Abstract
Background
Intracranial pressure (ICP) monitoring is central to the care of severe traumatic brain injury (TBI). External ventricular drains (EVD) allow ICP control via cerebrospinal fluid drainage, whereas intraparenchymal monitors (IPM) for ICP do not, but it is unclear whether EVD placement improves outcomes. To evaluate whether there exists a difference in patient outcomes with the use of EVD versus IPM in severe TBI patients, we conducted a retrospective cohort study using data from the Citicoline Brain Injury Treatment trial.
Methods
Adults with Glasgow Coma Score < 9 who had either an EVD or IPM placed within 6 h of study center arrival were included. We compared patients with EVD placement to those without on Glasgow Outcome Scale-Extended (GOS-E) and neuropsychological performance at 180 days, mortality, and intensive care unit length of stay. We used regression models with propensity score weighting for probability of EVD placement to test for association between EVD use and outcomes. Of 224 patients included, 45% received an EVD.
Results
EVD patients had lower GOS-E at 180 days [3.8 ± 2.2 vs 4.9 ± 2.2, p = 0.002; weighted difference − 0.97, 95% CI (− 1.58, − 0.37)], higher in-hospital mortality [23% vs 10%, p = 0.014; weighted OR 2.46, 95% CI (1.20, 5.05)], and did significantly worse on all 8 neuropsychological measures. Additional sensitivity analysis was performed to minimize confounding effects supported our initial results.
Conclusions
Our retrospective data analysis suggests that early placement of EVDs in severe TBI is associated with worse functional and neuropsychological outcomes and higher mortality than IPMs and future prospective trials are needed to determine whether these results represent an important consideration for clinicians.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Background
Traumatic brain injury (TBI) is a major public health issue in the USA and globally. Over 2.5 million emergency department (ED) visits, 250,000 hospitalizations and 50,000 deaths yearly are attributable to TBI in the USA [1]. In addition, brain injury results in significant financial burden to society with an estimated $13.1 billion in direct costs and $51.2 billion in indirect costs every year [2]. Complications from severe TBI remain substantial, with mortality rates as high as 40% despite improvements in critical care [3].
Intracranial pressure (ICP) monitoring is frequently employed to guide management of patients with severe TBI in high-income countries [4]. External ventricular drains (EVD) consist of a catheter placed into the lateral cerebral ventricle, tunneled through the scalp, and connected to a drainage and pressure monitoring system; removal of cerebrospinal fluid (CSF) through the catheter can be used to control ICP. Intraparenchymal monitors (IPM) are placed into the brain parenchyma and provide accurate measurement of ICP, but do not allow for CSF drainage. Although EVDs are generally considered the “gold standard,” studies comparing EVD-based management to intraparenchymal monitoring (IPM) techniques have produced variable results [5,6,7,8]. Current Brain Trauma Foundation (BTF) guidelines recommend ICP monitoring in the management of severe TBI but do not detail the choice of monitoring technique [9]. As these two monitoring approaches vary in terms of complications, cost, availability, and management difficulty, understanding their relationship with patient outcome is important. In the absence of randomization, observational analyses are confounded by various factors influencing the choice of monitoring technique in individual patients, as well as the utility of EVDs as therapeutic devices outside of their monitoring capabilities. This study examines the impact on in-hospital TBI care and long-term functional outcomes of EVD versus IPM as the initial ICP monitoring modality in an attempt to provide meaningful clinical guidance in choice of ICP monitor.
Objectives
We chose to examine the data collected as part of the Citicoline Brain Injury Treatment Trial (COBRIT) as it represents carefully collected data from multiple centers with established care paradigms for severe TBI and, in the absence of a randomized controlled trial, represents the best available data for analysis [10]. We hypothesized that the choice of ICP monitor would not alter patient outcomes, either in hospital or at follow-up.
Methods
Study Design
Prospectively collected data from the COBRIT were analyzed retrospectively. All data analyses were approved by the University of Washington IRB. COBRIT was a randomized, double-blind, placebo-controlled, multicenter trial of the effects of 90 days of citicoline on functional outcome in patients with complicated mild, moderate, and severe TBI [10]. Functional outcomes were assessed at 30, 90, and 180 days after the day of randomization. The primary outcome consisted of neuropsychological and functional measures analyzed as a composite measure using a global test procedure at 90 days. The trial showed no effect of citicoline on outcome,
Setting
Specific methodological details of COBRIT, including specific inclusion and exclusion criteria, were previously published [10]. Briefly, patients aged 18–70 years with nonpenetrating TBI were included. Computed tomography (CT) scans, vital signs, participant medical history, demographics, and injury information were obtained and reviewed prior to randomization. Information concerning other medical treatments, including surgical interventions, in-depth injury information, changes in clinical status, and vital signs, were collected within 24 h after randomization (noted here as Day 1). Routine laboratories and vital signs were obtained throughout days 2 through 7 of the hospitalization.
Patient Selection
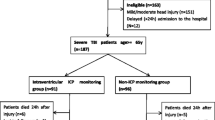
We compared severe TBI patients (Glasgow Coma Scale [GCS] < 9) who received an EVD within 6 h of arrival at the study hospital with or without an IPM, i.e., ICP monitoring with simultaneous drainage or at least the possibility to do so, to those who received an IPM with or without a late (placed after 6 h of study hospital arrival) EVD, i.e., ICP monitoring with parenchymal monitor without simultaneous drain placement. The purpose of this limitation on time to EVD placement was to analyze the use of EVD versus IPM as an initial ICP monitoring device and to exclude EVDs that may have been placed at a later time for therapeutic CSF drainage in cases of refractory ICP. The choice of 6 h as a time window was done to try and include as many patients with severe TBI as possible in the analysis and based upon a rough estimation of the amount of time it takes for initial ED analysis to the decision of whether ICP monitoring is necessary. We identified 224 patients in total; 123 with no EVD placement within 6 h, and 101 patients with an EVD placed within 6 h either exclusively or in combination with an IPM.
Demographics and Injury and Clinical Characteristics
Early information extracted from the COBRIT database includes participant demographics (age, sex, race, Hispanic ethnicity), injury characteristics (presenting GCS, pupillary exam at presentation [i.e., number fixed and number dilated], findings on initial CT, acute injury scale [AIS] –Head,) and clinical data (surgical intervention [craniotomy or craniectomy], indicators of coagulopathy, including prothombin time [PT], partial thromboplastin time [PTT] and international normalized ratio [INR]).
Outcomes
Primary outcome was Glasgow Outcome Scale-Extended (GOS-E) at 180 days. Secondary outcomes included in-hospital mortality, length of intensive care unit (ICU) stay, and measures of functional status and cognitive performance measured by neuropsychological tests performed around 180 days after injury. Neuropsychological testing included the California Verbal Learning Test, Controlled Oral Word Association Test, processing speed index (PSI), Trails A and B, digit span, and Stroop I and II [10].
Statistical Analysis
We compared EVD patients to IPM patients with respect to demographics, injury characteristics, and clinical data using Fisher’s exact and Mann–Whitney tests as appropriate. Given that type of ICP monitoring was not randomized, and the large number of potential predictors under consideration, we first used logistic regression to estimate the probability of receiving an EVD, the propensity model [11]. We considered all the variables mentioned above except PT, PTT and INR which had a high proportion of missing data and used forward selection with a significance cutoff of 0.30 to derive the propensity model. We used an unusually liberal variable inclusion cutoff because there is virtually no penalty for including extra unrelated variables in the propensity model [12]. The propensity model for the entire group included race, GCS motor, GCS eye plus verbal, number of fixed pupils, and presence of an epidural hematoma (EDH). We used inverse probability weighting based on probabilities from the logistic regression propensity model to decrease the confounding effect of imbalances in considered demographic and injury or clinical characteristics [13]. This method increases the weight of the data from participants who are under-represented in their treatment group compared to those who are over-represented while keeping the overall sum of the weights (sample size) unchanged. For example, to even out the groups, whites who are over-represented in the No-EVD group would get weights under 1 if they were in that group while they would get weights over 1 if they were in the EVD group. Both unadjusted (data from each participant gets a weight of 1) and inverse probability weighted results are presented, as are the unweighted and weighted descriptives. We evaluated differences in outcome using regression modeling with no covariates, both unadjusted and with inverse probability weighting. Confidence intervals were based on robust standard errors. We used logistic regression for in-hospital mortality, linear regression on ranks for ICU duration, number of neuroworsening events, 6-month GOS-E, and neuropsychological measures in which subjects who died or were too impaired to test were assigned the lowest ranks. We used the Holm–Bonferroni method to account for multiple comparisons when interpreting the p values for the outcomes [14]. Although the p values were obtained from rank regression, a corresponding linear regression was also performed and the estimate of the mean difference and its confidence interval are presented from these analyses to aid interpretability.
Although inverse probability weighting can overcome confounding by the variables considered for the propensity model, it does not account for unmeasured confounders or selection bias. In this multicenter study, 3 sites almost always used EVDs and 2 sites almost never did, with 2 or fewer cases treated by the method not favored at that site. To examine results uninfluenced by confounding by indication, we analyzed only the cases from these five hospitals and assigned cases to EVD or No-EVD based on the predominant monitoring method of the hospital rather than the monitoring technique actually used for individual patients. Forty-seven cases were treated in hospitals that predominantly inserted EVDs and 77 were treated in hospitals that predominantly did not. The model for propensity to be treated in a hospital that predominantly used EVDs included race, GCS motor, GCS eye plus verbal, AIS head, and presence of an EDH.
As sensitivity analyses, we looked at results requiring EVD placement within 3 h of arrival and at any time in the hospital course. As an additional sensitivity analysis for confounding by indication, we used as an instrumental variable the percent of cases in a hospital that were in the EVD group [15, 16]. This is analogous to the analysis by Cnossen et al. [17] and, as was done in that paper, excluded hospitals with fewer than 20 cases. Additional sensitivity analyses compared mortality and 6-month morbidity in each group to that expected according to the international mission for prognosis and analysis of clinical trials in TBI (IMPACT) laboratory model and corticosteroid randomisation after significant head injury (CRASH) high-income country Core model [18, 19]. We also performed logistic or rank regression without weighting but allowing the analyses to select potential confounding variables for adjustment in a stepwise manner using a p value of .05 for adding a variable and .10 for removing one.
Results
Participants
Two hundred twenty-four patients qualified for the analysis. Of those, 101 had an EVD placed within 6 h of arrival to the study hospital.
Demographics
We compare the demographic and injury characteristics of these groups in Table 1 and admission head CT and clinical data in Table 2. In the unweighted comparison, those who had EVD placed were more likely to be Black, and there was a trend for them to have a higher GCS but more fixed pupils and dilated pupils and a lower PTT. With the weighting, no differences were significant, with the lowest p value over 0.1.
Outcomes/Main Results
When comparing those monitored with EVD versus IPM without weighting, all differences were significant in the direction of better outcome in those who were monitored using IPMs (Table 3). For example, 67% of cases monitored using IPMs had GOS-E of 5 through 8 (favorable outcome) while only 45% of those monitored using EVDs did, whereas in-hospital mortality was 10% in those monitored using IPM while it was 23% in those monitored with EVDs. With inverse probability weighting, all outcomes were significantly better among those monitored by IPMs.
When we compared those treated in hospitals that primarily used EVDs versus those treated in hospitals that primarily used IPMs (in order to avoid possible confounding by indication), all differences were still in the direction of better outcome among those treated at hospitals that primarily used IPMs (Table 4). Although fewer differences were statistically significant, as would be expected just from the reduction in sample size, length of ICU stay and 6 of the 8 cognitive measures were significantly better in the IPM hospitals after accounting for multiple comparisons both before and after propensity adjustment. Weighted and unweighted sample characteristics are in Supplemental figure 1.
When we compared mortality and morbidity to that predicted by the IMPACT and CRASH models, poor outcomes were about as expected in the EVD group while they were about half of the expected rate in the group monitored initially without an EVD (Table 5). Of note, when time to EVD placement was lowered to 3 h or the time constraint was removed the significance of our analysis was not affected (Supplemental figure 2).
As additional sensitivity analyses, we evaluated the effect of the instrumental variable percent of participants in the individual’s hospital who received and EVD on 6-month GOS-E and got results comparable to that of the propensity weighted analysis (Supplemental figure 3).
We also used stepwise regression rather than propensity weighting to adjust for measured potential confounders, with significantly better outcome in the No EVD group for all outcomes (Supplemental figure 4).
We examined infection and intracranial bleed rates with EVD and IPM placement as possible causes of worse outcome in EVD placement patients. As recorded, however, the rates for both these complications were minimal and we were unable to analyze these effectively.
We performed an initial examination ICP control in the EVD vs IPM group and found no significant difference in the daily ICP average or highs over the first 5 days (Supplemental figure 5). However, due to the limitations of the COBRIT database, we were unable to look at significant trends or area under the curve calculations that would be helpful in future studies. In addition, we looked at whether CSF drainage was reported in the first day after EVD placement and found that in the majority of patients receiving an EVD did have CSF drainage on day 1. Whether this was intermittent or continuous drainage was not clear, nor is it clear from the database the reason for CSF drainage. (Supplemental figure 5).
Discussion
The hypothesis that choice of ICP monitor, IPM versus EVD, was not associated with differences in outcome was not supported by our analysis. The use of an EVD was associated with worse outcomes at 6 months and increased in-hospital mortality in this study. We controlled for selection bias and employed propensity weighting to control bias from observed confounders and still saw significant differences in patient outcomes comparing IPM to EVD placement in severe TBI. Our data suggests that the placement of EVD in post-traumatic patients needs further evaluation.
ICP monitoring has become a cornerstone in the management of severe TBI in high-income countries. It allows for the continuous reporting of ICP to facilitate interventions against potential secondary injuries that evolve over hours to days after a severe injury. Close evaluation of ICP allows for medical management of cerebral compliance, perfusion pressure and by proxy, cerebral oxygenation. Decreases in perfusion pressure have been linked to poor immediate and long-term outcomes and targeted cerebral profusion pressure management has shown mortality and outcome benefits [20, 21]. There remains uncertainty over whether ICP monitoring improves outcomes. Recent studies, both retrospective and prospective, have suggested that the use of an ICP monitor does not improve functional outcomes [22,23,24,25]. Regardless, current BTF guidelines recommend ICP monitor placement in severe TBI and ICP monitoring is often considered the standard of care in resource-rich medical systems. Choice of ICP monitor at initial presentation is a key clinical moment in the care of TBI.
ICP can be continuously and accurately monitored via EVD or IPM. Previous recommendations preferred the EVD, based on its accuracy and relative low cost [26]. Additionally, EVD placement allows for CSF drainage to manage elevated ICP. However, EVD placement is associated with a higher risk of complications when compared to IPM, including hemorrhage and infection [27, 28]. EVD accuracy is also sensitive to changes in the height of the pressure transducer and to clogging of the drainage system. This suggests a role for IPM as a simpler and safer alternative.
There have been a few studies examining the impact of EVD vs IPM after severe TBI, and the data have been inconclusive. The most recent retrospective analysis by Aiolfi et al. [5] demonstrated no difference in their primary outcome of 30-day mortality between EVD and IPM use in 2562 Trauma Quality Improvement Program patients with head AIS scores of ≥ 3. A retrospective review of 377 adults with ICP monitor placement showed that EVD use was associated with potential worsened complications and prolonged ICU stay compared to an IPM [6]. More recently, a study in a Chinese neuro-ICU suggested that placement of an EVD catheter versus an IPM was associated with improved mortality and glasgow outcome scale (GOS) at 6 months [7]. The results of this study can be partially explained by the use of the EVD to release CSF for treatment of refractory ICP and it is unclear how often this was necessary in their patient population. Recently Volovici et al. performed a meta-analysis of six studies examining outcomes of ventricular drains compared to IPM. They noted that each of the studies had considerable bias and did no report adequate outcomes, overall quality of the studies was poor [8]. However, they were able to analyze pooled outcomes of mortality and functional outcomes demonstrating no difference between modalities. Furthermore, they noted that ventricular drains demonstrated more complications with infection being the most significant. They concluded that due to a high risk of bias more studies were necessary to make any more meaningful comparison of EVD versus IPM. The study we present here attempts to address many of the concerns raised by Volovici’s meta-analysis. We have done robust analysis in multiple different ways to try and adjust for the inherent bias and confounders central to the difficulty comparing EVD to IPM placement. We have delineated clear timepoints of analysis (less than 6 h for placement time), and we have performed extensive sensitivity analysis. While we cannot address all of the concerns that come with a retrospective analysis, we believe that our study adds another compelling argument for the need for a clear well-defined randomized control trial to identify whether EVD placement versus IPM is potentially harmful or helpful in the severe TBI patients. With ICP monitoring playing such an important role in severe TBI clinical interventions, the question of how to monitor ICP is critical.
We present here a retrospective clinical analysis of the use of EVD versus IPM in severe TBI using data from a large, prospective, multicenter study wherein the choice of monitor was not specified. Unlike prior studies, we have limited our analysis to investigation of EVD vs IPM within the initial presenting period after injury in order to compare the use of these devices exclusively as first-line ICP measurement systems and to eliminate the impact of the CSF drainage capacity of an EVD as a therapeutic intervention. This differs from prior studies, where the insertion timeline was much longer, up to 48 h in the study done by Liu et al. [7]. The utility of CSF drainage as a method to lower ICP after a severe TBI has been reported in a number of studies. Nwachuku et al. [29] demonstrated that the use of an EVD to continuously drain CSF after severe TBI was associated with better ICP control and improved GOS at 6 months compared to intermittent drainage, but they did not compare this to patients who did not have an EVD placed. We attempted to separate the impact of CSF diversion from that of device-related monitoring on TBI-related outcomes and process variables.
However, we were not able to fully assess the utility of an EVD for CSF drainage as a tiered intervention to ICP management. While the COBRIT database does have some information regarding whether CSF was drained it does not indicate how much, continuous versus intermittent, or for what indication (i.e., infection analysis versus elevated ICP). This limits our ability to understand the importance of an EVD in the ongoing care of the patient after initial placement. We were able to do a rudimentary analysis of daily ICPs showing no difference between IPM and EVD in the first 5 days, which suggests that, at least initially, ICP control was similar between groups. What this does not assess is ICP spikes, time of elevated ICP or other adverse clinical events associated with or causative of increased ICP. This highlights another important question for a prospective trial, understanding the utility and necessity of CSF drainage as it relates to patient outcomes. At least initial analysis of the limited information in the COBRIT database would suggest that patients with EVDs did have CSF drained within the first day of injury (Supplemental figure 5). There are two possible hypotheses for this finding: (1) this may indicate that EVD placement was done in patients with a more severe injury needing CSF drainage or (2) that even with CSF drainage EVD placement did not improve outcomes in severe TBI patients. Understanding how EVDs are utilized by practitioners is a key clinical question to further studies.
Limitations
The major confounder in such a study is related to the difficulty in assessing those factors related to the managing physician’s choice of monitor in specific cases. While center-based protocols may influence choice of monitor, it is also likely that aspects related to an interaction between perceived injury severity or salvageability may have influenced this choice in individual patients. Such factors may have covaried with the physiologic likelihood of good outcome or other aspects such as intensity of treatment, limitation-of-care considerations, or other critical outcome determinants. Propensity score methods allow for the estimation of treatment effect by retrospectively accounting for covariates that predict receiving the treatment [30]. In essence, we utilized propensity analysis as a way to account for physician choice by predicting which patients should have received an EVD based upon their presenting clinical data, insofar as those data are recorded. Using this methodology to control confounding, we have demonstrated that early placement of an EVD after severe TBI is associated with worse GOS, worse neurocognitive scores, and a 2.5-fold increase in the odds of in-hospital mortality. There likely are unrecorded impressions that the clinicians used to decide who should get which type of monitor. We took advantage of sites using predominantly one monitoring method as another way to avoid confounding by indication. The results using this approach still favored IPM.
Secondly, in order to identify a decent number of patients for our analysis, we set our time constraint to EVD placement within 6 h of arrival at the hospital. This is largely an artificial timepoint that was determined based upon our own institutional experience as to the timeline of placement for an ICP monitor. Given that many patients with a severe TBI have concomitant injuries that may require treatment by other services prior to initial head CT or neurological evaluation, there are a subset of patients who do not receive an ICP monitor immediately upon recognizing brain injury. We attempted to address this concern by providing a second analysis at a 3-h timepoint which demonstrated no difference in our analysis. Future examinations should strongly consider analyzing timing of EVD placement with respect to outcomes as this initial attempt to do so is an imperfect attempt to address that consideration.
The reasons for this difference in patient outcome with EVD placement are unclear. Pupillary size and reactiveness is well known to correlate with outcomes and while not significant there was a tendency for patients who received an EVD to have a report of at least one enlarged pupil prior to ICP monitor placement. This could explain the difference in our outcomes as physicians may have chosen to place an EVD in patients with pupillary changes over an IPM. We employed multiple different statistical controls to force the inclusion of this difference into our propensity analysis and regression and the effect of EVD use remained. Prior studies have demonstrated differences in the bleed and infection rate between IPM and EVD which could account for worse outcomes. Within the COBRIT database, there were very few infections or secondary bleeds recorded; as such, we are unable to draw a conclusion on what the impact of EVD placement is on hemorrhage or infection rates in our study population. We did, however examine “neuroworsening events”, defined within the COBRIT database as a change in neurological status of the patient. Interestingly, there was a significant difference between EVD and IPM groups, with patients who received EVDs having a higher number of these events and these may represent new bleeds or infections that were not otherwise delineated. While limited in evaluation, this finding suggests that the placement of an EVD may have been associated with a subsequent increase in secondary events similar to what has been reported previously [6]. Unfortunately, we could not ascertain the nature of these events.
Conclusion
Our initial hypothesis that our analysis would show that choice of ICP monitor would not affect patient outcomes was incorrect. We had hypothesized that the data would show equivalent outcome with either EVD or IPM for ICP monitoring and thus provide physicians with reassurance that either modality is reasonable to use in the care of severe TBI.
This study suggests that placement of an EVD versus an IPM in the first 6 h after severe TBI is associated with a higher incidence of in-hospital mortality and worse functional and neurocognitive outcomes at 6 months post-injury. Utilizing propensity score methods to minimize confounding demonstrates that this effect is independent of recorded patient presentation including pupil size and reactivity. Using analysis by hospital using predominantly one method allowed control of confounding by indication. The causative factors for this difference in outcome are unclear at this time, but careful thought should be put into the indications for initial EVD placement for ICP monitoring in severely brain injured patients. This study does not address the use of an EVD for therapeutic drainage of CSF for ICP control and that is a variable that should be strongly considered in future evaluations. Our data coupled with past studies suggest the need for a more controlled analysis of the appropriate timing and indication for the use of EVDs in severe TBI.
References
Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16.
Dams-OʼConnor K, Mellick D, Dreer LE, et al. Rehospitalization over 10 years among survivors of TBI: a national institute on disability, independent living, and rehabilitation research traumatic brain injury model systems study. J Head Trauma Rehabil. 2017;32(3):147–57.
Nguyen R, Fiest KM, McChesney J, et al. The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43(6):774–85.
Piccinini A, Lewis M, Benjamin E, Aiolfi A, Inaba K, Demetriades D. Intracranial pressure monitoring in severe traumatic brain injuries: a closer look at level 1 trauma centers in the United States. Injury. 2017;48(9):1944–50.
Aiolfi A, Khor D, Cho J, Benjamin E, Inaba K, Demetriades D. Intracranial pressure monitoring in severe blunt head trauma: does the type of monitoring device matter? J Neurosurg. 2018;128(3):828–33.
Kasotakis G, Michailidou M, Bramos A, et al. Intraparenchymal vs extracranial ventricular drain intracranial pressure monitors in traumatic brain injury: less is more? J Am Coll Surg. 2012;214(6):950–7.
Liu H, Wang W, Cheng F, Yuan Q, Yang J, Hu J, Ren G. External ventricular drains versus intraparenchymal intracranial pressure monitors in traumatic brain injury: a prospective observational study. World Neurosurg. 2015;83(5):794–800.
Volovici V, Huijben JA, Ercole A, et al. Ventricular drainage catheters versus intracranial parenchymal catheters for intracranial pressure monitoring-based management of traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2019;36(7):988–95.
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury. Neurosurgery. 2017;80(1):6–15.
Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: citicoline brain injury treatment trial (COBRIT). JAMA. 2012;308(19):1993–2000.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22:278–95.
Hernan MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60:578–86.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70.
Brookhart MA, Schneeweiss S. Preference-based instrumental variable methods for the estimation of treatment effects: assessing validity and interpreting results. Int J Biostat. 2007;3(1):14.
Agoritsas T, Merglen A, Shah ND, O’Donnell M, Guyatt GH. Adjusted analyses in studies addressing therapy and harm: users’ guides to the medical literature. JAMA. 2017;317(7):748–59.
Cnossen MC, van Essen TA, Ceyisakar IE, et al. Adjusting for confounding by indication in observational studies: a case study in traumatic brain injury. Clin Epidemiol. 2018;18(10):841–52.
Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329–37.
MRC CRASH Trial Collaborators, Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336(7641):425–9.
Xie Q, Wu H-B, Yan Y-F, Liu M, Wang E-S. Mortality and outcome comparison between brain tissue oxygen combined with intracranial pressure/cerebral perfusion pressure-guided therapy and intracranial pressure/cerebral perfusion pressure-guided therapy in traumatic brain injury: a meta-analysis. World Neurosurg. 2017;100:118–27.
Petkus V, Preiksaitis A, Krakauskaite S, et al. Benefit on optimal cerebral perfusion pressure targeted treatment for traumatic brain injury patients. J Crit Care. 2017;41:49–55.
Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471–81.
Bennett TD, DeWitt PE, Greene TH, et al. Functional outcome after intracranial pressure monitoring for children with severe traumatic brain injury. JAMA Pediatr. 2017;171(10):965–71.
Cremer OL, van Dijk GW, van Wensen E, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit Care Med. 2005;33(10):2207–13.
Farahvar A, Gerber LM, Chiu Y-L, Carney N, Härtl R, Ghajar J. Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg. 2012;117(4):729–34.
Brain Trauma Foundation. American association of neurological surgeons, congress of neurological surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106.
Khan SH, Kureshi IU, Mulgrew T, Ho SY, Onyiuke HC. Comparison of percutaneous ventriculostomies and intraparenchymal monitor: a retrospective evaluation of 156 patients. Acta Neurochir Suppl. 1998;71:50–2.
Tsioutis C, Karageorgos SA, Stratakou S, et al. Clinical characteristics, microbiology and outcomes of external ventricular drainage-associated infections: the importance of active treatment. J Clin Neurosci. 2017;42:54–8.
Nwachuku EL, Puccio AM, Fetzick A, et al. Intermittent versus continuous cerebrospinal fluid drainage management in adult severe traumatic brain injury: assessment of intracranial pressure burden. Neurocrit Care. 2014;20(1):49–53.
D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81.
Funding
None.
Author information
Authors and Affiliations
Contributions
WB, RB, RB, JB, NT, RC—Manuscript preparation and data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval/Informed Consent
All research contained here in was approved by an institutional review board as meeting the standards for retrospective data collection and analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental figure 1
. Demographics and injury characteristics for those patients analyzed by site as presented in Table 4 (DOCX 28 kb)
Supplemental figure 2
. Alternate samples. This table shows analysis done to examine the effect of limiting our consideration for EVD placement to less than 3 hrs or considering all patients to have ever received an EVD. Here, we demonstrate that there is no difference in outcomes if we change our inclusion criteria for timing of EVD placement. (DOCX 19 kb)
Supplemental figure 3.
As additional sensitivity analyses, we evaluated the effect of the instrumental variable percent of participants in the individual’s hospital who received and EVD on 6-month GOSE and got results comparable to that of the propensity weighted analysis. (DOCX 15 kb)
Supplemental figure 4.
Demonstration of a stepwise regression rather than propensity weighting to adjust for measured potential confounders, with significantly better outcome in the No EVD group for all outcomes. (DOCX 21 kb)
Supplemental figures 5.
Brief evaluation of average and high ICP values within the first five days after admission demonstrating no significant difference in ICP values. In addition whether CSF drainage was performed within the first day after admission has been examined. Data demonstrates that the majority of patients with an EVD placed had CSF drainage but it is unclear for what reason and if this drainage was continuous or intermittent. Further studies will need to delineate this information more completely. (DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Bales, J.W., Bonow, R.H., Buckley, R.T. et al. Primary External Ventricular Drainage Catheter Versus Intraparenchymal ICP Monitoring: Outcome Analysis. Neurocrit Care 31, 11–21 (2019). https://doi.org/10.1007/s12028-019-00712-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00712-9