Abstract
Background
Delirium is common during sepsis, although under-recognized. We aimed to assess the value of continuous electroencephalography (cEEG) to aid in the diagnosis of delirium in septic patients.
Methods
We prospectively evaluated 102 consecutive patients in a medical intensive care unit (ICU), who had sepsis or septic shock, without evidence of acute primary central nervous system disease. We initiated cEEG recording immediately after identification. The median cEEG time per patient was 44 h (interquartile range 21–99 h). A total of 6723 h of cEEG recordings were examined. The Confusion Assessment Method for the ICU (CAM-ICU) was administered six times daily to identify delirium. We analyzed the correlation between cEEG and delirium using 1252 two-minute EEG sequences recorded simultaneously with the CAM-ICU scorings.
Results
Of the 102 included patients, 66 (65%) had at least one delirium episode during their ICU stay, 30 (29%) remained delirium-free, and 6 (6%) were not assessable due to deep sedation or coma. The absence of delirium was independently associated with preserved high-frequency beta activity (> 13 Hz) (P < 10−7) and cEEG reactivity (P < 0.001). Delirium was associated with preponderance of low-frequency cEEG activity and absence of high-frequency cEEG activity. Sporadic periodic cEEG discharges occurred in 15 patients, 13 of whom were delirious. No patient showed clinical or electrographic evidence of non-convulsive status epilepticus.
Conclusions
Our findings indicate that cEEG can help distinguish septic patients with delirium from non-delirious patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Delirium and sepsis are serious complications of critical illness. Within intensive care units (ICUs), delirium and sepsis affect an estimated 60–80% of mechanically ventilated patients and 20–50% of patients receiving noninvasive mechanical ventilation [1]. Delirium is associated with increased 6-month mortality, longer duration of ICU and hospital stay, and severe persisting cognitive decline [2,3,4,5,6]. Bedside clinical screening tests for delirium, such as the Confusion Assessment Method for the ICU (CAM-ICU) [7, 8], do not reliably assess the fluctuating course of delirium [9].
In delirious patients, routine electroencephalography (EEG) commonly show increased slow-wave activity and a slowed and disrupted alpha rhythm [10,11,12,13,14]. However, it remains unclear whether delirium affects continuous EEG (cEEG) activity in the high-frequency range that reflects information processing [15, 16].
Here, we used cEEG to assess time-variant brain function among septic patients and examined the hypothesis that cEEG results could distinguish patients with delirium from non-delirious patients. We report data from power spectral cEEG analysis, simultaneously acquired CAM-ICU scores [11, 13], and data from manual cEEG analysis [17]. Our results revealed that delirious episodes in septic patients were associated with disappearance of high-frequency electrographic cEEG activity and increased power of low-frequency activity. Seizure-like activity was not observed in any septic patient in this cohort.
Methods
Patients
This single-center prospective observational study included critically ill patients, without known acute central nervous system affliction, who were admitted to a medical ICU with sepsis or septic shock (defined by the 2012 sepsis guidelines) [18] and mono- or multi-organ failure. Respiratory insufficiency, circulatory shock, and hepatic or renal failure, respectively, were indicated by the need for invasive or noninvasive mechanical ventilation, vasoactive drugs, or continuous renal replacement therapies (i.e., dialysis). Inclusion criteria were age > 18 years and expected stay in the ICU of > 24 h. Exclusion criteria were expected death within 24 h, psychiatric illness, preexisting delirium, severe dementia, cerebrovascular event within 6 months, major surgery within 2 months, and not speaking Danish or English.
We screened 726 patients who were admitted between July 2013 and September 2015. A total of 117 patients were included, of whom 15 were subsequently excluded (Fig. 1), leaving 102 patients for analysis. Of the 15 excluded patients, 8 had a medical history of cerebrovascular events, 2 suffered clinical and computed tomography-verified cerebrovascular events after inclusion, and 7 withdrew consent or died within the first few hours after inclusion.
This study was approved by the National Committee on Health Research Ethics (file number 1300778) and the Danish Data Protection Agency (file number 2007-58-0015). We performed cEEG at the earliest opportunity after admission, in accordance with Danish legislation permitting deferred consent for emergency research. Informed consent was obtained as soon as possible from the patient’s next of kin and general practitioner, or from patients who were able to make decisions.
Methods
cEEG
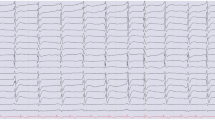
We initiated cEEG as soon as clinically feasible after admission (median 9 h, interquartile range [IQR] 6–31 h). Depending on sedation level and patient compliance, cEEG was continued until ICU discharge or for 7 days, whichever occurred first. CEEG recordings were obtained using 19 silver/silver chloride electrodes positioned according to the international 10–20 system with full scalp coverage. Cap electrodes were applied when tolerated by patients, and when the expected length of ICU stay was < 48 h. For patients expected to stay in the ICU for ≥ 48 h, we used subdermal wire electrodes (SWE) or conductive plastic electrodes (CPE) [19]. Bilateral electro-oculograms and electromyography electrodes were applied in sedated patients. Electrode–skin impedance was kept < 10 kΩ for cap electrodes, and < 5 kΩ for SWE and CPE. We recorded cEEG data using the NicoletOne™ EEG system, version 5.71 (Nicolet®, Natus Medical Incorporated) with a sampling rate of 1 kHz, online band-pass filtering from 1 to 120 Hz, 12 dB/octave, and referenced to an fronto-central sagittal electrode. EEG technicians and trained ICU nurses ensured the quality of cEEG recordings.
EEG Reactivity Tests
Eye-opening and eye-closing were performed three times daily in all patients. ICU nurses used the CAM-ICU to perform delirium screening six times daily at fixed intervals from 8 a.m. until 10 p.m. Suction was applied in deeply sedated, mechanically ventilated patients. These maneuvers provided a fair assessment of cEEG reactivity. The reactivity was defined as change of amplitude, frequency of the EEG, including attenuation [17]. The cEEG reactivity protocol and results are included as Supplemental Digital Content.
EEG and Epilepsy
An epileptic seizure is defined as “a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain” [20, 21]. Electrographically, we defined a seizure based on the revised terminology for rhythmic and periodic patterns in critically ill patients with coma/stupor [17].
Protocol for Managing Pain, Agitation, and Delirium
Pain, agitation, and delirium were managed following the department’s protocol, in accordance with recommendations of the Society of Critical Care Medicine, the PAD (pain, agitation, and delirium) guidelines, and the ABCDEF bundle [7]. Spontaneous Awakening and Breathing Trials were performed daily at morning rounds. Pain was assessed using the Numeric Rating Scale and treated with intravenous opioids as the first-line drug of choice. Sedation was assessed using the Richmond Agitation–Sedation Scale (RASS). Light sedation levels (RASS 0 to − 2) were primarily maintained with opioids, α2-agonists, and propofol, supplemented with benzodiazepines only when this strategy proved insufficient. Delirium was assessed using the CAM-ICU and treated with α2-receptor agonists and, when needed, antipsychotics (haloperidol or olanzapine) to overcome motor agitation. Mobility and daily exercise with a physical therapist were established early. This protocol was not changed due to study participation.
Delirium Screening
The CAM-ICU flowsheet tests the following features in a standardized fashion: (1) acute onset or fluctuating course of a change from mental status baseline, (2) inattention, (3) altered level of consciousness, and (4) disorganized thinking [22]. Patients were scored as CAM-ICU positive (delirious) or CAM-ICU negative (non-delirious). Heavily sedated or unconscious patients (RASS −3 to −5) were rated as non-assessable. All nurses were experienced with the CAM-ICU, as this test was implemented as daily routine in this ICU in 2008.
Data Collection
We recorded baseline demographic data, including age, gender, medical history, neurologic and cognitive status, medication, smoking, and alcohol and/or substance abuse. Predicted mortality and disease severity were assessed using the Simplified Acute Physiology Score and Acute Physiology and Chronic Health Evaluation (APACHE II).
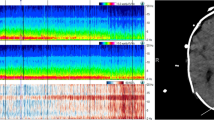
Visual Analysis of cEEG Data (Fig. 2)
The cEEG data were described using American Clinical Neurophysiology Society terminology [17] with appropriate adaption for a medical study population. Predictor variables were predominant EEG background frequencies categorized as follows: high (beta 13–30 Hz), low (delta 0.5–3.9 Hz; theta 4–7.9 Hz), normal (posterior dominant alpha 8–12.9 Hz), reactivity (defined as a change in background activity upon eye-opening or stimulation), suppression (< 10 µV), burst suppression, frontally predominant generalized rhythmic delta activity (GRDA), and periodic discharges.
We performed visual analysis of the entire recording from all patients for two purposes: to qualitatively assess the entire recording for the occurrence and localization of predictor variables, including seizure activity; and to analyze 2-min epochs correlating in time with CAM-ICU scores to represent time covariation of the predictor variables in statistical analysis. The cEEG recordings were manually screened by three experienced EEG technicians. Sequences with severe artifact contamination were excluded. We allowed minor artifacts (e.g., electrocardiogram, eye blinking, or pulse/sweat artifacts) in a maximum of 10% of the 2-min epochs. Subsequently, the recordings were described according to the protocol by two clinical neurophysiologists blinded to delirium and sedation scores (K Benedek and O Urdanibia-Centelles). These two readers reached a consensus on predictor variables as described.
We assessed the prevalence of the predictor variables per 24-h period focusing in periodic discharges, seizures and status epilepticus, and all predictor variables mentioned above during the hour of CAM-ICU scoring for the qualitative analysis. For the quantitative analysis, we isolated the 2-min sequences from the exact times of the six daily CAM-ICU scores (8 a.m., 11 a.m., 3 a.m., 6 p.m., 8 p.m., and 10 p.m.), totaling 1252 two-minute sequences for statistical analysis.
EEG and Delirium
Due to deaths during the observation period, the data were right censored and unsuitable for standard regression analysis. Furthermore, the presence of delirium was non-assessable in some observations because of sedation, which makes the data interval censored when analyzing for predictors of delirium. Multi-state Markov models are used to model state occupancy- and state transition probabilities and can handle interval censored data. In addition, they can be used to estimate the effects of covariates on the state transition hazard rate.
To assess the association between electrographic patterns and delirium, we applied a multi-state Markov model, with the states being defined as non-delirious or delirious, and non-assessable observations as being censored. All states were defined as reversible. Univariate regression was performed to identify predictor variables significantly associated with delirium, with a statistical threshold of α = 0.05. Subsequently, all variables retained from univariate analysis were subjected to multivariate stepwise regression using backward selection. Predictor variables included EEG delta, theta, and beta activity; normal background; burst suppression; suppression < 10 µV; reactivity; periodic discharges; GRDA; and illness severity at admission estimated by APACHE. EEG data were analyzed using R version 3.3.1 [23] with the msm package [24].
Survival Analysis
The Cox proportional hazards model can be used for censored data, but can only model time to the first event, typically death. Thus, variations of the original Cox model and new types of models [25] have been developed for analyzing the effects of time-dependent covariates (predictors that change over time) on the risk of having an event as outcome. Using time-dependent Cox proportional hazards regression, we assessed the associations between electrographic patterns and death, and between delirium and death. We applied backward selection, and the predictive variables included delta, theta, and beta activity; normal background; burst suppression; suppression < 10 µV; reactivity; periodic discharges; GRDA; and APACHE II and CAM-ICU scores. Descriptive data were analyzed using IBM SPSS statistics version 22. Continuous variables were presented as median and interquartile range (IQR), and categorical variables as proportions. The analysis was done using the survival package [26]. P < 0.05 was considered statistically significant.
Results
Patient Demographics, Clinical Characteristics, and Medication (Table 1)
The median APACHE II score was 22 (IQR 16–28), and the median length of ICU stay was 5.6 days (IQR 3–9 days). A total of 66 patients (65%) experienced at least one delirious episode during admission, whereas 30 (29%) remained delirium-free. Six patients (6%) could not be assessed at any time due to a RASS of < −2. Nineteen patients (19%) died in the ICU, with a 1-month mortality rate of 35%, and 6-month mortality rate of 54%.
Qualitative Analysis
We visually analyzed a total of 6723 h (280 days) of EEG recording. The time elapsed from admission to monitoring was median 9 h (IQR 6–31 h). The median cEEG time per patient was 44 h (IQR 21–99 h). Continuous, nearly continuous, or intermittent low-frequency delta and theta activity were associated with delirium and occurred only intermittently in non-delirious patients. Normal CAM-ICU scores were associated with continuous or nearly continuous high-frequency cEEG beta activity, preserved cEEG reactivity, and normal cEEG background activity. Comparatively, 28 patients with delirious CAM-ICU scores exhibited suppressed background cEEG activity, which was not observed in any non-delirious patients. Delirious patients received propofol and benzodiazepines (Table 1), while non-delirious patients did not and showed preserved beta activity. Thus, in non-delirious patients, cEEG beta activity reflected the ability to produce high-frequency electrographic activity, not sedation.
We found no evidence of non-convulsive status epilepticus (NCSE) in our patient cohort, but we detected sporadic periodic discharges (PDs) in 15 patients, of whom 13 (87%) were delirious, 1 was non-delirious, and 1 was not assessable due to coma. PDs were rare, with only 22 episodes, each lasting < 2 min, in 6723 h of recording. Thirteen PDs were lateralized, nine were bilateral, and none were generalized. We observed no runs of PDs reaching 3–4 per second, no spatiotemporal evolution, no evolving pattern, and no decrementing termination. Of the 15 patients with PDs, 9 (60%) died in the ICU or elsewhere in the hospital. The patients with PDs who died had a higher median APACHE II score (28; IQR 26–29) than those who survived (23; IQR 20–24). We suggest that the PDs may represent brain pathology rather than epilepsy. Our dataset could not confirm the notion that NCSE and PDs are common in severe sepsis [27].
Quantitative Analysis
Delirium (Table 2)
Transition from non-delirium to delirium revealed by univariate analysis that delirium was significantly associated with the presence of continuous cEEG delta activity (hazard ratio [HR] 1.6; 95% confidence interval [95% CI] 1.2–2.1; P < 10−5), cEEG theta activity (HR 1.6; 95% CI 1.2 − 2.0; P < 0.05), and suppression of cEEG background (HR 1.7; 95% CI 1.1–2.6; P < 10−6). On the other hand, the absence of delirium was significantly associated with continuous cEEG beta activity (HR 0.7; 95% CI 0.5–0.9; P < 10−6), normal cEEG background activity (HR 0.3; 95% CI 0.1–0.5; P < 10−6), and preserved cEEG reactivity (HR 0.22; 95% CI 0.09–0.52; P < 10−9). Delirium was not correlated with APACHE II, burst suppression, generalized rhythmic delta activity, or PDs in univariate analysis. Multivariate analysis included cEEG delta, theta, and beta activity; normal background; suppression; and reactivity. The absence of delirium remained significantly associated with preserved cEEG beta activity (HR 0.7; 95% CI 0.5–0.9; P < 10−9), and EEG reactivity. On the other hand, the termination of delirium was significantly associated with the reoccurrence of beta (HR 1.6; 95% CI 1.2–2.1; P < 10−6) and the decrease in delta (HR 0.7; 95% CI 0.5–1.0; P < 10−5).
Mortality (Table 3)
We analyzed cEEG reactivity in every 2-min epoch during the entire cEEG recording from each patient. The presence or absence of cEEG reactivity was recorded every 8 h upon manual eye-opening, when talking to the patient, and during arousal six times daily during CAM-ICU scoring. All cEEG epochs were used included in data analysis. Preserved cEEG reactivity during all instances of arousal was the only statistically significant marker for reduced mortality (HR 0.4; 95% CI 0.2–0.9; P < 0.05), in accordance with previous studies [27] and recent data [28].
Discussion
Our present results suggest that preserved cEEG power in the beta band is a simple indicator of non-delirium in awake or lightly sedated ICU patients with sepsis. With regard to indicators of delirium, reduced high-frequency cEEG beta activity was superior to increased cEEG delta activity [11, 13]. Preserved cEEG reactivity was associated with reduced mortality and preservation of cognitive function. Periodic discharges were rare, and treatment-requiring epileptic activity was not observed in 6723 h of cEEG recordings from 102 patients.
High-Frequency Activity
Cerebral rhythmic cEEG activity reflects the cortical neurons’ ability to synchronize input from thalamo-cortical and cortico-cortical neurons. Specifically, cortical beta activity generation requires fast spiking of highly energy-demanding somatostatin-positive interneurons [29]. We posit that sepsis interferes with brain blood flow and, consequently, with energy supply—thus causing neuronal desynchronization expressed as loss of power in the beta band [30]. Further studies are needed to elucidate this relationship.
When interpreting the present data, we must consider the patients’ exposure to sedative drugs since both propofol and benzodiazepines can induce beta activity [31], interfere with cEEG reactivity testing, or inhibit PDs [27]. Notably, sedatives may be more frequently prescribed for hyperactive delirious patients—thus inducing suppression-burst, low-voltage, unreactive cEEG, or beta-enriched patterns. Sedation-induced beta activity is primarily prefrontal, appearing as spindles with diffuse polymorph high delta activity, and diffuse spontaneous beta activity [32]. In our population, sedation with propofol and/or benzodiazepines in delirious patients was not associated with increased beta activity upon arousal for confusion assessment method for the intensive care unit testing. This suggests that even if these drugs may induce beta activity in normal brains, delirious brains are unable to produce this type of cEEG activity. Based on the clinical and electrographic correlation between non-delirious lightly sedated patients and diffuse high-frequency activity, it seems unlikely that the beta activity was caused by sedation. We propose that cEEG beta activity was prominent in non-delirious brains because they were able to produce fast network activity. The data in Table 1 show that treatment with propofol and benzodiazepines was not associated with cEEG beta activity; patients with preserved cEEG beta activity did not receive propofol or benzodiazepines. Thus, we posit that beta activity in our patient cohort reflected preserved perception and cognition rather than an effect of medication [15, 16].
Periodic Discharges
PDs represent cerebral dysfunction and may occur as ictal, postictal, or peri-ictal phenomena, and as a signature of septic encephalopathy [27]. PDs were rare in our population, occurring in only 15% of the patient cohort with sepsis, primarily in delirious patients [33,34,35]. EEG is indispensable for diagnosing NCSE because the clinical signs (if any) are often subtle and nonspecific [36]. NCSE may resemble hypoactive delirium, potentially involving confused thinking, reduced environmental awareness, reduced motor activity, sluggishness, or abnormal drowsiness. Therefore, delirious patients commonly undergo routine EEGs due to suspicion of NCSE. In the 6723 h of analyzed EEG recordings, we observed no episodes of seizures or NCSE. Previous studies show an 8–48% prevalence of NCSE among medical and neurologic ICU patients [37,38,39]. This can partly be explained by selection bias since prior studies have retrospectively analyzed EEG data recorded upon clinical suspicion of NCSE. Nevertheless, one previous study showed results consistent with our findings [40]. One prospective observational study found PDs associated with NCSE in a mixed neurologic–medical ICU population [27], and PDs and NCSE are reportedly common among patients in a surgical ICU [41], while our present study only included patients without signs of neurologic disease. These observations imply that the cEEG pattern during sepsis and delirium reflects the disease etiology.
Reactivity
EEG reactivity is a powerful indicator of the overall prognosis in coma, but there is high interrater variability in the visual detection of reactivity. Previous studies show that visual analysis of EEG reactivity has an overall sensitivity of 40–85% and specificity around 20–90%, while analysis using quantitative computerized methods shows a sensitivity of around 40% and specificity of 100% [42]. Here, cEEG reactivity was assessed by two certified clinical neurophysiologists, and consensus between them was considered the basis for identifying a change in background cEEG activity upon eye-opening or stimulation. Preserved cEEG activity was associated with reduced mortality, which is consistent with previous findings [28].
Limitations
The risk of confounders, or that a common cause/reason to two effects are correlated remains. This could be, for example, that the severity of the illness drives delirium, and at the same time the chance of getting treated with a drug X. If drug X decreases beta activity, then the effect of the drug and the effect of delirium are confounded resulting in a false correlation between delirium and reduced beta cEEG activity. It is not possible based on the information we had available to correct for this type of confounding conditions.
Conclusions
We performed a prospective observational study of cEEG in a cohort of patients with sepsis in a medical ICU. In 6723 h of analyzed cEEG data, NCSE was not observed at any time. Preserved cEEG high-frequency activity was the strongest marker for wakeful absence of delirium. Delirium was characterized by neuronal desynchronization and loss of power in high-frequency EEG activity, as confirmed by bedside clinical screening with the CAM-ICU test. Early detection of cerebral deterioration by cEEG [43] may provide a means for identifying an early window for intervention to prevent lethargy, coma, or agitated delirium and brain pathology in septic patients.
Abbreviations
- ICU:
-
Intensive care unit
- cEEG:
-
Continuous electroencephalography
- CAM-ICU:
-
Confusion Assessment Method for the ICU
- RASS:
-
Richmond Agitation–Sedation Scale
- CRRT:
-
Continuous renal replacement therapies
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation
- GRDA:
-
Generalized rhythmic delta activity
- IQR:
-
Interquartile range
- NCSE:
-
Non-convulsive status epilepticus
- PD:
-
Periodic discharges
- HR:
-
Hazard ratio
References
Brummel NE, Vasilevskis EE, Han JH, Boehm L, Pun BT, Ely EW. Implementing delirium screening in the ICU. Crit Care Med. 2013;41(9):2196–208.
Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16. https://doi.org/10.1056/NEJMoa1301372.
Ely E, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–900. https://doi.org/10.1007/s00134-001-1132-2.
Ely EW. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753. https://doi.org/10.1001/jama.291.14.1753.
Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–8.
Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients*. Crit Care Med. 2004;32(4):955–62.
Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Pharm. 2013;70(1):53–8.
Meagher DJ, Morandi A, Inouye SK, et al. Concordance between DSM-IV and DSM-5 criteria for delirium diagnosis in a pooled database of 768 prospectively evaluated patients using the delirium rating scale-revised-98. BMC Med. 2014;12(1):164. https://doi.org/10.1186/s12916-014-0164-8.
Van Eijk MM, Van Den Boogaard M, Van Marum RJ, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med. 2011;184(3):340–4.
Koponen H, Partanen J, Paakkonen A, Mattila E, Riekkinen PJ. EEG spectral analysis in delirium. J Neurol Neurosurg Psychiatry. 1989;52:980–5.
Van Der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94–101.
van Dellen E, van der Kooi AW, Numan T, et al. Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology. 2014;121(2):328–35.
van der Kooi AW, Leijten FSS, van der Wekken RJ, Slooter AJC. What are the opportunities for EEG-based monitoring of delirium in the ICU? J Neuropsychiatry Clin Neurosci. 2012;24(4):472–7. https://doi.org/10.1176/appi.neuropsych.11110347.
Koponen H. Electroencephalographic indices for diagnosis of delirium. Int Psychogeriatr. 1991;3(2):249–51.
Iijima M, Mase R, Osawa M, Shimizu S, Uchiyama S. Event-related synchronization and desynchronization of high-frequency electroencephalographic activity during a visual Go/No-Go paradigm. Neuropsychobiology. 2015;71(1):17–24.
Chung JW, Ofori E, Misra G, Hess CW, Vaillancourt DE. Beta-band activity and connectivity in sensorimotor and parietal cortex are important for accurate motor performance. Neuroimage. 2017;144(Pt A):164–73.
Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology society’s standardized critical care EEG terminology. J Clin Neurophysiol. 2013;30(1):1–27.
Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. https://doi.org/10.1007/s00134-012-2769-8.
Alvarez V, Rossetti AO. Clinical use of EEG in the ICU. J Clin Neurophysiol. 2015;32(6):481–5.
Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):522–30. https://doi.org/10.1111/epi.13670.
Falco-Walter JJ, Scheffer IE, Fisher RS. The new definition and classification of seizures and epilepsy. Epilepsy Res. 2018;139:73–9.
Amirfarzan H, Pun BT, Ely EW. CAM-ICU Flowchart CAM-ICU. 2006. https://www.icudelirium.org.
R: The R project for statistical computing. 2016 [cited 2018 Aug 21]. https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf.
Software CJ-J of statistical, 2011 undefined. Multi-state models for panel data: the msm package for R. jstatsoft.org [cited 2019 Feb 3]. https://www.jstatsoft.org/article/view/v038i08/v38i08.pdf.
Therneau TM, Grambsch PM. The cox model. New York: Springer; 2000. p. 39–77. https://doi.org/10.1007/978-1-4757-3294-8_3.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. https://doi.org/10.18637/jss.v067.i01.
Gilmore EJ, Gaspard N, Choi HA, et al. Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med. 2015;41(4):686–94. https://doi.org/10.1007/s00134-015-3709-1.
Azabou E, Navarro V, Kubis N, et al. Value and mechanisms of EEG reactivity in the prognosis of patients with impaired consciousness: a systematic review. Crit Care. 2018;22(1):184.
Chen G, Zhang Y, Li X, et al. Distinct inhibitory circuits orchestrate cortical beta and gamma band oscillations. Neuron. 2017;96(6):1403–1418.e6.
Kann O. The interneuron energy hypothesis: implications for brain disease. Neurobiol Dis. 2015. https://doi.org/10.1016/j.nbd.2015.08.005.
San-juan D, Chiappa KH, Cole AJ. Propofol and the electroencephalogram. Clin Neurophysiol. 2010;121(7):998–1006.
Schomer DL, da Silva FL. Niedermeyer’s electroencephalography: basic principles, clinical applications, and related fields. 6th ed. Philadelphia: Wolters Kluwer Health; 2010.
García-Morales I, García MT, Galán-Dávila L, et al. Periodic lateralized epileptiform discharges. J Clin Neurophysiol. 2002;19(2):172–7.
Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22(2):79–91.
Pedersen GL, Rasmussen SB, Gyllenborg J, Benedek K, Lauritzen M. Prognostic value of periodic electroencephalographic discharges for neurological patients with profound disturbances of consciousness. Clin Neurophysiol. 2012. https://doi.org/10.1016/j.clinph.2012.06.010.
Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54(SUPPL. 6):28–9.
Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37(6):2051–6.
Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009;109(2):506–23.
Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54(2):340. https://doi.org/10.1212/WNL.54.2.340.
Young GB, Bolton CF, Archibald YM, Austin TW, Wells GA. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol. 1992;9(1):145–52.
Kurtz P, Gaspard N, Wahl AS, et al. Continuous electroencephalography in a surgical intensive care unit. Intensive Care Med. 2014;40(2):228–34.
Henri C, Duez V, Qvist Ebbesen M, et al. Large inter-rater variability on EEG-reactivity is improved by a novel quantitative method. Clin Neurophysiol. 2018;129:724–30. https://doi.org/10.1016/j.clinph.2018.01.054.
Claassen J, Taccone FS, Horn P, Holtkamp M, Stocchetti N, Oddo M. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39(8):1337–51.
Funding
This study was funded by Research grant from the Grosserer Jakob Ehrenreich and wife Grete Ehrenreichs Foundation, Rigshospitalet and University of Copenhagen, Denmark.
Author information
Authors and Affiliations
Contributions
RMN conceived the study and was responsible for data acquisition and drafted the manuscript; OUC carried out the interpretation and data analyses and drafted the manuscript; EVL performed the statistical analysis and drafted the manuscript; KJT, KSO, and AØL helped with data collection and revised the manuscript; KM helped with the study coordination and revised the manuscript; HSE helped with de data acquisition and revised the manuscript. ML and KB conceived the study and coordinated the study, also revised and helped to draft the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the National Committee on Health Research Ethics (file number 1300778) and the Danish Data Protection Agency (file number 2007-58-0015). Informed consent was obtained from all individual participants included in the study.
Registered at ClinicalTrials.gov ID
NCT01980251.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nielsen, R.M., Urdanibia-Centelles, O., Vedel-Larsen, E. et al. Continuous EEG Monitoring in a Consecutive Patient Cohort with Sepsis and Delirium. Neurocrit Care 32, 121–130 (2020). https://doi.org/10.1007/s12028-019-00703-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00703-w