Abstract
Patients with prolonged or rapidly recurring convulsions lasting more than 5 min are in status epilepticus (SE) and require immediate resuscitation. Although there are relatively few randomized clinical trials, available evidence and experience suggest that early and aggressive treatment of SE improves patient outcomes, for which reason this was chosen as an Emergency Neurological Life Support protocol. The current approach to the emergency treatment of SE emphasizes rapid initiation of adequate doses of first-line therapy, as well as accelerated second-line anticonvulsant drugs and induced coma when these fail, coupled with admission to a unit capable of neurological critical care and electroencephalography monitoring. This protocol will focus on the initial treatment of SE but also review subsequent steps in the protocol once the patient is hospitalized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Each year in the United States, emergency departments (EDs) experience an average of one million seizure-related visits based on International Classification of Diseases (ICD)-9 coding. These visits represent approximately 20 % of ED visits for neurological problems and 1 % of all ED visits [1–3]. Approximately, 200,000 US patients per year have prolonged or rapidly recurring convulsions lasting more than 5 min—the defining features of status epilepticus (SE).
The 30-day mortality of patients with generalized convulsive SE ranges from 19 to 27 % [4–8], while more recent randomized controlled trials suggest that this rate might be lower (hospital discharge mortality of 9.4 %) with more inclusive definitions of SE [9]. Prolonged seizures are associated with higher mortality and worse clinical outcomes [7, 8, 10–12]. Adverse effects of SE include both indirect systemic problems arising from the convulsive state (e.g., impaired ventilation, pulmonary aspiration, and metabolic aberrations) and direct neuronal cellular injury from excitotoxicity, causing both immediate neuronal loss and delayed programmed cell death.
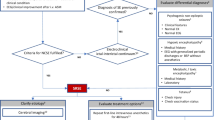
Rapid control of seizures is fundamental to the emergency treatment of SE (Fig. 1). Earlier termination of SE reduces neuronal injury in animal models of SE, and is associated with improved clinical outcomes in human observational studies. In experimental SE, benzodiazepines are more likely to terminate seizures when given closer to seizure onset and decrease in effectiveness as seizure duration increases. This is most likely related to changes in the neuronal gamma-aminobutyric acid (GABA) receptor subunit composition as a function of time [13]. Rapid seizure cessation may also prevent duration-dependent kindling and adverse cytokine-mediated effects in experimental models [14, 15].
The ENLS-suggested algorithm for the initial management of SE is shown in Fig. 1. Suggested items to complete within the first hour of evaluating a patient with SE are shown in Table 1.
Diagnosis
The importance of more rapid treatment of prolonged convulsions is reflected in the current definitions of SE. Outdated definitions of SE required that convulsions persist or recur for greater than 30 min without a return to the pre-seizure neurological baseline. The rationale for this definition has been challenged, and the more useful and clinically relevant duration of greater than five minutes of unrelenting seizure is more appropriate [16, 17].
As in patients with any other critical illness, diagnostic evaluation of patients with SE is pursued concurrently with treatment and stabilization. However, it is important to ensure that diagnostic testing does not interfere with or delay control of seizures in the ED.
Approximately, two-thirds of patients evaluated in the ED for SE have a history of a prior seizure. Approximately, half of these patients will be found to have poor adherence to anti-epileptic drugs (AEDs) or withdrawal from AEDs or other substances, and half will have idiopathic breakthrough seizures. A smaller portion have other diagnoses that lower seizure threshold.
The importance of early rapid treatment has been demonstrated in the prehospital phase, where intramuscular (IM) administration of midazolam is more successful in stopping seizures than the intravenous (IV) route; presumably the result of delays in obtaining IV access [18]. The prehospital treatment of SE is discussed below.
In the ED, a detailed neurological evaluation including a description of ongoing convulsions, automatisms, focal deficits, pupillary changes, and level of arousal is also useful. Hypoglycemia, hypoxia, and hemodynamic instability are addressed during the patient’s initial evaluation and stabilization. Oxygen saturation and cardiac monitoring are typically initiated during this phase.
Blood and serum laboratory evaluation typically includes a complete blood count, basic metabolic panel, and calcium and magnesium determinations. Selected laboratory studies that may be useful in some patients include liver enzymes, cardiac injury markers, toxicology screen, and arterial blood gas determinations. AED levels for specific medications, such as phenytoin/fosphenytoin, valproate, and carbamazepine, may be obtainable on an acute basis and can be helpful to direct management.
The need for neuroimaging should be determined for each individual but is generally warranted in patients who do not return to a normal level of consciousness, have new focal neurological findings, or have new onset SE without an otherwise obvious identifiable etiology. Non-contrast computed tomography (CT) of the brain will identify most immediate threats and is the most typical initial imaging study obtained in the ED. Chest X-rays and electrocardiograms should also be obtained selectively. A lumbar puncture should be performed in febrile patients and when there is suspicion of central nervous system infection or subarachnoid hemorrhage, preferably after obtaining the CT scan.
As noted in sections on hospital treatment below, electroencephalography (EEG) is necessary to identify non-convulsive SE [19] in patients who do not return to a normal level of consciousness. EEG may also guide therapy in these patients and provide other diagnostic information.
Non-epileptic spells simulating SE (“pseudostatus”) occur in patients with or without genuine seizure disorders and may be difficult to differentiate from SE. These may represent volitional behavioral problems or non-volitional somatization disorders. Indicators suggestive of non-epileptic spells include preserved consciousness or purposeful movements, poorly coordinated thrashing, back arching, eyes held shut, head rolling, and pelvic thrusting.
Emergent Initial Therapy in the Prehospital Setting
The initial minutes after seizure onset offer the most effective opportunity for pharmacological termination of SE seizures. The Prehospital Treatment of Status Epilepticus (PHTSE) trial randomized SE patients to paramedic treatment with IV diazepam, lorazepam, or placebo. PHTSE demonstrated that emergency medical services (EMS) delivery of benzodiazepines resulted in a higher rate of cessation of seizures prior to arrival in the ED than compared to placebo. Early termination of seizures was associated with better clinical outcomes in PHTSE, and there was also a trend toward better clinical outcomes with EMS delivery of benzodiazepines compared to placebo [9]. Although 2–4 mg of IV lorazepam was more effective in the PHTSE trial [9], it is usually impractical for EMS use because of a short shelf life outside of refrigeration. The IM route of midazolam may eventually replace the previously recommended 5–10 mg of IV diazepam. More recently, IM administration of midazolam (10 mg in adults, 5 mg in children) was compared to IV administration of lorazepam (4 mg in adults, 2 mg in children) in a randomized, prehospital, prospective trial [18]. Midazolam administered IM was at least as good as IV administered lorazepam in terminating SE. This has advantages as midazolam does not need refrigeration, and the administration of an IM drug is significantly easier and quicker than obtaining IV access for administration.
Airway adjuncts and/or supplemental oxygen may be needed during SE. An IV or intraosseous (IO) line should be established to allow for correction of hypotension with fluid resuscitation. IO lines provide rapid access to the vascular space for medication administration when IV access is difficult to achieve. Other alternatives include midazolam given intramuscularly or across the nasal or buccal membranes, or diazepam given rectally. Extremes in serum blood glucose can result in seizures and should be rapidly identified and treated.
The most important modifiable element of therapy determining successful termination of seizures is the time to initiating benzodiazepines (emergent initial therapy). When treatment with IV agents was begun within 30 min of seizure onset, the initial AED was successful in terminating SE patients’ seizures in 80 % of cases, yet this rate decreased to 40 % if treatment was started 2 h after seizure onset [20]. This was amply documented in the recent trial of field-administered midazolam, in which IM administration was superior to IV but almost entirely on the basis of the extra time expended in inserting an IV catheter.
Paramedics should be prepared to treat respiratory depression in patients with SE. Benzodiazepine doses recommended for SE are relatively higher than those used for many other indications in the ED and may contribute to respiratory depression. However, continued seizures are more likely to cause respiratory problems than are side effects of the benzodiazepines [9].
Emergent Initial Therapy in the Hospital Setting
Initial treatment of SE in the ED continues or completes the elements that should have been initiated by EMS. Airway, breathing, and circulation should be re-evaluated and supportive care continued. If IV access has not already been obtained, it should be achieved upon arrival in the ED. If hypoglycemia has not been excluded either clinically or by measuring blood glucose, this should also occur upon ED arrival. For hospitalized patients, both emergent initial therapy and urgent control therapy should proceed seamlessly.
In patients who continue to convulse or do not regain consciousness following emergent initial therapy due to electrographic seizures, additional benzodiazepines may be administered. The Veterans Administration (VA) cooperative trial identified 4 mg of IV lorazepam as the preferred initial AED, but IV diazepam was also efficacious in terminating seizures [4]. If adequate doses of benzodiazepines had already been administered by EMS, then in the ED, management should proceed to use of a second-line AED (urgent control therapy), as noted in the next section. If the patient did not get benzodiazepines prior to ED arrival and is still seizing, the first dose of lorazepam can be followed by a repeat dose 5–10 min after the first, which in turn is immediately followed by urgent control therapy with a second-line AED.
Benzodiazepines are frequently under-dosed because the labeled 4 mg initial dosing of lorazepam for SE is greater than the initial dose used for most indications other than seizures in the ED. Initial treatment failure is often a result of using inadequate initial doses of IV benzodiazepines and then waiting too long to repeat benzodiazepine doses and advance to second-line agents or general anesthesia and drug-induced coma.
Urgent Control Therapy in the Hospital
The best choice of second-line AEDs for patients with established SE unresponsive to benzodiazepines is uncertain since the second-line AEDs have not been adequately compared in randomized controlled trials. Options include phenytoin/fosphenytoin, phenobarbital, valproate sodium, and levetiracetam [4].
Among second-line AEDs, the preferred medications in most units have been IV 20 mg/kg of phenytoin at rates up to 50 mg/min or 20 mgPE/kg of fosphenytoin, the latter given at rates of up to 150 mg/min [21–23]. Phenytoin and fosphenytoin are FDA labeled for the treatment of SE in adults. Fosphenytoin is a water-soluble prodrug that is converted to phenytoin by plasma esterases. Phenytoin, but not fosphenytoin, is labeled for SE in children. They act at the sodium channel rather than the gamma-aminobutyric acid (GABA) receptor and, therefore, represent a rational choice for treating patients whose seizures do not terminate with the benzodiazepine GABA agonists. Bradycardia and hypotension may occur at high infusion rates with the phenytoins, especially in the elderly or those with significant cardiac disease.
Although phenytoin-related adverse events are usually self-limited, some physicians who treat SE prefer alternative approaches. A small, randomized study suggested that IV valproate sodium may have similar efficacy in SE when compared to phenytoin [21]. Sodium valproate 20–40 mg/kg is given intravenously over 10 min, with an additional 20 mg/kg given over 5 min if the patient is still seizing. Although adverse events were not statistically significantly different in the randomized study, sodium valproate probably has less cardiopulmonary side effects than phenytoin and may be preferred in patients with hypotension or respiratory distress.
IV phenobarbital is also FDA labeled for the treatment of SE in both adults and children and remains a reasonable option, but it is now less commonly chosen in adults unless other agents are contraindicated or unavailable. Phenobarbital 20 mg/kg of IV is given at 50–100 mg/min. An additional 5–10 mg/kg may be given after 10 min, if needed. Phenobarbital also acts at the GABA receptor and may be a less rational choice in those who have not responded to benzodiazepines, although there is a paucity of data addressing this topic. Levetiracetam is often used off-label as a second-line agent to treat SE and can be given as a 1–3 g dose IV over five minutes or infused at 2–5 mg/kg min [24].
Second-line AEDs are also typically used in the ED, in the same doses, to suppress recurrent seizures in patients after SE has ended. If a patient stops convulsing but does not wake up or does not return to the pre-convulsive state, an EEG should be obtained to detect continuing non-convulsive SE.
Second-line AEDs may be less effective, and may sometimes be contraindicated, for urgent control therapy in patients with SE that is secondary to intoxication or poisoning. SE known to result from isoniazid or organophosphates should preferentially be treated with specific antidotes. Cardiac effects of tricyclic antidepressant poisoning may be exacerbated by attempting to prevent seizures with some second-line anticonvulsants.
Anticonvulsant Dosing
Urgent control therapy is used to prevent seizure recurrence after SE has been terminated, or to terminate SE in those with persistent or recurring seizures without awakening. If seizures have stopped and the patient has awakened, loading doses of anti-epileptic medications with longer half-lives should be initiated and can be given either intravenously or orally. A single oral loading dose of phenytoin 20 mg/kg can result in therapeutic levels within 3 h. For those requiring IV loading, fosphenytoin 20 mgPE/kg IV at a rate not exceeding 150 mgPE/min, or sodium valproate 40 mg/kg IV over 10 min with an additional 20 mg/kg over five minutes if still seizing, can be used.
EEG monitoring is useful if the patient has not awakened. The reasons for persistent seizures should be established by determining AED levels, neuroimaging, urine toxicology, and any other appropriate testing. The most important cause of persistent stupor after convulsive SE is ongoing electrical seizures (subclinical or electrographic seizures) that can only be detected by EEG monitoring.
Treatment of Refractory SE
SE will typically be terminated by the primary and secondary drugs described above. If the patient remains in the ED and seizures have not stopped despite urgent and emergent drug therapy, SE is considered refractory. General anesthesia and drug-induced coma are recommended in these circumstances.
It is not necessary, and is usually not advisable, to delay advanced therapy with repeated trials of alternative second tier AEDs. Some period, generally shorter than an hour and perhaps even 30 min, is adequate to determine if the above-described conventional approach will be successful.
Endotracheal intubation is necessary to allow induction of coma and should be quickly performed in refractory SE. Because pharmacologic paralysis performed for purposes of intubation and mechanical ventilation will mask ongoing convulsions, it is appropriate to obtain continuous EEG monitoring.
The agents most commonly used to induce a general anesthetic state of coma are continuous infusions of midazolam or propofol [25–28]. IV midazolam infusions usually are preceded by a loading dose of 0.2 mg/kg at 2 mg/min, with repeated boluses of 0.2–0.4 mg/kg every 5 min until the seizures stop, up to a maximum loading dose of 2 mg/kg. A continuous infusion should then be started at 0.05–2 mg/kg h. IV propofol infusions usually include a loading dose of 1–2 mg/kg IV over 3–5 min, with repeated boluses of the same amount every 3–5 min until the seizures stop, up to maximum total loading dose of 10 mg/kg. The propofol infusion should then be maintained at a rate of 30–200 mcg/kg min. Hypotension may be seen with higher doses.
Pentobarbital (or thiopental in some countries) is an alternative agent for the treatment of refractory SE. It has significant side effects, including hypotension, and a prolonged effective half-life, but it is still a reasonable option when other agents have failed or are contraindicated. Use of a pentobarbital infusion requires close monitoring and should be undertaken in a care environment with appropriate nursing and monitoring resources. IV pentobarbital infusions include a loading dose of 5–15 mg/kg IV at a rate of up to 50 mg/min, with repeated 5–10 mg/kg boluses until seizures stop, and then maintenance of 0.5–5 mg/kg h.
Sedatives and anesthetics used for treatment of SE may have a number of side effects and will frequently be associated with dose-dependent hypotension requiring IV vasopressor support [25]. Hypotension may be more frequently seen with pentobarbital, while prolonged use of propofol is associated with the rare-but-often-fatal propofol-related infusion syndrome (PRIS). PRIS is characterized by rhabdomyolysis, metabolic acidosis, and cardiac and renal failure [28]. Pentobarbital is used more frequently in children with refractory SE because of this adverse effect with propofol.
IV valproate sodium may be preferentially used for patients with refractory SE who cannot or should not be intubated [29–32]. Valproate sodium is not recommended in children under the age of 2 due to its association with fatal hepatotoxicity. Other potentially useful but unproven therapeutic options in refractory SE include ketamine, lacosamide, and induced mild hypothermia.
In the ED, sedative IV agents will usually be titrated to the cessation of clinical manifestations of convulsive or subtle SE. When continuous EEG monitoring is available, the administration rate can be titrated to the desired electroencephalographic findings, ranging from suppression of frank seizures to burst suppression or a completely suppressed background. Few data are available to identify the optimal treatment level of suppression.
It is appropriate to continue second-line AEDs to attain therapeutic serum levels during the treatment of refractory SE. Expeditious admission to an intensive care setting, preferably with continuous EEG monitoring, is advisable for patients treated for refractory SE.
Status Epilepticus in Children
Generally, the emergent treatment of status epilepticus in children is identical to that in adults. Central nervous system infections or underlying genetic or metabolic disorders are more commonly the source of SE in children, especially in the infant and the younger child. There is also a higher incidence of febrile status epilepticus in infants and children.
The agents used for the emergent treatment of status epilepticus are the same as in adults. The agents used to treat refractory status epilepticus are also similar, although pentobarbital and midazolam are preferred over propofol because of concerns regarding PRIS. Continuous intravenous infusions in children with refractory SE are often not used until after the failure of three first- or second-line agents. Because of a rare genetic disorder of pyridoxine (B6) metabolism, it is recommended that children with refractory SE, especially the infant and younger child, receive intravenous B6 (Table 2) early in the course of resuscitation. Pyridoxine should also be used to treat SE from isoniazid poisoning in children or adults.
Escalation of anti-epileptic dosing may result in transient respiratory depression and hemodynamic instability. Careful attention to airway management and blood pressure will prevent complications and the risk of secondary brain injury.
Communication
When communicating to an accepting or referring physician about a SE patient, consider including the key elements listed in Table 3.
References
Pallin DJ, Goldstein JN, Moussally JS, Pelletier AJ, Green AR, Camargo CA Jr. Seizure visits in US emergency departments: epidemiology and potential disparities in care. Int J Emerg Med. 2008;1:97–105.
Farhidvash F, Singh P, Abou-Khalil B, Arain A. Patients visiting the emergency room for seizures: insurance status and clinic follow-up. Seizure. 2009;18:644–7.
Pitts SR, Niska RW, Xu J, National Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2006;2008:1–38.
Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–8.
Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Short-term mortality after a first episode of status epilepticus. Epilepsia. 1997;38:1344–9.
Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994;35:27–34.
Legriel S, Mourvillier B, Bele N, et al. Outcomes in 140 critically ill patients with status epilepticus. Intensive Care Med. 2008;34:476–80.
Legriel S, Azoulay E, Resche-Rigon M, et al. Functional outcome after convulsive status epilepticus. Crit Care Med. 2010;38:2295–303.
Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–7.
Scholtes FB, Renier WO, Meinardi H. Generalized convulsive status epilepticus: causes, therapy, and outcome in 346 patients. Epilepsia. 1994;35:1104–12.
Claassen J, Hirsch LJ, Emerson RG, Bates JE, Thompson TB, Mayer SA. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology. 2001;57:1036–42.
Rossetti AO, Hurwitz S, Logroscino G, Bromfield EB. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry. 2006;77:611–5.
Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2 + sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–40.
Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60.
Ravizza T, Vezzani A. Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neuroscience. 2006;137:301–8.
Lowenstein DH. Status epilepticus: an overview of the clinical problem. Epilepsia. 1999;40:S3–8.
Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–6.
Silbergleit R, Durkalski V, Lowenstein DH, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600.
DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833–40.
Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993;43:483–8.
Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure. 2007;16:527–32.
Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38:202–7.
Misra UK, Kalita J, Patel R. Sodium valproate vs phenytoin in status epilepticus: a pilot study. Neurology. 2006;67:340–2.
Berning S, Boesebeck F, van Baalen A, Kellinghaus C. Intravenous levetiracetam as treatment for status epilepticus. J Neurol. 2009;256:1634–42.
Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002;43:146–53.
Rossetti AO, Lowenstein DH. Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol. 2011;10:922–30.
Rossetti AO, Milligan TA, Vulliemoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care. 2011;14:4–10.
Iyer VN, Hoel R, Rabinstein AA. Propofol infusion syndrome in patients with refractory status epilepticus: an 11-year clinical experience. Crit Care Med. 2009;37:3024–30.
Limdi NA, Shimpi AV, Faught E, Gomez CR, Burneo JG. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64:353–5.
Sinha S, Naritoku DK. Intravenous valproate is well tolerated in unstable patients with status epilepticus. Neurology. 2000;55:722–4.
Tripathi M, Vibha D, Choudhary N, et al. Management of refractory status epilepticus at a tertiary care centre in a developing country. Seizure. 2010;19:109–11.
Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, Laroche SM, Riviello JJ Jr, Shutter L, Sperling MR, Treiman DM, Vespa PM, Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit Care. 2012;17:3–23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Claassen, J., Riviello, J.J. & Silbergleit, R. Emergency Neurological Life Support: Status Epilepticus. Neurocrit Care 23 (Suppl 2), 136–142 (2015). https://doi.org/10.1007/s12028-015-0172-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0172-3