease and multiple sclerosis [23, 29].
Although deep gray matter structures appear to be relatively preserved in the entire cohort of SIBD patients, specific basal ganglion nuclei and thalamus show significantly reduced volumes in certain subgroups. Caudate nuclei, putamen, and thalamus showed lower volume values in non-survivors than in survivor SIBD patients and healthy controls. Likewise, left putamen and right thalamus regions of SIBD patients with coma showed a more pronounced volume reduction than those of the patients with encephalopathy. These results might suggest that basal ganglia involvement may be an indicator of increased severity of sepsis-associated brain involvement and unfavorable outcome, as exemplified by previous case reports [30].
Limitations
Our study has limitations, including small sample size. Power analysis showed that our study groups contained sufficient number of patients for SIBD versus healthy control comparisons. However, for subgroup analysis (e.g., normal versus abnormal MRI; patients with encephalopathy versus coma) our sample size was rather low. Also, our study, as well as other ICU neuroimaging studies, is limited by the lack of pre-ICU imaging and assessment of the presence of atrophy before SIBD. This is mostly due to the high risk of transporting sepsis patients to the neuroimaging unit, which reduces the chances to provide multiple MRIs from the same patient. The same factor also restricts the sample size of the study. Based on the results of this preliminary study, power analysis may now be performed in future studies to calculate the precise number of patients needed to be included. Since around 5% volume change was observed in hippocampal and cortical volumes of subgroups, sample size required to detect this magnitude of alteration with a power of 90% and a significance level of 5% would be more than 200 patients/subgroup. Thus, our results may be confirmed by prospective multicenter studies with a larger sample size. Finally, since our aim was to evaluate volumetric changes of major anatomical locations that were previously shown to be affected by SIBD, such as hippocampus, deep gray matter nuclei, and cerebral cortex, we did not choose anatomical locations manually and instead we analyzed the default anatomical regions determined by an automated volumetric analysis system. This approach may limit to show changes in the regions not defined in the automatic segmentation process and changes in the subregions of the predefined areas.
Conclusions
In conclusion, our results suggest that volumetric analysis of the brain stands out as a sensitive measure of volume change especially in hippocampi and cerebral white matter. Volume differences of specific brain regions may be used as a marker of prognosis and outcome. Serial volumetric evaluations may be used to assess the efficacy of treatment response in SIBD. Further studies comparing brain volume values with follow-up clinical findings and cognitive test scores are required to better assess the clinical benefits of this method in SIBD. Furthermore, glial activity, neurotransmitter imbalance, endothelial cell activation, and blood–brain barrier disruption may be more precisely assessed by specific positron emission tomography methods and other new imaging techniques and correlated with volumetric data and levels of inflammation markers.
References
Iacobone E, Bailly-Salin J, Polito A, et al. Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med. 2009;37(10):S331–6.
Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513.
Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–62.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94.
Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–7.
Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–66.
Polito A, Eischwald F, Maho AL, et al. Pattern of brain injury in the acute setting of human septic shock. Crit Care. 2013;17(5):R204.
Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27(10):2179–90.
Suchyta MR, Jephson A, Hopkins RO. Neurologic changes during critical illness: brain imaging findings and neurobehavioral outcomes. Brain Imaging Behav. 2010;4(1):22–34.
Orhun G, Tüzün E, Özcan PE, et al. Association between inflammatory markers and cognitive outcome in patients with acute brain dysfunction due to sepsis. Arch Neuropsychiatry. 2019;56(1):63.
Orhun G, Esen F, Ozcan PE, et al. Neuroimaging findings in sepsis-induced brain dysfunction: association with clinical and laboratory findings. Neurocrit Care. 2019;30(1):106–17.
Semmler A, Widmann CN, Okulla T, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013;84(1):62–9.
Heming N, Mazeraud A, Verdonk F, et al. Neuroanatomy of sepsis-associated encephalopathy. Crit Care. 2017;21(1):65.
Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228.
Sutter R, Chalela JA, Leigh R, et al. Significance of parenchymal brain damage in patients with critical illness. Neurocrit Care. 2015;23(2):243–52.
Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–10.
Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44.
Posner JB, Plum F, Saper CB, Schiff N. Plum and Posner’s diagnosis of stupor and coma, vol. 17. Oxford: OUP USA; 2007.
Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55.
Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2022–32.
Finke C, Kopp UA, Pajkert A, et al. Structural hippocampal damage following anti-N-methyl-d-aspartate receptor encephalitis. Biol Psychiatry. 2016;79(9):727–34.
Finke C, Pruss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol. 2017;74(1):50–9.
Yoneda Y, Mori E, Yamashita H, Yamadori A. MRI volumetry of medial temporal lobe structures in amnesia following herpes simplex encephalitis. Eur Neurol. 1994;34(5):243–52.
Anderson VM, Fisniku LK, Khaleeli Z, et al. Hippocampal atrophy in relapsing-remitting and primary progressive MS: a comparative study. Mult Scler. 2010;16(9):1083–90.
Semmler A, Hermann S, Mormann F, et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38.
Peng QY, Wang YM, Chen CX, et al. Inhibiting the CD38/cADPR pathway protected rats against sepsis associated brain injury. Brain Res. 2018;1678:56–63.
Fu Q, Wu J, Zhou X-Y, et al. NLRP3/Caspase-1 pathway-induced pyroptosis mediated cognitive deficits in a mouse model of sepsis-associated encephalopathy. Inflammation. 2019;42:306–18.
Zaghloul N, Addorisio ME, Silverman HA, et al. Forebrain cholinergic dysfunction and systemic and brain inflammation in murine sepsis survivors. Front Immunol. 2017;8:1673.
Femminella GD, Ninan S, Atkinson R, et al. Does microglial activation influence hippocampal volume and neuronal function in Alzheimer’s disease and Parkinson’s Disease dementia? J Alzheimers Dis. 2016;51(4):1275–89.
Kondo A, Sugiura C, Fujii Y, et al. Fulminant sepsis-associated encephalopathy in two children: serial neuroimaging findings and clinical course. Neuropediatrics. 2009;40(4):157–61.
Acknowledgements
The authors thank the personnel of the Multidisciplinary Critical Care Unit at the University of Istanbul for support and are indebted to Bio. Fatma Vildan Adali for assistance.
Funding
This study was funded by Scientific Research Projects Coordination Unit of Istanbul University (Grant Nos. 35165 and 2793/53037).
Author information
Authors and Affiliations
Contributions
GO, ET, BB, SS, and FE contributed to conception and design of the study. GO, PEÖ, and FE contributed to acquisition, analysis, and interpretation of data from sepsis patients. BB contributed to acquisition, analysis, and interpretation of volumetric analysis of brain regions. SS and MB contributed to acquisition, analysis, and interpretation of brain magnetic resonance imaging. ET and BB performed the statistical analysis. GO, ET, BB, and FE were involved in drafting the manuscript or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Ethical Approval/Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board (approval number: 2013/98), and informed consent was obtained from all individual participants or their relatives included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orhun, G., Tüzün, E., Bilgiç, B. et al. Brain Volume Changes in Patients with Acute Brain Dysfunction Due to Sepsis. Neurocrit Care 32, 459–468 (2020). https://doi.org/10.1007/s12028-019-00759-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00759-8