Abstract
Delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage is a cause of considerable morbidity and mortality. Magnesium sulfate has been proposed as a prophylactic intervention for angiographic vasospasm and to improve clinical outcomes. A systematic review was conducted to determine the evidence for the prophylactic use of magnesium sulfate in aneurysmal subarachnoid hemorrhage. Medline, Embase, Cochrane library, clinicaltrials.gov, and controlled-trials.com were searched with a comprehensive search strategy. 2,035 records were identified in the initial search and 1,574 remained after removal of duplicates. Randomized, parallel group, controlled trials of magnesium sulfate in patients with aneurysmal subarachnoid hemorrhage were included. A total of ten studies were included. Review Manager and GRADE software were used to synthesize the results. The summary effect for Glasgow outcome scale and the modified Rankin scale is a risk ratio (RR) of 0.93 [95 % confidence interval (CI) 0.82–1.06]. The RR for mortality is 0.95 [95 % CI 0.76–1.17]. Delayed cerebral ischemia has a RR of 0.54 [95 % CI 0.38–0.75], which is the only outcome with a statistically significant summary effect measure favoring magnesium treatment. Delayed ischemic neurological deficit has a RR of 0.93 [95 % CI 0.62–1.39]. Transcranial doppler vasospasm has a RR of 0.72 [95 % CI 0.51–1.03]. Current evidence does not support the prophylactic use of magnesium sulfate in aneurysmal subarachnoid hemorrhage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Subarachnoid hemorrhage (SAH) secondary to rupture of intracranial aneurysms is a cause of significant morbidity and mortality. Treatment is directed at early aneurysm repair, administration of prophylactic nimodipine, and intensive care support. Various therapeutic interventions have been studied over the past few decades, some demonstrating promise in pilot trials, though failing to demonstrate any appreciable beneficial effect in subsequent clinical trials. Recently, there has been renewed interest in the role of magnesium sulfate as a prophylactic intervention for patients with aneurysmal subarachnoid hemorrhage.
The clinical research question that forms the basis of this systematic review is whether administration of prophylactic magnesium sulfate versus placebo improves short-term (angiographic vasospasm, delayed cerebral ischemia, and delayed ischemic neurological deficits) and long-term (Glasgow outcome scale/modified Rankin scale and mortality) outcomes in patients with aneurysmal subarachnoid hemorrhage.
Methods
A research protocol was developed in advance and detailed all aspects of the conduct of this systematic review. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) were used for protocol development and manuscript preparation. We did not register this review. Randomized parallel group, placebo-controlled trials evaluating magnesium sulfate in patients with aneurysmal subarachnoid hemorrhage were included. Non-English studies were not excluded. As it was anticipated that there would be fewer than 12 randomized controlled trials for inclusion based on prior systematic reviews that have been completed, there was no specific restriction on study methodological quality beyond selecting studies that were identified as randomized controlled trials. This was done to prevent being overly restrictive at the study selection stage. Studies with different doses, routes of administration, and duration of treatment were included in the analysis with further subgroup analysis specified if warranted.
Pre-specified outcome measures included mortality, functional outcome scales [Glasgow outcome scale (GOS) and modified Rankin scale (MRS)], delayed cerebral ischemia (DCI), delayed ischemic neurological deficit (DIND), angiographic vasospasm, and transcranial doppler (TCD) vasospasm. Delayed cerebral ischemia as defined as “the presence of cerebral infarction on CT or MRI scan of the brain within 6 weeks after SAH, not present on the scan between 24 and 48 h after early aneurysm occlusion, and not attributable to other causes” [1]. Delayed ischemic neurological deficit is clinical deterioration secondary to cerebral ischemia and is defined as “the occurrence of focal neurological impairment, or a decrease of at least 2 points on the Glasgow Coma scale, lasting for at least 1 h, is not apparent immediately after aneurysm occlusion, and cannot be attributed to other causes” [1].
Electronic databases searched included Medline, Embase, and the Cochrane library. Clinical trials registries www.controlled-trials.com and www.clinicaltrials.gov were searched for ongoing and recently completed clinical trials. Searches for unpublished studies were done in consultation with a health sciences librarian and included a review of key conference proceedings. Searches were conducted in Medline (1946-January week 4, 2012), Embase (1980–2012 week 5), and the Cochrane Central Register of Controlled Trials (-Jan 2012). Additional searches were conducted in two clinical trials registries, www.clinicaltrials.gov and www.controlled-trials.com. Our comprehensive search strategies are available for review on request. A gray literature search was also conducted in the WHO ICTRP Search Portal, National Research Register Archive, OCLC PapersFirst and ProceedingsFirst. Two reviewers, Deven Reddy (DR) and Aria Fallah (AF), independently performed the title and abstract review for study selection for full-text review. Kappa statistics were done to evaluate reviewer agreement. Differences were resolved by discussion and consensus.
DR and AF performed data extraction, risk of bias assessments, and evidence quality evaluation independently with resolution of discrepancies by discussion and consensus. A modified Cochrane risk of bias tool was used to evaluate the risk of bias in individual studies. The evaluation of evidence quality by outcome, to determine confidence in estimates of treatment effect, was done using the GRADE tool combining the risk of bias assessment with assessments of inconsistency, indirectness, imprecision, and other considerations including publication bias [2].
Data are presented using forest plots generated by Review Manager [3] software.
Risk ratios (RR) were used as measures of treatment effect and were reported with 95 % confidence intervals (CI) and pooled where appropriate. The Mantel–Haenszel random effects model was used for meta-analysis. Absolute and relative measures of treatment effect were reported in the GRADE summary of findings table. Functional outcomes (GOS and MRS) were dichotomized into favorable and unfavorable outcomes at the same cut-point with unfavorable outcomes counting as events (moderate disability to no symptoms/good recovery = favorable outcome). Inconsistent reporting and differing study specific thresholds for the diagnosis of angiographic vasospasm outcomes presented a challenge for data analysis. As a result, there is no meta-analysis reported for angiographic vasospasm diagnosed by digital subtraction angiography, CT angiography, and MR angiography.
Chi square and I 2 statistics were applied to evaluate the presence and extent of heterogeneity among included studies. A Chi square p value of <0.1 and/or an I 2 > 40 % was the selected thresholds for determining heterogeneity of studies pooled in meta-analyses. Forest plots generated by Review Manager [3] were examined visually to evaluate each outcome.
Results
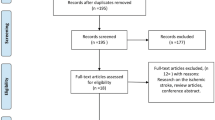
The initial database searches yielded 2,035 records. Following removal of duplicates, 1,574 records remained. We excluded 1,538 records after title and abstract review. We were left with 36 records for full-text review (Fig. 1). The kappa statistic for reviewer agreement for title and abstract review was 0.8 (record 1–500) and 0.75 (record 501–1,574).
Following full-text review, ten studies were included for quantitative data synthesis (Table 1). The kappa statistic for reviewer agreement for full-text review was 0.75. At full-text review, 27 records were excluded for not being randomized controlled trials, for including patients with non-aneurysmal subarachnoid hemorrhage, for not having at least one pre-specified outcome measure or for having an active comparator that is considered standard of care (Fig. 1).
A modified Cochrane risk of bias tool was used to evaluate risk of bias in the included studies. High risk of bias was identified in the areas of allocation concealment (selection bias), blinding of participants and healthcare personnel (performance bias), and blinding of outcome assessment (detection bias). High risk of bias was detected in five of the ten included studies (Fig. 2).
The overall quality of evidence for each outcome is reported in the summary of findings table (Table 2). This provides an evaluation of the confidence that can be placed on the estimate of treatment effect. Overall, for the functional outcome scales (GOS and MRS) and mortality, the summary effect measure was not statistically significant. The quality of evidence was high.
DCI summary effect measure was statistically significant demonstrating benefit with magnesium administration and the quality of evidence was moderate. DIND summary effect measure showed no benefit of magnesium treatment and the quality of evidence was low. TCD vasospasm summary effect measure was not statistically significant and the quality of evidence was low.
Outcomes were evaluated with forest plots with summary effect measures in the form of pooled risk ratios (Figs. 3, 4, 5, 6, 7). The functional outcome scales (GOS and MRS) were pooled together, dichotomized into unfavorable and favorable outcomes, with unfavorable outcomes reported as events. The functional outcome scales were dichotomized at the same cut-points and pooled for the meta-analysis.
The summary effect for GOS and MRS was a RR of 0.93 (95 % CI 0.82–1.06). The RR for mortality was 0.95 (95 % CI 0.76–1.17). Delayed cerebral ischemia had a RR of 0.54 (95 % CI 0.38–0.75), which was the only outcome with a statistically significant summary effect measure, demonstrating benefit with magnesium administration. Delayed ischemic neurological deficit had a RR of 0.93 (95 % CI 0.62–1.39). TCD vasospasm had a RR of 0.72 (95 % CI 0.51–1.03). The outcome-dependent GRADE quality of evidence ranged from low to high though it was high for patient important outcomes of mortality and functional outcome.
Discussion
Following subarachnoid hemorrhage, a complex cascade of events is triggered including the intracellular influx of calcium, inhibition of nitric oxide, and the production of endothelin-1. Nitric oxide is a vasodilator and endothelin-1 is a vasoconstrictor. Magnesium has been explored as a therapeutic intervention as it is thought to target the vasospasm process at multiple levels from its’ calcium antagonist effect to inhibiting the production of endothelin-1. It is also thought to have additional neuro-protective effects and improve cerebral blood flow.
Delayed cerebral ischemia is a cause of significant morbidity and mortality following aneurysmal subarachnoid hemorrhage. The 2012 American Heart Association guidelines for the management of aneurysmal subarachnoid hemorrhage summarize the evidence for treatment interventions in aneurysmal subarachnoid hemorrhage [4]. Based on their established classification and evidence grading system, oral nimodipine is a class I, level of evidence A recommendation, maintenance of euvolemia and hypertension is a class I, level of evidence B recommendation, and cerebral angioplasty and/or selective intra-arterial vasodilator therapy is a class IIA, level of evidence B recommendation.
This systematic review is a current evaluation of the available evidence for the use of magnesium sulfate in this clinical situation. Prior reviews have been more enthusiastic in their support of this intervention based on summary statistics that suggested benefit though not conclusively [5–7]. The quality assessment associated with the summary effect measure by outcome allows for a more objective overall evaluation. The quality of evidence was evaluated with the GRADE outcome specific quality assessment tool. This included assessment of the risk of bias, inconsistency, indirectness, imprecision, and other considerations including publication bias. Factors that resulted in downgrading are summarized in the evidence profile table. Risk of bias issues included the lack of blinding and allocation concealment. Indirectness of evidence occurred with outcome assessment at different times e.g., 3 and 6 months for functional outcome scales.
Several steps in this review process were done in duplicate to reduce the likelihood of systematic biases. This included title and abstract review, full-text review, data abstraction, risk of bias assessment, and quality of evidence assessment. The gray literature search was not done in duplicate due to time constraints, though it is unlikely to change confidence in estimates of effect. The application of the GRADE quality of evidence assessment enhances the reader’s ability to determine the confidence that can be placed in estimates of treatment effect.
The meta-analysis did not demonstrate any statistically significant difference between the magnesium administration group and placebo group for all the assessed outcomes with the exception of DCI. The total number of study participants assessed for the DCI outcome is 468, which represents less than 25 % than that of the functional outcome (GOS and MRS). The GRADE quality of evidence for DCI is moderate suggesting that further research may change the estimate of the summary effect measure. It could be worthwhile to explore further with a larger study, though given DCI would be considered a surrogate outcome; it would also have to demonstrate that an improvement in DCI results in a measurable improvement in functional outcome or a reduction in mortality.
Limitations of this review include variability in dose and duration of magnesium administration (Table 1). There was also between study variability in timing of outcome assessment with follow-up ranging from 3 months to 1 year. Thresholds for the diagnosis of TCD vasospasm varied substantially. Despite these limitations, the quality of evidence for mortality and functional outcome is high. This meta-analysis represents the most comprehensive and current review of the use of magnesium sulfate in aneurysmal subarachnoid hemorrhage.
Conclusion
Current evidence does not support the routine use of magnesium sulfate in aneurysmal subarachnoid hemorrhage. The assessed evidence quality ranges from low to high though is high for patient important outcomes of mortality and functional outcome.
References
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a Multidisciplinary Research Group. Stroke. 2010;41:2391–5.
GRADEpro. [Computer program]. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711–37.
Chen T, Carter BS. Role of magnesium sulfate in aneurysmal subarachnoid hemorrhage management: a meta-analysis of controlled clinical trials. Asian J Neurosurg. 2011;6(1):26–31.
Ma L, Liu W, Zhang J, Chen G, Fan J, Sheng H. Magnesium sulphate in the management of patients with aneurysmal subarachnoid hemorrhage: a meta-analysis of prospective controlled trials. Brain Inj. 2010;24(5):730–5.
Wong GK, Chan MT, Gin T, Poon WS. Intravenous magnesium sulfate after aneurysmal subarachnoid hemorrhage: current status. Acta Neurochir Suppl. 2011;110(Pt 2):169–73.
Aidaros M, Goda T, El-Sharkawy KAM. The role of magnesium sulphate in treatment of subarachnoid hemorrhage and its effect on outcome. Egypt J Neurol Psychiatry Neurosurg. 2011;48(1):79–83.
Akdemir H, Kulakszoglu EO, Tucer B, Menku A, Postalc L, Gunald O. Magnesium sulfate therapy for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg Q. 2009;19(1):35–9.
Baiocchi M, Grassetto A, Ori C. Potential role of continuous magnesium infusion to prevent vasospasm and consequent ischaemic deficit correlated to subarachnoid haemorrhage. Acta Anaesthesiol Italica. 2006;57(2–3):131–45.
Dorhout Mees SM, Algra A, Vandertop WP, van Kooten F, Kuijsten HAJM, Boiten J et al. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. Lancet. 2012;6736(12)60724–60727. doi:10.1016/S0140-.
Muroi C, Terzic A, Fortunati M, Yonekawa Y, Keller E. Magnesium sulfate in the management of patients with aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, dose-adapted trial. Surg Neurol. 2008;69(1):33–9 (discussion 39).
van den Bergh WM, Algra A, van Kooten F, Dirven CM, van Gijn J, Vermeulen M, et al. Magnesium sulfate in aneurysmal subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2005;36(5):1011–5.
Veyna RS, Seyfried D, Burke DG, Zimmerman C, Mlynarek M, Nichols V, et al. Magnesium sulfate therapy after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;96(3):510–4.
Westermaier T, Stetter C, Vince GH, Pham M, Tejon JP, Eriskat J, et al. Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, clinical study. Crit Care Med. 2010;38(5):1284–90.
Wong GK, Chan MT, Boet R, Poon WS, Gin T. Intravenous magnesium sulfate after aneurysmal subarachnoid hemorrhage: a prospective randomized pilot study. J Neurosurg Anesthesiol. 2006;18(2):142–8.
Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, Zee BC. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled. Multicenter Phase III trial. Stroke. 2010;41:921–6.
Conflict of interest
Deven Reddy, Aria Fallah, Jo-Anne Petropoulos, Forough Farrokhyar, and Draga Jichici declare that they have no conflict of interest. R.L. Macdonald receives grant support from the Physicians Services Incorporated Foundation, Brain Aneurysm Foundation, Canadian Stroke Network, and the Heart and Stroke Foundation of Ontario. R.L. Macdonald is a consultant for Actelion Pharmaceuticals and Chief Scientific Officer of Edge Therapeutics, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, D., Fallah, A., Petropoulos, JA. et al. Prophylactic Magnesium Sulfate for Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-analysis. Neurocrit Care 21, 356–364 (2014). https://doi.org/10.1007/s12028-014-9964-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-9964-0