Abstract
Background and Purpose
As survival rates have increased for intracerebral hemorrhage (ICH) patients, there is limited information regarding recovery beyond 3–6 months. This study was conducted to examine recovery curves using the modified Rankin Scale (mRS) and Barthel Index (BI) up to 12 months post-injury.
Methods
We prospectively enrolled 173 patients admitted with ICH who were subsequently evaluated using the mRS and BI at discharge as well as 3, 6, and 12 months. Repeated measures nonparametric testing was conducted to assess functional trajectories across time.
Results
The mRS scores showed significant improvement between discharge (median 4) and 3 (median 4), 6 (median 4), and 12 months (median 3) (p values <0.001). However, the mRS scores did not differ between follow-up time-points (i.e., 3–6, 6–12 months). There was significant improvement in scores using the BI (p values <0.001), showing improvement between discharge (mean 43.0) and 3 (mean 73.0), 6 (mean 78.2), and 12 months (mean 83.4). Additionally, there were differences in the BI between 3 and 12 months (p = 0.013), as well as between 6 and 12 months (p = 0.025).
Conclusions
The BI may be a more sensitive measure of long-term recovery post-injury than the mRS, which shows minimal improvement for some survivors after 3 months. BI scores indicate survivors continually improve till 12 months post-injury. These results may have implications for the prognostication of ICH and design of clinical trial outcome measures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Translating clinical research into patient care requires the ability to properly interpret clinical outcome scales. The modified Rankin Scale (mRS) and Barthel Index (BI) are designed for use in stroke patients to evaluate discharge disability [1], understand stroke prognosis [2], and assess stroke recovery [1, 3, 4]. With improvements in clinical practice leading to higher survival rates for intracerebral hemorrhage (ICH) patients [5], there is a growing emphasis on long-term recovery. Central to this understanding is how these clinical scales can capture recovery in survivors.
The mRS is a seven-point scale commonly used to assess global disability [1, 6], where 0 represents no symptoms nor disability and 6 represents death. Conversely, the BI assesses the extent of disability as a score from 0 to 100 across ten domains of activities of daily living [7]: bathing, bladder, bowels, dressing, feeding, grooming, mobility, stairs, toilet use, and transfer [3]. Among ischemic stroke patients, the mRS has been shown to better differentiate mild-to-moderate disability over 12 months [8]. Additionally, it has been suggested that ischemic stroke patients plateau in recovery on the BI between 2 and 5 months post-injury [9,10,11], suggesting maximal recovery within the first 6 months. However, this analysis of recovery has not been demonstrated in ICH survivors, whom exhibit much greater initial disability compared to ischemic stroke survivors [12], with studies in rehabilitation settings [12,13,14] suggesting differences in recovery profiles.
It has been proposed that 37.7% of ICH patients regain functional independence (i.e., BI ≥ 95) at 100 days post-injury [15]; however, to our knowledge the BI has not been used to evaluate survivors beyond this time-point in any prospective observational ICH study. Additionally, while the mRS is commonly used to assess outcome in ICH patients [16], there has been little focus on understanding the burden of disability at various time-points. As new interventions for ICH patients are being developed, choosing proper end points as well as clinically relevant assessments is important for well-designed trials. The current study was designed to prospectively evaluate the improvement in mRS and BI scores of ICH survivors across 1 year post-injury. The specific goal was to analyze the recovery profile of ICH survivors using both measures to understand how well these scales capture long-term recovery and how this may impact research practices. Our initial hypothesis was that both measures would show substantive improvement in scores between all follow-up time-points, suggesting a sustained recovery of ICH survivors up to 12 months post-injury.
Methods
This prospective observational study was conducted at a single medical center, Yale New Haven Hospital, between July 1, 2014, and October 5, 2016. Patients provided informed consent within 96 h of admission and evaluated at discharge, 3, 6, and 12 months using multiple neurological outcome measures. Inclusion criteria included patients greater than 18 years old, a computed tomography (CT) scan consistent with ICH, and a documented last known normal (LKN) time. The study subject’s medical decision maker was consented in cases in which the subject was intubated or incapacitated. Exclusion criteria included a clinical history of recent trauma or hemorrhagic conversion of an ischemic stroke.
Baseline Recordings
All demographic data were abstracted from Epic (Epic Systems Corporation, Verona, WI, USA) or obtained directly from study subjects. Intensive care unit hospitalization days, total hospitalization days, and likely etiology of ICH were all documented upon hospital discharge. If an etiology was not clearly established during the hospitalization (e.g., pending additional imaging or two potential diagnosis), the etiology was classified as “unknown.” All clinical information was stored in a REDCap™ database (Research Electronic Data Capture, Nashville, TN, USA). Two researchers (A.S. and A.L.) completed data abstraction, with an inter-rater reliability of 87.79% of the initial five subjects enrolled in the study.
Imaging Analysis
Baseline CT or CT angiography defined as the scan closest to LKN, was evaluated by two blinded independent raters (A.S. and A.L.) using SYNAPSE (Fujifilm, Tokyo, Japan). ICH location was classified as either: lobar, deep, cerebellum, brainstem, or unable to specify. All location classifications were validated by the official radiology report, and any discrepancies were adjudicated by the study P.I. (K.S.). ICH volume was determined using Analyze 11.0 (AnalyzeDirect, Overland Park, KS, USA) as previously described [17]. Volumes were determined by either of the independent raters (A.S. and A.L.), with a Pearson correlation between raters for the first ten measurements of r = 1.000, p < 0.001 for all measurements.
Outcomes Assessments
Baseline mRS and BI were collected at day 7 or discharge (±3 days). Follow-up assessments were conducted either in person or by phone at 3, 6, and 12 months (±2-week window). All evaluators completed online training regarding mRS administration and in-person staff training regarding BI administration. The mRS was collected via a semi-structured interview, and the BI was assessed using a pre-defined set of questions provided in the assessment form. Unclear cases were brought to group discussion among evaluators and adjudicated by the P.I. (K.S.).
Statistics
Initially, descriptive statistics were used to summarize demographic and baseline characteristics of the entire cohort. While mortality was included in the initial outcome characteristics, these data were subsequently excluded from any successive analyses in order to only focus on ICH survivors as stated in the overall goals. To directly compare differences between the mRS and BI, percentages of “good outcomes” were calculated using mRS < 3 and BI ≥ 85 as respective cutoffs. Cutoffs of good outcome were chosen based on their commonality within ICH research and clinical trials [16, 18, 19]. To specifically assess significant differences in the distribution of mRS scores (i.e., 0–5) between all time-points (i.e., 7 days, 3, 6, and 12 months), repeated measures nonparametric testing was conducted using the Friedman test. This test is analogous to a nonparametric version of an ANOVA, examining differences in the distribution of scores across multiple different time-points. If a significant difference was found in the distribution of scores across time, follow-up statistics were conducted using the Wilcoxon signed-rank Test. This specific test assesses for differences between individual time-points (i.e., 7 days to 3 months, 3–6 months). The same series of nonparametric tests were conducted to look for differences in the distribution of BI scores (i.e., 0–100) between time-points. Statistics were conducted using SPSS v.19 software (SPSS Inc., Chicago, IL, USA).
Ethics Approval
The study was approved by the Yale University School of Medicine IRB (HIC Approval #1405014045 and #1509016433).
Results
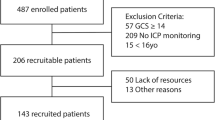
A total of 173 subjects were enrolled, with 83 subjects completing all follow-up time-points. We achieved follow-up rates of 88.5, 89.1, and 86.7% at 3, 6, and 12 months, respectively (electronic supplement Figure I). All demographics and descriptive statistics of the cohort are provided in Table 1. This cohort had 55.5% of participants over the age of 65, and 49.1% were male. The majority of subjects were Caucasian (69.4%), with hypertension (67.1%), hyperlipidemia (30.1%), and diabetes (19.7%) as the most common medical comorbidities. Additionally, 10.4% of subjects had a prior stroke, and 56.3% of these were previous ICHs. This resulted in 11.0% having a pre-admission mRS ≥ 2. At admission, the median NIHSS was 9 (±8.7), and the median GCS was 14 (±4.5).
The average admission ICH volume was 26.1 ml (±31.9), with 24.9% of subjects with an additional intraventricular hemorrhage component. Additionally, 38.2% of patients exhibited a midline shift on admission CT, which was measured at 6.9 mm (±4.1) for this subset. The majority of ICHs were deep (41.0%), with almost an equivalent percentage of lobar ICHs (39.3%); a full breakdown of ICH locations for the cohort is included in electronic supplement Table I. During hospitalization, 19.7% required decompression and 23.7% were made comfort measures only (electronic supplement Table II). Hypertension (46.8%) was the leading identifiable cause of ICH; however, there were a large proportion of “undetermined” etiologies (27.8%).
Discharge mortality was 17.3%, which increased to 26.0, 28.3, and 30.6% at 3, 6, and 12 months, respectively. Outcomes for all surviving subjects are outlined in Table 2. By 3 months, the majority of survivors were home (64.2%). This proportion increased to 83.4% by 12 months. While the proportion of subjects with a good outcome on the mRS (mRS < 3) increased from 20.0% at 7 days to 62.0% at 12 months, these proportions were more pronounced for the BI (BI ≥ 85), 20.9% to 76.6% at 7 days and 12 months, respectively (Fig. 1).
Using repeated measures nonparametric testing, there was a significant difference between time-points with the mRS, χ 2(3) = 17.37, p = 0.001 (Fig. 2). Follow-up testing showed differences between 7 days and 3 (z = −5.28, p < 0.001), 6 (z = −5.48, p < 0.001), and 12 months (z = −3.93, p < 0.001). However, there were no differences between all follow-up time-points (i.e., 3–6, 3–12, 6–12 months). The BI showed significant differences between all time-points in nonparametric testing, χ 2(3) = 40.170, p < 0.001 (Fig. 3). There were also differences between most follow-up time-points, including 3 and 12 months (z = −2.49, p = 0.013) as well as 6 and 12 months (z = −2.23, p = 0.025). There was no significant difference between 3 and 6 months (z = −1.22, p = 0.222).
Discussion
This study was designed to prospectively assess functional status of ICH survivors at repeated time-points to better characterize their recovery curves. Our results suggest significant differences in mRS scores for ICH subjects between discharge and follow-up time-points, however, not among the later time-points (e.g., 3–6, 6–12 months) (Fig. 2). Conversely, we were able to show differences on the BI between all short- and long-term time-points, except between 3 and 6 months (Fig. 3). Additionally, proportions of good outcome on either the mRS (mRS < 3) or BI (BI ≥ 85) showed more pronounced improvement via BI measurements compared to the mRS (Fig. 1). The majority of all ICH survivors were back home at 3 months, with 83.4% of living ICH subjects at home by the end of the study. Taken as a whole, these results suggest that survivors of the initial ICH injury will show improvement in overall outcomes over the course of 1 year post-injury.
The recovery profiles of our cohort suggest maximal recovery is observed on the mRS at 3 months post-ICH, but seen up to a year on the BI. Differences noted between the two measures likely stem from discrepancies in how survivors are evaluated with either measure. The mRS has a limited approach to assigning scores and lacks the specificity of the domains within the BI. For instance, the major discriminating factor within the mRS is the ability to walk, separating a score of 3 from 4. This excludes most other functional improvement from factoring into an evaluation. Conversely, the inclusion of specific activities of daily livings within the BI allows for evaluation of finer motor skills (i.e., brushing teeth, combing hair), which may take subjects longer to recover or adapt. While technically an ordinal scale, the BI is more consistent with an interval scale given the wide range of scores a subject could attain. This results in the ability to more easily discriminate outcome and achieve statistical significance. Our results suggest that the mRS is optimal for measuring short-term gross motor recovery for most ICH survivors, while the BI seems better suited to measure long-term finer motor recovery.
The utility of the BI in measuring long-term recovery in ICH patients seems to contradict research of ischemic stroke patients who plateau in recovery between 2 and 5 months [9,10,11]. It is likely that the higher initial morbidity of ICH patients [12] accounts for a delay in recovery, resulting in improved outcomes up to 1 year post-ICH. Ischemic stroke patients must compensate and undergo neuronal plasticity in the presence of dead cellular tissue. Conversely, hematoma formation in ICH may actually result in a lower burden of dead tissue. It has been suggested that neuronal plasticity only occurs once the hematoma resolves in ICH patients [20], which may take upward of months depending on volume. Consequently, ICH survivors may experience an overall good recovery that is simply delayed due to hematoma resolution.
There is currently no consensus on how these scales should be interpreted (i.e., ordinal scales or dichotomizations) [21]. Many mRS studies dichotomize outcomes [16, 22,23,24,25] into good vs. poor to improve the statistical power [26] and simplify analysis. Similarly, while some studies have used the BI with cutoffs [15, 27], others analyze the measure as a scale [28,29,30]. We chose not to dichotomize our statistical analysis, because of research suggesting that this process fails to capture smaller improvements in functional status [31] and limits applicability to other clinical trials [21]. By examining the distribution and shifts in distribution, this study is better able to evaluate finer improvements in functional status as evidenced by the improvements noted on the BI.
This study should be interpreted noting some limitations regarding its sample size. All subjects were enrolled at a single institution and reflect the clinical practices and outcomes of that institution. Additionally, effects of interventions (i.e., surgery) or baseline differences (i.e., previous stroke) were not considered in the analysis or interpretation of results. While these factors may affect overall outcomes, they would not account for the differences observed between time-points. Mortality in our cohort was approximately 30%; slightly lower than previous studies [32], but within the range of other larger cohort studies [33]. Additionally, our low initial discharge mortality may be attributable to the changes in clinical practice regarding aggressive care for ICH patients within 24 h as outlined in 2015 AHA/ASA ICH management guidelines. These were released during the course of this study [34]. This study did not include deceased subjects (mRS 6) in the analysis, which would skew overall outcomes at each of the time-points. Due to the fact that the focus of this study was on functional recovery of ICH survivors and not mortality, these results should only be applied to ICH survivors. Finally, since mRS and BI were assigned to patients at the same follow-up time-points, there could possibly be a bias when assigning scores together (i.e., rating higher on one scale can result in a favorability for higher rating on the other). This bias was minimized to an extent via semi-structured or standardized questions in evaluation. Conversely, the strengths of this study include its low loss to follow-up, with response rates over 85% for most time-points when controlling for mortality. While this study was unable to follow all its enrolled subjects to the 12-month time-point, the recovery profiles of the existing cohort already suggest a sustained recovery. We did not attempt any statistical method to impute missing data in order to avoid potential biases that may either strengthen or weaken overall conclusions.
Given the results of this observational study, further work is warranted regarding ICH recovery. Since our cohort showed improvement on the BI up to 1 year post-ICH, a longer-term study beyond 1 year would be required to evaluate the potential peak recovery in ICH survivors. These results should also prompt the inclusion of 12-month end points in ICH clinical trials, which commonly use 3- or 6-month primary end points. If the natural course of ICH injury requires a minimum at least 12 months to recover, these shorter-term end points will fail to evaluate peak efficacy of a potential intervention. Since most BI domains are scored up to 10 points, the noted improvement between 3 and 12 months (from 73.0 to 83.4; approximately 10-point difference) represents a clinically significant improvement. Finally, both of these measures focus on motor disability and do not examine quality of life, cognition, or emotional distress. Further work is still needed to evaluate non-motor-based recovery in this population.
Understanding when each of these neurological assessments should be used within the recovery process is important for both patient care and clinical research. This is the first study to our knowledge that tracks changes in both the mRS and BI in ICH survivors out to 12 months post-injury. Our results suggest that the mRS may be better suited for short-term recovery, while the BI could potentially be used up to 1 year post-injury. However, both measures are important for assessing functional status and specifically evaluate different aspects of recovery, gross versus fine motor recovery. The inclusion of both measures in clinical research seems warranted given the differences observed in ICH patients.
References
Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–12. doi:10.1016/s1474-4422(06)70495-1.
Huybrechts KF, Caro JJ. The Barthel Index and modified Rankin Scale as prognostic tools for long-term outcomes after stroke: a qualitative review of the literature. Curr Med Res Opin. 2007;23(7):1627–36. doi:10.1185/030079907x210444.
Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J. 1965;14:61–5.
Granger CV, Dewis LS, Peters NC, Sherwood CC, Barrett JE. Stroke rehabilitation: analysis of repeated Barthel Index measures. Arch Phys Med Rehabil. 1979;60(1):14–7.
McKinney JS, Cheng JQ, Rybinnik I, Kostis JB. Comprehensive stroke centers may be associated with improved survival in hemorrhagic stroke. J Am Heart Assoc. 2015;. doi:10.1161/jaha.114.001448.
New PW, Buchbinder R. Critical appraisal and review of the Rankin scale and its derivatives. Neuroepidemiology. 2006;26(1):4–15. doi:10.1159/000089536.
Lee JY, King C, Stradling D, Warren M, Nguyen D, Lee J, Riola MA, Montoya R, Patel D, Le VH, Welbourne SJ, Cramer SC. Influence of hematoma location on acute mortality after intracerebral hemorrhage. J Neuroimaging. 2014;24(2):131–6. doi:10.1111/j.1552-6569.2012.00766.x.
Weimar C, Kurth T, Kraywinkel K, Wagner M, Busse O, Haberl RL, Diener HC. Assessment of functioning and disability after ischemic stroke. Stroke. 2002;33(8):2053–9.
Pan SL, Wu SC, Wu TH, Lee TK, Chen TH. Location and size of infarct on functional outcome of noncardioembolic ischemic stroke. Disabil Rehabil. 2006;28(16):977–83. doi:10.1080/09638280500404438.
Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76(5):406–12.
Kong KH, Lee J. Temporal recovery of activities of daily living in the first year after ischemic stroke: a prospective study of patients admitted to a rehabilitation unit. NeuroRehabilitation. 2014;35(2):221–6. doi:10.3233/nre-141110.
Kelly PJ, Furie KL, Shafqat S, Rallis N, Chang Y, Stein J. Functional recovery following rehabilitation after hemorrhagic and ischemic stroke. Arch Phys Med Rehabil. 2003;84(7):968–72.
Chae J, Zorowitz RD, Johnston MV. Functional outcome of hemorrhagic and nonhemorrhagic stroke patients after in-patient rehabilitation. Am J Phys Med Rehabil. 1996;75(3):177–82.
Paolucci S, Antonucci G, Grasso MG, Bragoni M, Coiro P, De Angelis D, Fusco FR, Morelli D, Venturiero V, Troisi E, Pratesi L. Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation: a matched comparison. Stroke. 2003;34(12):2861–5. doi:10.1161/01.str.0000102902.39759.d3.
Weimar C, Roth M, Willig V, Kostopoulos P, Benemann J, Diener HC. Development and validation of a prognostic model to predict recovery following intracerebral hemorrhage. J Neurol. 2006;253(6):788–93. doi:10.1007/s00415-006-0119-x.
Sreekrishnan A, Dearborn JL, Greer DM, Shi FD, Hwang DY, Leasure AC, Zhou SE, Gilmore EJ, Matouk CC, Petersen NH, Sansing LH, Sheth KN. Intracerebral hemorrhage location and functional outcomes of patients: a systematic literature review and meta-analysis. Neurocrit Care. 2016;. doi:10.1007/s12028-016-0276-4.
Urday S, Beslow LA, Goldstein DW, Vashkevich A, Ayres AM, Battey TW, Selim MH, Kimberly WT, Rosand J, Sheth KN. Measurement of perihematomal edema in intracerebral hemorrhage. Stroke. 2015;46(4):1116–9. doi:10.1161/strokeaha.114.007565.
Winkler J, Shoup JP, Czap A, Staff I, Fortunato G, McCullough LD, Sansing LH. Long-term improvement in outcome after intracerebral hemorrhage in patients treated with statins. J Stroke Cerebrovasc Dis. 2013;22(8):e541–5. doi:10.1016/j.jstrokecerebrovasdis.2013.06.015.
Zweifel C, Katan M, Schuetz P, Ernst A, Mariani L, Muller B, Christ-Crain M. Growth hormone and outcome in patients with intracerebral hemorrhage: a pilot study. Biomarkers. 2011;16(6):511–6. doi:10.3109/1354750x.2011.599074.
Kwon H, Jang SH. Delayed recovery of gait function in a patient with intracerebral haemorrhage. J Rehabil Med. 2012;44(4):378–80. doi:10.2340/16501977-0962.
Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538–41.
Qureshi AI, Palesch YY. Antihypertensive treatment of acute cerebral hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15(3):559–76. doi:10.1007/s12028-011-9538-3.
Kim KH. Predictors of 30-day mortality and 90-day functional recovery after primary intracerebral hemorrhage: hospital based multivariate analysis in 585 patients. J Korean Neurosurg Soc. 2009;45(6):341–9. doi:10.3340/jkns.2009.45.6.341.
Matsukawa H, Shinoda M, Fujii M, Takahashi O, Yamamoto D, Murakata A, Ishikawa R. Factors associated with lobar vs. non-lobar intracerebral hemorrhage. Acta Neurol Scand. 2012;126(2):116–21. doi:10.1111/j.1600-0404.2011.01615.x.
Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y, Li H, Zhao X, Wang Y. A novel risk score to predict 1-year functional outcome after intracerebral hemorrhage and comparison with existing scores. Crit Care. 2013;17(6):R275. doi:10.1186/cc13130.
Young FB, Lees KR, Weir CJ. Strengthening acute stroke trials through optimal use of disability end points. Stroke. 2003;34(11):2676–80. doi:10.1161/01.str.0000096210.36741.e7.
Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, Yan Y, Huang D, Yu C, Shi FD. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71(9):1092–101. doi:10.1001/jamaneurol.2014.1065.
Li Q, Yang CH, Xu JG, Li H, You C. Surgical treatment for large spontaneous basal ganglia hemorrhage: retrospective analysis of 253 cases. Br J Neurosurg. 2013;27(5):617–21. doi:10.3109/02688697.2013.765938.
Agnihotri S, Czap A, Staff I, Fortunato G, McCullough LD. Peripheral leukocyte counts and outcomes after intracerebral hemorrhage. J Neuroinflammation. 2011;8:160. doi:10.1186/1742-2094-8-160.
Chen YC, Chen CM, Liu JL, Chen ST, Cheng ML, Chiu DT. Oxidative markers in spontaneous intracerebral hemorrhage: leukocyte 8-hydroxy-2′-deoxyguanosine as an independent predictor of the 30-day outcome. J Neurosurg. 2011;115(6):1184–90. doi:10.3171/2011.7.jns11718.
Lai SM, Duncan PW. Stroke recovery profile and the modified Rankin assessment. Neuroepidemiology. 2001;20(1):26–30. doi:10.1159/000054754.
Rathor MY, Rani MF, Jamalludin AR, Amran M, Shahrin TC, Shah A. Prediction of functional outcome in patients with primary intracerebral hemorrhage by clinical-computed tomographic correlations. J Res Med Sci. 2012;17(11):1056–62.
Koch S, Elkind MS, Testai FD, Brown WM, Martini S, Sheth KN, Chong JY, Osborne J, Moomaw CJ, Langefeld CD, Sacco RL, Woo D. Racial-ethnic disparities in acute blood pressure after intracerebral hemorrhage. Neurology. 2016;87(8):786–91. doi:10.1212/wnl.0000000000002962.
Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–60. doi:10.1161/str.0000000000000069.
Funding
This study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR000141. The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mr. Sreekrishnan reports grants from National Center for Advancing Translational Sciences, during the conduct of the study. Ms. Leasure, Dr. Shi, Dr. Hwang, Dr. Schindler, Dr. Petersen, Dr. Gilmore, Dr. Kamel, Dr. Sansing, Dr. Greer, and Dr. Sheth have nothing to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sreekrishnan, A., Leasure, A.C., Shi, FD. et al. Functional Improvement Among Intracerebral Hemorrhage (ICH) Survivors up to 12 Months Post-injury. Neurocrit Care 27, 326–333 (2017). https://doi.org/10.1007/s12028-017-0425-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-017-0425-4