Abstract
Background/Purpose
Primary intracerebral hemorrhage (ICH) studies often use hematoma location rather than ICH etiologies when assessing outcome. Characterizing ICH using hematoma location is effective/reproducible, but may miss heterogeneity among these ICH locations, particularly lobar ICH where competing primary ICH etiologies are possible. We subsequently investigated baseline characteristics/outcome differences of spontaneous, primary ICH by their etiologies: cerebral amyloid angiopathy (CAA) and hypertension.
Methods
Primary ICH clinical/outcomes data were prospectively collected between 2009 and 2015. Modified Boston criteria were used to identify “probable/definite” and “possible” CAA-ICH, which were evaluated separately. SMASH-U criteria were used to identify hypertension ICH. Medication and systemic disease coagulopathy ICH were excluded. Baseline characteristics/outcomes among “probable/definite” CAA-ICH, “possible” CAA-ICH, and hypertension ICH were compared using logistic regression. Mortality models using ICH etiologies compared to hematoma location as predictor variables were assessed.
Results
Two hundred and four hypertension ICHs, 55 “probable/definite” CAA-ICHs, and 46 “possible” CAA-ICHs were identified. Despite older age and larger ICH volumes, lower hospital mortality was seen in “probable/definite” CAA-ICH versus hypertension ICH (OR 0.2; 95% CI 0.05–0.8; p = 0.02) after adjusting for female gender, components of ICH score, and EVD placement. There were no mortality differences between “possible” CAA-ICH and hypertension ICH. However, lower hospital mortality was seen in “probable/definite” versus “possible” CAA-ICH (OR 0.2; 95% CI 0.04–0.7; p = 0.02). When using ICH etiology rather than hematoma location, hospital mortality models significantly improved (χ2: [df = 2, N = 305] = 6.2; p = 0.01).
Conclusions
Further investigation is required to confirm the mortality heterogeneity seen within our primary ICH cohort. Hematoma location may play a role for these findings, but the mortality differences seen among lobar ICH using CAA-ICH subtypes and a failure to identify mortality differences between “possible” CAA-ICH and hypertension ICH suggest the limitations of accounting for hematoma location alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebral amyloid angiopathy intracerebral hemorrhage (CAA-ICH) is a common etiology of lobar ICH in the elderly due to β-amyloid deposition in cortical and leptomeningeal arteries. Hypertension is a common etiology of deep ICH, but can also cause lobar ICH due to degeneration of small perforating end arteries resulting in lipohyalinosis and arteriosclerosis [1]. Despite lobar ICH having these competing etiologic diagnoses, primary ICH studies often categorize ICH by hematoma location as location has become an increasingly recognized factor in outcome/mortality [2, 3]. Subsequently, primary ICH has often become simplified to CAA-ICH: lobar, hypertension ICH: deep.
However, by using ICH location over ICH etiology categorization, it is assumed that ICH location primarily drives outcome differences seen between ICH etiologies and that baseline characteristic/outcomes are similar within ICH locations. Studies evaluating ICH by etiology rather than ICH location have shown outcome differences between groups, but these were largely driven by differences in medication and systemic disease coagulopathy etiologies and no differences were seen between CAA-ICH and hypertension ICH [4]. However, it is difficult to assess whether there truly are no outcome differences between CAA-ICH and hypertension ICH as many of these studies included “possible” CAA-ICH using Boston criteria when analyzing CAA-ICH. Though sensitive, “possible” CAA-ICH has suboptimal specificity for CAA but is often included in CAA-ICH analysis [5]. We subsequently sought to evaluate risk factors and outcomes among CAA and hypertension ICH, specifically in the absence of therapeutic anticoagulation and systemic disease coagulopathy while accounting for differences in diagnostic specificities of Boston criteria. Additionally, we compared mortality models using predictor variables: ICH etiology versus hematoma location.
Methods
Primary ICH was analyzed from an ongoing prospective cohort study of ICH patients admitted to Columbia University Medical Center. This institutional review board approved study: ICH Outcomes Project (ICHOP), collects demographics, clinical/neuroimaging characteristics, ICH etiology, interventions, and outcomes which are adjudicated by consensus in weekly meetings of study physicians. Further details regarding ICHOP have been described previously [6]. Informed consent was obtained by patients/family.
Patient Selection and Data Collection
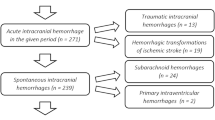
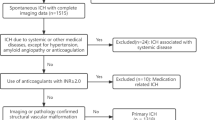
Adult (> 18 years old) ICH patients were enrolled in ICHOP between 2009 and 2015. Traumatic ICH, hemorrhagic conversion of ischemic stroke, subarachnoid hemorrhage, subdural/epidural hematoma, and secondary ICH were excluded. Four hundred and twenty-five spontaneous, primary ICHs within ICHOP were identified. CAA-ICH was diagnosed based on modified Boston diagnostic criteria [5, 7] (Supplement 1). Given the lower diagnostic specificity of “possible” CAA-ICH (compared to excellent specificity of “probable” and “definite” CAA-ICH) [5], this was assessed separately from “definite/probable” CAA-ICH. Hypertension ICH was diagnosed using SMASH-U criteria (pre-ICH blood pressure ≥ 160/100 mmHg either on or off antihypertensive therapy, mention of pre-ICH elevated blood pressure with left ventricular hypertrophy, or pre-ICH use of blood pressure medication) [4]. Lobar and deep hemorrhages that did not meet criteria for CAA and hypertension ICH, respectively, were adjudicated as “unknown” etiology and excluded. ICH patients with systemic disease coagulopathy or therapeutic anticoagulation (defined as warfarin with INR ≥ 2, novel oral anticoagulants within 3 days, or therapeutic heparin per SMASH-U criteria) [4] were also excluded to effectively compare outcomes between only CAA and hypertension ICH without any confounding medication or systemic disease effect on outcome.
Patients were managed according to American Heart Association/American Stroke Association. Stroke guidelines [8] and enrolled prior to the release of ATACH2 [9]. Treatment involved strict systolic blood pressure control < 140 mmHg, appropriate reversal of antithrombotic medications, extraventricular drain (EVD) placement for hydrocephalus or intraventricular hemorrhage (IVH), and hemicraniectomy ± clot evacuation for refractory elevated intracranial pressure. Medical (hemorrhage reversal transfusions, hyperosmolar therapy) and surgical interventions (hemicraniectomy, hematoma evacuation, EVD) were prospectively recorded.
ICH was diagnosed via admission non-contrast head computed tomography, and semiautomatic hematoma size measurements (MIPAV, NIH: Bethesda, MD) were obtained. An MRI was obtained where structural lesions or CAA-ICH was suspected. Modified Rankin Scale outcomes were obtained at discharge. Full methodological details have been described previously [6].
Statistical Analysis
Analyses were performed using SPSS (ver23). Intergroup differences were determined using Mann–Whitney U or t test for continuous variables and Chi-square or Fisher exact test for categorical variables. Univariable differences were entered into logistic regression models to determine associations with “probable/definite” CAA-ICH, hypertension ICH, and “possible” CAA-ICH. Additional logistic regression models assessed ICH etiology association with mortality after adjusting for established covariates of ICH outcome [10] and significant intergroup differences. Model performance using hematoma location (lobar vs non-lobar) versus ICH etiology (“probable/definite,” “possible” CAA-ICH, hypertension ICH) as predictor variables for mortality was performed via − 2 log-likelihood statistic. Statistical significance was judged at p value < 0.05.
Results
Study Population
Of 425 primary ICH patients, 305 spontaneous, small-vessel disease (non-anticoagulated) ICH patients were identified (Fig. 1). One hundred and one (33%) CAA-ICH (55 “probable/definite” and 46 “possible”) and 204 (67%) hypertension ICHs were identified with intergroup comparisons as shown in Table 1. No differences in severity scores, medication use (antiplatelet, sub-therapeutic anticoagulant, statins), do not resuscitate, or withdrawal of care were seen between groups.
Among the 46 “possible” CAA-ICH patients, 20 received an magnetic resonance imaging (MRI) scan and 3 received an MRI with intraoperative biopsy without evidence of CAA. All patients with “possible” CAA-ICH that received MRI did not have any evidence of lobar microbleeds. An MRI was obtained in all but 3 patients with “probable/definite” CAA-ICH (all MRIs had evidence of lobar microbleeds). The 3 “probable” CAA-ICH patients that did not receive MRI had intraoperative (non-autopsy) pathological evidence of CAA. Of 204 hypertension ICHs, 186 (91%) had a prior diagnosis of hypertension, and the other 18 (9%) had reports of uncontrolled blood pressure with left ventricular hypertrophy on echocardiogram. One hundred and five (51%) of the hypertension ICH patients received MRI.
“Probable/Definite” CAA-ICH Versus Hypertension ICH
Multivariable logistic regression revealed older age (OR 1.1; 95% CI 1.03–1.1; p < 0.0001), prior ICH (OR 4.1; 95% CI 1.3–12.6; p = 0.01), lower hypertension (OR 0.2; 95% CI 0.1–0.5; p = 0.001) were associated with “probable/definite” CAA-ICH compared to hypertension ICH. Female predominance in the “probable/definite” CAA-ICH group was not significant (OR 1.9; 95% CI 0.94–3.8; p = 0.07). Hematoma size was significantly larger in “probable/definite” CAA-ICH compared to hypertension ICH (21 vs 7 mL) (Table 1).
There were expected ICH location differences between “probable/definite” CAA-ICH and hypertension ICH. The majority of hypertension ICHs were deep (82%), followed by infratentorial (12%), and brainstem (6%). No infratentorial CAA-ICH was identified using Boston criteria, and all “probable/definite” CAA-ICH patients were lobar in location. Subsequently, “probable/definite” CAA-ICH had lower IVH and EVDs compared to hypertension ICH, but no other surgical/medical intervention differences were seen. Multivariable logistic regression revealed lower hospital mortality in “probable/definite” CAA-ICH compared to hypertension ICH (OR 0.2; 95% CI 0.05–0.8; p = 0.02) after adjusting for female gender, EVD differences, and known predictors of ICH mortality from ICH score: age > 80, ICH volume > 30 mL, Glasgow Coma Scale (GCS) < 13, infratentorial location, and IVH (Table 2).
“Possible” CAA-ICH Versus Hypertension ICH
Multivariable logistic regression revealed older age (OR 1.06; 95% CI 1.03–1.1; p < 0.0001) and increased odds of female gender (OR 3.5; 95% CI 1.7–7.1; p = 0.001) in “possible” CAA-ICH compared to hypertension ICH (Table 1). Similar to “probable/definite” CAA-ICH, larger hematoma sizes and less IVH and EVD placements were seen in “possible” CAA-ICH compared to hypertension ICH. However, logistic regression did not identify hospital mortality differences between “possible” CAA-ICH and hypertension ICH (OR 1.1; 95% CI 0.4–3.1; p = 0.9) after adjusting for female gender, EVD placement, and the same covariates and components of ICH score (Table 2).
Lobar ICH: “Probable/Definite” Versus “Possible” CAA-ICH
Outside of dyslipidemia, no risk factor differences were identified when evaluating lobar ICH, specifically “probable/definite” and “possible” CAA-ICH. However, “probable/definite” CAA-ICH unexpectedly had smaller hematoma volumes (21 vs 32 mL) compared to “possible” CAA-ICH. No EVD/IVH differences were seen (Table 1). Logistic regression revealed lower hospital mortality in “probable/definite” compared to “possible” CAA-ICH (OR 0.2; 95% CI 0.04–0.7; p = 0.02) after adjusting for the same covariates and components of ICH score (Table 2).
Mortality models using ICH location (2 separate ICH location definitions used: (a) lobar versus non-lobar: defined as brainstem, infratentorial, and deep; (b) lobar versus deep only) as a predictor variable did show lower mortality in lobar ICH but was not statistically significant (Table 2). However, when substituting ICH etiology instead of ICH location, the model significantly improved (χ2 (df = 2, N = 305) = 6.2; p = 0.01) in assessing associations with hospital mortality.
Discussion
We identified different baseline characteristics/outcomes when comparing “probable/definite” CAA-ICH to both hypertension ICH and “possible” CAA-ICH. Our cohort’s overall ICH hospital mortality of 20% was better than previously reported outcomes [11] likely due to the exclusion of medication/systemic disease coagulopathy ICH from the analysis. Despite this, our cohort had similar vascular risk factors as compared to other ICH cohorts [12], but higher proportions of black (29%) and Hispanic (38%) patients were seen [12, 13]. Even with this diversity, our comprehensive adjudication process for ICH etiology resulted in a similar distribution of modified Boston criteria subcategories compared to previously validated, predominantly white CAA-ICH cohorts [14]. Although “probable” and “definite” CAA-ICH have excellent diagnostic specificities, “possible” CAA-ICH’s 60% specificity (potentially lower in multiethnic cohorts) may cause inadvertent inclusion of non-CAA-ICH to CAA-ICH analysis which we forwent by analyzing “possible” CAA-ICH separately.
All CAA-ICH (both “probable/definite” and “possible”) patients had lobar ICH location and no infratentorial/cerebellar ICH patients were identified as CAA-ICH within our cohort. CAA-ICH patients were expectedly older given age > 55 requirements in the diagnostic framework of the Boston criteria. However, female gender was associated with “possible” CAA-ICH (and approached statistical significance for “probable/definite” CAA-ICH) when compared to hypertension ICH even after adjusting for age. Although prior population-based studies have shown lower overall ICH incidence in females, these gender-related differences are complex as they depend on age, ethnicity, and hematoma location. Our findings may be analogous to European studies on gender-related differences in ICH that have shown associations of female gender to lobar ICH [15].
Despite larger hematoma size and older age, “probable/definite” CAA-ICH had lower hospital mortality compared to hypertension ICH unrelated to any differences in clinical severity or withdrawal-of-care/do-not-resuscitate orders. There were no differences in mechanical ventilation, hospital length of stay, other clinical characteristics, or treatment with the exception of lower IVH and EVD placement in “probable/definite” CAA-ICH compared to hypertension ICH. Though these IVH/EVD differences may suggest the inherent importance of deep ICH location driving these hospital mortality differences [4, 12], these IVH/EVD differences were adjusted for and “probable/definite” CAA-ICH continued to be independently associated with less hospital mortality.
Additionally, “possible” CAA-ICH, despite having similarly lower IVH and EVD placement, did not have lower hospital mortality compared to hypertension ICH. Though the lower EVD placement among “possible” CAA-ICH patients encountering IVH may argue for the possibility of less aggressive measures taken in this group (contributing to the higher mortality seen), there were no differences in withdrawal-of-care or do-not-resuscitate orders seen. Furthermore, the aforementioned lower EVD placement in “possible” CAA-ICH may have been attributable to the lower IVH volume in this group compared to hypertension ICH not necessitating EVD placement.
Further suggesting that location was not the sole driving factor for mortality in our cohort was our subgroup analysis of modified Boston criteria diagnostic subgroups: “probable/definite” versus “possible” CAA-ICH. This “inter-lobar” ICH location comparison revealed lower mortality in “probable/definite” compared to “possible” CAA-ICH. Lastly, when evaluating hospital mortality by ICH location, rather than ICH etiology as a predictor variable, no significant associations were identified reiterating the heterogeneity of lobar ICH that may be inadequately captured by characterizing lobar ICH by location alone.
Limitations of our study included its single-center design, paucity of pre-admission neuroimaging, absent Apolipoprotein E genotyping, lack of postmortem examinations, lack of validation or testing of etiologic frameworks used, inherent significant interaction of ICH-etiology and location (given that all our CAA-ICH patients were lobar), and high volumes of outside hospital transfers which may not reflect a community-based ICH sample. Furthermore, our strict inclusion of only CAA and hypertension ICH created a large exclusion group. Specifically, the medication-related coagulopathy ICH etiology subgroup often includes patients that do have underlying CAA or hypertension ICH. However, given our focus on risk factors and outcome, this group was excluded to account purely for CAA or hypertension etiologies for outcome. Age diagnostic requirements for CAA-ICH (age > 55) were an inherent limitation that contributed to the older ages seen in our CAA-ICH cohort, but age adjustment was performed for all statistical models. Lastly, specific quantitative MRI analysis of markers of small-vessel disease was lacking from our current dataset in addition to the often seen bias that MRI was more frequently obtained in lobar ICH compared to deep ICH.
Conclusion
Given our findings, further investigation is warranted to confirm the risk factors, clinical/radiographic characteristics, and outcome differences seen in our cohort that distinguish “probable/definite” CAA-ICH from both hypertension ICH and “possible” CAA-ICH. The heterogeneity of lobar ICH in our cohort requires further study to better identify etiology among those with a “possible” CAA-ICH designation. This may emphasize the importance of evaluating lobar ICH by etiology rather than location alone in future studies.
References
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701.
Sreekrishnan A, et al. Intracerebral hemorrhage location and functional outcomes of patients: a systematic literature review and meta-analysis. Neurocrit Care. 2016. https://doi.org/10.1007/s12028-016-0276-4.
Delcourt C, et al. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology. 2017;88:1408–14.
Meretoja A, et al. SMASH-U a proposal for etiologic classification of intracerebral hemorrhage. Stroke. 2012;43:2592–7.
Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–9.
Witsch J, et al. Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology. 2015;84:989–94.
Linn J, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–50.
Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60. https://doi.org/10.1161/STR.0000000000000069.
Qureshi AI, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033–43.
Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke J Cereb Circ. 2001;32:891–7.
van Asch CJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
Falcone GJ, Biffi A, Brouwers H, et al. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol. 2013;70:988–94.
Woo D, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190–5.
Charidimou A, et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2016;86:505–11.
Roquer J, et al. Sex-related differences in primary intracerebral hemorrhage. Neurology. 2016;87:257–62.
Author information
Authors and Affiliations
Contributions
DR contributed to acquisition of data, analysis and interpretation, draft of the manuscript, critical revision of the manuscript for important intellectual content. CS and ESC contributed to acquisition of data and critical revision of the manuscript for important intellectual content. JMS contributed to analysis and interpretation and critical revision of the manuscript for important intellectual content. EG, SM, SP, and SA contributed to critical revision of the manuscript for important intellectual content. JC contributed to critical revision of the manuscript for important intellectual content and study supervision. David Roh, MD, takes full responsibility for the data, the analyses and interpretation. This author has full access to all of the data, and this author has the right to publish any and all data separate and apart from any sponsor. All authors have read and approved the submitted manuscript, and the manuscript has not been submitted elsewhere nor published elsewhere in whole or in any part.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roh, D., Sun, CH., Schmidt, J.M. et al. Primary Intracerebral Hemorrhage: A Closer Look at Hypertension and Cerebral Amyloid Angiopathy. Neurocrit Care 29, 77–83 (2018). https://doi.org/10.1007/s12028-018-0514-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-018-0514-z