Abstract
Background
To determine neurologic outcome in patients with out-of-hospital cardiac arrest (OHCA) and treatment with mild therapeutic hypothermia (MTH).
Methods
Seventy-three consecutive OHCA patients treated with MTH were retrospectively analyzed. Serum neuron-specific enolase (NSE) was measured 24, 48, and 72 h after admission. In patients with no motor response 48 h after termination of analgosedation (n = 40), clinical neurological examination and evoked potentials (EPs) were determined. Neurological outcome was assessed after 2 months based on the cerebral performance categories (CPC), and categorized as good (CPC 1–3) or poor (CPC 4 and 5).
Results
Forty-three patients had a CPC score of 1–3 and 30 patients had a CPC 4–5. The best predictive value for poor neurologic outcome was an increase of NSE by ≥4.3 ng/mL between day 1 and day 2 (sensitivity 80 %, specificity 100 %, positive predictive value (PPV) 100 %, negative predictive value 86 %). Absolute NSE values were less reliable in the prediction of poor outcome with the highest sensitivity (88 %) and specificity (95 %) if values reached ≥36.3 ng/mL on day 3. Somatosensory EPs (SSEPs) showed a specificity of 100 % and PPV of 100 %; however, sensitivity for evoked potentials was low (29 %). Intriguingly, two initially comatose patients with excessive NSE values (24 h NSE: 101 and 256 ng/mL, and 48 h NSE: 93 and 110 ng/mL, respectively) had physiological SSEPs and regained a CPC score of 1.
Conclusion
In patients treated with MTH after OHCA changes in NSE are more suitable than its absolute serum levels for the prediction of poor neurologic outcome. Since unequivocal prediction of poor neurologic outcome is of utmost importance in these patients the decision to limit therapy must be based on several prediction tools with the highest PPV and specificity including SSEPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although scientific and organization efforts in cardiopulmonary resuscitation (CPR) have led to an improved rate of restoration of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest (OHCA), it remains a fatal condition [1–3]. After successful initial resuscitation, the patient′s outcome is predominantly dependent on neurological recovery following temporary anoxia [4]. Early prediction of neurologic outcome in patients having survived OHCA is eminently important to determine the extent of post CPR care or the time point to potentially withdraw further treatment [5]. Reliable and unequivocal prediction of poor neurologic outcome is of utmost importance to avoid futile escalation of intensive care in these patients on one hand and to avoid fatal limitation of therapy in patients who might potentially recover.
Mild therapeutic hypothermia (MTH) has been shown to ameliorate brain damage and reduce mortality and morbidity in patients with ROSC after OHCA due to ventricular fibrillation (VF) and thus has become standard of care in these patients [6, 7].
Early prediction of neurologic outcome in comatose patients is based on the assessment of clinical variables with differing sensitivities and specificities, including neurological examination, the assessment of evoked potentials (EPs), and biochemical markers like the neuron-specific enolase (NSE). With several reports confirming reliable prediction of poor neurologic outcome with NSE, this enzyme has attracted most attention in recent years (refer to [8] for review). NSE is a dimeric enzyme composed of 2 γ subunits with a molecular weight of 78 kDa and biologic half-life of ≈24 h, present in neurons and other cells of neuroectodermal origin. Elevations of NSE are associated with increased severity of postanoxic neuronal injury. The PROgnosis in PostAnoxic Coma (PROPAC) study group determined peak NSE more than 33 ng/mL within the first 72 h of postanoxic coma to be predictive of poor outcome, with a false-positive rate of 0–3 % [9]. This cut-off value was consecutively adopted in the 2006 guidelines of the American Association of Neurology [4]. Even with the introduction of MTH as standard of care in patients after cardiac arrest, elevated NSE seemed to remain a valid marker of the severity of postanoxic injury [10]. In a prospective study including 90 patients treated with MTH after OHCA, peak NSE >33 ng/mL at 48 h had 100 % specificity for poor outcome [11]. Similar results have been reported by several investigators [12, 13], including a multicenter study with 102 patients, in which even a lower cut-off >28 ng/mL at day 2 for poor outcome was found [14]. However, others determined higher NSE values for prediction of poor outcome with an FPR of 0 % (>78 μg/L, [15]) or showed good survival rates even in patients with NSE levels >33 ng/mL [16], thus raising important concerns about the ability of NSE in predicting outcome in patients treated with MTH.
The present study, therefore, aimed at investigating the predictive value of NSE in comparison to other clinical factors in a homogenous cohort of patients admitted to a cardiac intensive care unit after OHCA treated with mild therapeutic hypothermia.
Methods
Patients
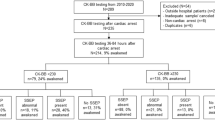
Seventy-nine consecutive patients admitted to our university hospital cardiology department with successful resuscitation after OHCA were retrospectively analyzed. From these patients, six patients died of non-neurologic causes (septic and cardiogenic shock) before analgosedation was terminated and were excluded from further analysis. All surviving patients (n = 73) with ROSC after OHCA, Glasgow-come scale <8, and without contraindications were referred to MTH described in detail below, after major contraindications (intracerebral hemorrhage or other uncontrolled bleedings) were excluded. All patients were initially analgosedated with midazolam and sufentanil to a Richmond agitation and sedation (RASS) score of −4 until completed rewarming. In case of myocardial ischemia as the suspected cause for cardiac arrest, patients were transferred to the catheter lab for coronary angiography and revascularization when indicated prior to admission to the ICU. Only routinely collected clinical data were used.
None of the patients were neurologically disabled prior to OHCA. Upon induction of MTH all patients were sedated for at least 40 h post CPR and sedation was stopped at the earliest possible time after reaching normothermia. The Acute Physiology and Chronic Health Evaluation (APACHE) IV score [17] was calculated based on patient′s demographics, admission source, primary admission diagnosis, and detailed laboratory and physiologic variables collected over the first 24 h. The patients’ baseline characteristics are outlined in Table 1.
This investigation was performed as a quality control measure, it was strictly retrospective and no identifying data were part of the analysis. As such, it was approved by the local ethics committee of the University of Cologne and conforms to the principles outlined in the Declaration of Helsinki.
Treatment Protocol
Peripheral cooling was initiated in OHCA patients by the first responding emergency medical service by means of rapid infusion of 2 L ice cold saline (4 °C) and/or applying cool-packs to the femoral or neck area. Emergency medical doctors joined the patients either on site or during transport to the clinic to improve medical care, as a usual routine in German emergency medical service. Cooling to the target temperature of 33 °C was continued in hospital using an endovascular cooling device (Coolgard 3000/ICY® catheter, Zoll Medical Corp., Chelmsford, Massachusetts, USA). Core temperature was continuously registered in the bladder through a thermal sensor at the tip of a transurethral urinary catheter. A target temperature of 33 °C was maintained for 24 h. MTH was terminated by rewarming through the same endovascular device at a controlled rate of 0.3 °C per hour until the physiologic body temperature of 37 °C was reached. All patients were initially analgosedated with midazolam and sufentanil to a RASS score of −4 by emergency medical staff and continued on the ICU until rewarming was completed.
After admission to the ICU, patients were monitored by continuous recording of ECG, pulse oxymetry, and intravascular blood pressure measurement (Drägerwerk AG, Lübeck, Germany). Blood gas analysis was performed every 3 h (ABL800 FLEX, Radiometer Medical ApS, Brønshøj, Denmark). All patients included in the present analysis showed signs of cardiogenic shock and initially required circulatory support with crystalloid fluids and continuous infusion of vasopressors and inotropes adapted to a target mean arterial pressure (MAP) ≥65 mmHg. In unstable patients hemodynamic monitoring was completed by transpulmonary thermodilution and arterial pulse contour analysis (PiCCO, Pulsion Medical Systems AG, Munich, Germany). Nutritional support was provided parenterally and/or enterally according to expected demand. Intravenous insulin was administered if plasma glucose levels exceeded 180 mg/dL. All OHCA survivors were oxygenated by pressure-controlled ventilation (Evita 4 or Evita XL, Drägerwerk AG) until they were able to be weaned from mechanical ventilation. The decision to wean patients from invasive mechanical ventilation was solely based on clinical aspects such as the underlying cardiac disease, infectious complications, seizures, or myoclonus status, among others.
Clinical Assessment of Neurological Outcome
Neurological outcome was registered in all patients 2 months after CPR based on the Glasgow-Pittsburgh cerebral performance categories (CPC) of the Utstein recommendations [18]. CPC 1 represents conscious and alert, CPC 2 conscious and alert with moderate cerebral disability, CPC 3 conscious with severe cerebral disability precluding independent existence, CPC 4 comatose or in persistent vegetative state, and CPC 5 brain dead. The results of clinical examination, serum NSE, EP analysis, and standard CPC evaluations were assessed independently of one another. For statistical analyses, neurologic outcome was dichotomized into good (CPC 1–3) and poor (CPC 4–5). We defined a CPC of 4 and 5 after 2 months as “poor outcome” based on the clinical observation that the chance of recovery of consciousness in patients who were still unconscious 2 months after CPR is virtually zero (refer to [19]). The outcome at 2 months was either assessed during a routine follow up visit in the outpatient clinic or by a phone interview with the attending physician if the patient had been discharged to a different hospital or rehabilitation center.
Blood Sampling and Measurement of Serum NSE Levels
Samples for NSE were collected from consecutive hypothermia-treated OHCA patients on day 1 (≤24 h), day 2 (24–48 h), and day 3 (48–72 h) in standard serum tubes (Sarstedt AG, Nuembrecht, Germany). Samples with visible hemolysis or chemical indication for hemolysis were excluded. Samples were processed routinely by the institute for clinical chemistry of the university hospital of Cologne, and serum NSE values were determined using an electrochemiluminescence immunoassay according to the manufacturer’s instructions (COBAS®, Roche Diagnostics, Mannheim, Germany). The range of detection of this specific assay ranges between 0.050 and740 ng/mL (including dilution), with normal values <16.3 ng/mL. Since the present investigation is based on retrospective analyses, NSE values were not available at all time points for some patients (Table 4).
Clinical Neurological and Electrophysiological Examinations
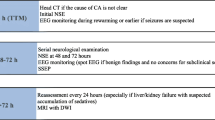
Neurological assessment was performed 48 h after termination of analgosedation by the attending neurologist in comatose patients with no motor activity (n = 40). As part of neurologic examination brainstem reflexes (pupillary light reflexes, eye movements, and corneal reflexes), and the presence of seizures or myoclonus status epilepticus were documented. Motor response was determined as spontaneous or absent, with or without painful stimuli. Eye movements were classified as absent or spontaneous upon cervico-ocular testing.
The neurologic electrophysiological assessment consisted of EPs, including somatosensory evoked potentials (SSEPs) as well as brainstem auditory evoked potentials (BAEPs) according to standard techniques [20, 21]. Registration of SSEPs was performed from the somatosensory cortical areas and at the spinal C7 level to exclude peripheral nerve conduction failure. The results were evaluated using the laboratory`s standard normal values. N20-Latencies and N20/P25 amplitude of the SEP and wave I, III, and V latency and wave V/Va amplitude of the brainstem AEP were used for further analysis. Latencies of EP were considered abnormal when they exceeded the 2.5-fold SD of established normal values. Amplitudes of EP were considered abnormal when the side-to-side difference exceeded 50 % or when the amplitude was below the 2.5-fold value of the established standard value. Evoked potentials were documented for each side separately and graded according to the following classification: (1) normal, (2) pathological amplitudes and/or latencies, and (3) absent. All EPs were assessed by a certified EP examiner, who was aware that the patient had a postanoxic coma, but blinded as to the clinical course.
Data Analysis
Quantitative data are presented as mean ± standard error of the mean (SEM). Continuous data are reported with medians and interquartile range (IQR). Groups were dichotomized into good (CPC 1–3) and poor (CPC 4–5) outcome and differences were compared using the Student’s t test or Fisher’s exact test. The discriminative ability of NSE or EPs (BAEPs or SSEPs) to predict outcome at 2 months from hospital admission following OHCA was evaluated using receiver operating characteristic (ROC) to calculate cut-off levels (closest distance to the upper left corner or Youden-Index). Area under the curve (AUC), SEM, and 95 %-confidence interval (95 % CI) were calculated. Calculations were performed using GraphPad Prism for Windows (GraphPad Prism version 5.0, La Jolla, CA, USA). A p value <0.05 was considered statistically significant.
Results
Patient’s Characteristics
Gender distribution was 9/73 females and was not different between outcome groups (6/43 in CPC 1–3, 3/30 in CPC 4–5, p = 0.73). Overall mortality at 2 months was 26 % (19 patients). The distribution of underlying causes for cardiac arrest was 54/73 myocardial infarction, 18/73 primary rhythm disorders, and 1/73 with pulmonary embolism. These underlying causes for OHCA were significantly different between outcome groups with a rate of myocardial infarction between outcome groups (83.8 % (36/43) in CPC 1–3 vs. 60 % (18/30) in CPC 4–5, p = 0.03, Table 1), and primary arrhythmia occurring significantly more often in the poor outcome group (14 % in CPC 1–3 vs. 40 % in CPC 4–5). All patients received MTH as part of standard care. An initial non-shockable rhythm (asystole) as recorded by EMS occurred in 13/73 (17.8 %) patients and resulted in poor outcome in all of these patients (p < 0.01, Table 1). General resuscitation variables, such as frequency of bystander CPR, time to ROSC, and time-interval from collapse to EMS arrival were not different between outcome groups (Table 1).
Neurological Outcome
33/73 patients showed early recovery with movement of the limbs within 48 h after cessation of analgosedation, which was accompanied by a good neurologic outcome (CPC 1–3). 40/73 patients had no signs of motor activity and were subjected to further neurological examination to evaluate prognosis. Out of these 40 patients, 10 recovered and were successively categorized as good outcome (CPC 1–3), resulting in poor outcome in 30/73 patients. Dichotomization resulted in a total of 43/73 patients categorized CPC 1–3 (n = 22; CPC 2 n = 14; CPC 3 n = 7) and 30/73 with CPC 4–5 (CPC 4 n = 11; CPC 5 n = 19, Table 1).
NSE in the Prediction of Poor Neurological Outcome
Between both outcome groups, absolute NSE values were not significantly different at the 24-h time point (median CPC 1–3: 34.4 ng/mL vs. CPC 4–5: 41.7 ng/mL, n.s.), with a great range of values detected, especially in the good-prognosis group (CPC 1–3: 12.3–255.8 ng/mL vs. CPC 4–5: 15.0–192.2 ng/mL). At 48 h, absolute NSE values were significantly higher in the poor prognosis group (median CPC 1–3: 26.0 ng/mL vs. CPC 4–5: 86.6 ng/mL, p < 0.0001). This significant difference was preserved throughout day 3. Within the good-prognosis group, absolute NSE were not different between day 2 and day 3 (p = 0.08), but exhibited a significant decrease when compared between day 1 and day 2 (p = 0.02). Out of the cohort, two patients exhibited excessive NSE values (NSE on day 1 of 101 and 256 ng/mL, and NSE values of 93 and 110 ng/mL on day 2, respectively). Focal ischemia was excluded by computer tomography scan or magnetic resonance imaging. These two patients regained consciousness and showed a good neurologic outcome (CPC 1 and physiological SSEPs). Data are presented in Fig. 1a; Table 2.
At day 2, the NSE value with 100 % specificity and 100 % PPV was 112.4 ng/mL for the prediction of poor neurologic outcome, with a sensitivity of 30 % (Fig. 3a). A cut-off value of 42.4 ng/mL was calculated with a specificity of 86 %, sensitivity of 85 %, PPV of 82 %, and NPV of 88 %. On day 3, a NSE value of 65.4 ng/mL exhibited a specificity of 100 %, sensitivity of 76 %, PPV of 100 %, and NPV of 83 %. The calculated cut-off value was 36.3 ng/mL with a specificity of 95 %, sensitivity of 88 %, PPV of 94 %, and NPV of 90 % (Fig. 3a; Table 4). The AUC for the 72-h time point was 0.94 ± 0.05, 95 % CI 0.84–1.0, p < 0.0001 (Fig. 3a).
Delta NSE values. a Delta NSE values between day 1 and day 2 and between day 2 and day 3 between groups. b Change of NSE values in CPC 1–3 between day 1 and day 2. c Change of NSE values in CPC 4–5 between day 1 and day 2. Groups were dichotomized into good outcome (CPC 1–3, n = 43) and poor outcome (CPC 4–5, n = 30). NSE values are presented in ng/mL as mean ± SEM
With regard to relative changes, NSE decreased within the good-prognosis group from day 1 to day 2 (mean −15.2 ± 5.7 ng/mL, p = 0.17) and further decreased in the period between day 2 and day 3 (mean −12.4 ± 3.3 ng/mL). In the poor prognosis group, NSE values significantly increased from day 1 to day 2 (mean +31.2 ± 8.6 ng/mL) to a median value of 86.6 ng/mL (p = 0.01). Up to day 3, values slightly but not significantly decreased by a mean of −2.0 ± 4.8 ng/mL (p = 0.48, Fig. 2; Table 3). Between groups, the change in NSE from day 1 to day 2 was significantly different (−15.2 ± 5.7 vs. +31.2 ± 8.6 ng/mL, p < 0.0001), whereas no difference was found from day 2 to day 3 (−12.4 ± 3.3 vs. −2.0 ± 4.8 ng/mL, p = 0.09).
Receiver Operating Characteristic analyses revealed that an increase in NSE of ≥4.3 ng/mL between day 1 and 2 provides the best predictive values with a specificity of 100 %, sensitivity of 80.0 %, PPV 100 %, and NPV of 86 % to predict poor outcome (AUC 0.86 ± 0.07, 95 % CI 0.72–1.0, p < 0.0001, Fig. 3b). Between day 2 and 3, an increase of mean NSE value of ≥8.0 ng/mL was associated with a specificity of 100 %, sensitivity of 37.5 %, PPV 100 %, and NPV of 62 % to predict poor outcome (Fig. 3b). The calculated best cut-off value was an increase of ≥4.3 ng/mL between day 1 and 2, with a specificity of 100 %, sensitivity of 80 %, PPV 100 %, and NPV of 86 % (Fig. 3b; Table 4). From day 2 to day 3, the best cut-off value was a decrease of ≤−6.2 ng/mL with a specificity of 81 %, sensitivity of 63 %, PPV 71 %, and NPV of 68 % (Fig. 3b; Table 4). The AUC was 0.70 ± 0.01 (95 % CI 0.51–0.89, p = 0.06) for this time point.
Evoked Potentials in the Prediction of Poor Neurologic Outcome
In the present analysis, the predictive value of evoked potentials only applies to those patients who did not show any motor response 2 days after termination of analgosedation (40/73 patients). In these patients, the absence of SSEPs had a specificity of 100 %, sensitivity of 29 %, PPV of 100 %, and NPV of 48 % in the prediction of poor outcome, as ten patients recovered to a CPC 1–3 (Table 4). BAEPs were calculated to have a specificity of 100 %, sensitivity of 26 %, PPV of 100 %, and NPV of 47 % to predict poor outcome (Table 4).
Clinical Examination in the Prediction of Poor Neurologic Outcome
40/73 patients showed no early motor response. As mentioned above, out of these patients, ten recovered and were categorized CPC 1–3. Two days after termination of analgosedation the absence of motor response had a specificity of 23 %, sensitivity of 72 %, PPV of 57 %, and NPV of 38 % to predict poor outcome, whereas the absence of brainstem reflexes at the same time displayed a specificity of 85 %, sensitivity of 26 %, PPV of 71 %, and NPV of 44 % (Table 4).
Discussion
The present investigation shows the higher predictive value of the relative changes in NSE between day 1 and 2 after OHCA compared to its absolute levels in MTH-treated patients. To date research on outcome prediction focused on the definition of peak NSE values; however, reports with constantly rising cut-off levels questioned the validity and reliability of this biomarker as clinical aid for the crucial decision of treatment termination in comatose patients. Before the adoption of MTH as standard of care in patients with ROSC following cardiac arrest, several groups reported that peak NSE levels ≥33 ng/mL within 48 h were predictive of poor neurologic outcome (categorized by CPC 3–5) in patients with postanoxic coma [4]. However, later reports variably proposed higher cut-off values. A critical issue in this controversy is the varying frequency, in which MTH was applied in the respective studies. MTH is known to improve neurologic prognosis, but it may also have a direct effect on absolute NSE levels. While some studies report similar cut-off values in patients treated with MTH [11, 14] compared to normothermic conditions [9], others found lower absolute NSE levels in the subset of patients subjected to MTH [13, 15]. Most prominently, Steffen et al. [15] report a NSE cut-off value of 78.9 ng/mL after MTH and 27 ng/mL measured 3 days after admission in patients with normothermia. Another recent study further elevated the proposed cut-off value, reporting a 100 % predictive value for a cut-off value of serum NSE of >97 ng/mL for the highest observed NSE value to predict poor neurologic outcome, defined as death or vegetative state. Importantly, two third of patients were treated with MTH, but a differentiation between patients with or without MTH treatment with regard to prognostic values was not proposed in this study [8]. The frequent increase of NSE cut-off values in recent years for the assessment of neurologic outcome raises doubts regarding their reliability. This is underlined by the finding of two patients in our collective with excessive NSE values (day 1 NSE of 101 and 256 ng/mL, and day 2 NSE values of 93 and 110 ng/mL, respectively), who regained consciousness and showed a good neurologic outcome (CPC score of 1 and physiological SSEPs). From our data we calculated a cut-off value of serum NSE determined on day 2 of 42.4 ng/mL; however, an 86 % specificity and 85 % sensitivity of this value questions its clinical relevance. In contrast, the relative change in NSE levels between day 1 and day 2 ≥4.3 ng/mL predicted death or vegetative state (CPC 4–5) in our study population with absolute specificity and high sensitivity (Table 4).
It has been suggested that the magnitude of NSE serum levels may vary depending on the biochemical assay. With regard to the patients with excessive NSE values in the present study, the rise and decay of serum concentrations followed the typical kinetics for NSE in repeated measures. A relevant modification of the values by hemolysis or the analytical method is, therefore, unlikely. In addition, the assessment of delta NSE values would largely level out methodological differences, which may result in a better comparability of results between individual centers.
According to the 2006 guidelines of the American Association of Neurology, bilaterally absent cortical responses (N20) on SSEP 24 h after cardiac arrest are 100 % predictive of poor outcome [4]. Therefore, the assessment of EPs (including SSEPs and BAEPs) in patients who do not show motor response 2 days after termination of analgosedation is part of clinical routine in survivors of OHCA at our institution. However, the studies supporting these guideline recommendations were carried out before hypothermia was established as standard treatment. The present findings confirm the reliability of SSEPs even after MTH treatment with a specificity and PPV of 100 % for the prediction of poor prognosis. In a prospective study, Bouwes and colleagues [22] recently reported similar findings when SSEPs were performed during hypothermia (32–34 °C) as well. On the other hand, a retrospective analysis by Leithner et al. [23] raised some caution when interpreting “poor” signs on SSEPs in MTH-treated patients: in a retrospective analysis of 58 patients with bilaterally pathological or absent N20 responses they identified one patient with a severe reduction of N20 amplitudes and another with absent N20 responses determined in normothermia 3 days after CA both of which regained full neurologic recovery (CPC 1).
With regard to clinical examination, the absence of motor response 48 h after termination of analgosedation was per se not suitable to predict poor outcome. Additionally, the absence of brainstem reflexes was also not considered suitable in the prediction of poor outcome, although displaying a somewhat higher specificity (Table 4). These findings are in line with recent studies, reporting a high false-positive rate for the absence of motor response to painful stimulation with [24, 25] or without specific MTH treatment [8]. Intriguingly, a recently published case report describes the transient reversal of diagnosed brain death shortly after rewarming, indicating that clinical examination for the assessment of neurologic prognosis should at least be performed at a core body temperature of ≥37 °C [26].
In the present study population with underlying cardiac disease we found that all patients with a first recorded non-shockable rhythm (asystole) had a poor prognosis although MTH treatment was consistently applied (Table 1). This finding is supported by the generally lower rate of survival in these patients [2, 27], but fuels the ongoing debate of possible protective effects of MTH in patients with an initial rhythm other than VF [28]. There is some evidence for a beneficial effect of MTH in patients presenting with other than VF and, therefore, MTH is also recommended for this group of patients although the supporting body of evidence is weak [2, 29]. In 2009, a subgroup analysis of patients with MTH and initial non-shockable CA found that MTH did not have a significant effect on neurologic outcome in patients with non-shockable rhythm [30]. On the other hand, another recent study reports improved neurologic outcome and reduced risk of death in patients treated with MTH, who suffered a witnessed OHCA due to non-shockable rhythm [31]. In a recently published meta-analysis, the authors report a slightly reduced in-hospital mortality in patients with MTH after a non-shockable CA, but as stated by the authors, the evidence is limited, especially as most studies had substantial risk of bias and quality of evidence was very low [28]. Our data represent a typical population with OHCA due to an underlying cardiac disease suggesting that such patients with non-shockable rhythm rather do not benefit from MTH treatment. Whether, in contrast, MTH is harmful under these conditions can only be speculated and would have to be answered in randomized trials. A cardiac rhythm primarily documented as asystole could in fact be a degenerated VF and, therefore, only resembles a deterioration of the patient′s condition by prolonged hypoxia. This may conflict assumptions regarding the relevance of a non-shockable rhythm per se. Careful evaluation of these aspects has to involve differences between in- and out-of-hospital cardiac arrest and differences between initial shockable, converted-to-shockable, and never-shockable rhythms.
Conclusions
Among the various tests used in clinical routine to predict neurologic outcome in comatose patients after OHCA treated with MTH, the present study identified the change in NSE on day 2, SSEPs, and the initial presence of a non-shockable rhythm to have the highest predictive value. Since unequivocal prediction of poor neurologic outcome is of utmost importance to avoid fatal limitation of therapy in patients who might potentially recover this decision must always be based on several prediction tools and be viewed in the overall clinical context.
References
Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O’Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL. Part 1: Executive summary: 2010 american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S640–56.
Nolan JP, Soar J, Zideman DA, Biarent D, Bossaert LL, Deakin C, Koster RW, Wyllie J, Bottiger B. European resuscitation council guidelines for resuscitation 2010 section 1. Executive summary. Resuscitation. 2010;81(10):1219–76.
Bradley SM, Gabriel EE, Aufderheide TP, Barnes R, Christenson J, Davis DP, Stiell IG, Nichol G. Survival increases with CPR by emergency medical services before defibrillation of out-of-hospital ventricular fibrillation or ventricular tachycardia: Observations from the resuscitation outcomes consortium. Resuscitation. 2010;81(2):155–62.
Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the quality standards subcommittee of the american academy of neurology. Neurology. 2006;67(2):203–10.
Lippert FK, Raffay V, Georgiou M, Steen PA, Bossaert L. European resuscitation council guidelines for resuscitation 2010 section 10. The ethics of resuscitation and end-of-life decisions. Resuscitation. 2010;81(10):1445–51.
The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Daubin C, Quentin C, Allouche S, Etard O, Gaillard C, Seguin A, Valette X, Parienti JJ, Prevost F, Ramakers M, Terzi N, Charbonneau P, du Cheyron D. Serum neuron-specific enolase as predictor of outcome in comatose cardiac-arrest survivors: A prospective cohort study. BMC Cardiovasc Disord. 2011;11:48.
Zandbergen EG, Hijdra A, Koelman JH, Hart AA, Vos PE, Verbeek MM, de Haan RJ. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66(1):62–8.
Shinozaki K, Oda S, Sadahiro T, Nakamura M, Abe R, Nakada TA, Nomura F, Nakanishi K, Kitamura N, Hirasawa H. Serum s-100b is superior to neuron-specific enolase as an early prognostic biomarker for neurological outcome following cardiopulmonary resuscitation. Resuscitation. 2009;80(8):870–5.
Oksanen T, Tiainen M, Skrifvars MB, Varpula T, Kuitunen A, Castren M, Pettila V. Predictive power of serum nse and ohca score regarding 6 months neurologic outcome after out-of-hospital ventricular fibrillation and therapeutic hypothermia. Resuscitation. 2009;80(2):165–70.
Cronberg T, Rundgren M, Westhall E, Englund E, Siemund R, Rosen I, Widner H, Friberg H. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology. 2011;77(7):623–30.
Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron-specific enolase and s-100b protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34(12):2881–6.
Rundgren M, Karlsson T, Nielsen N, Cronberg T, Johnsson P, Friberg H. Neuron specific enolase and s-100b as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation. 2009;80(7):784–9.
Steffen IG, Hasper D, Ploner CJ, Schefold JC, Dietz E, Martens F, Nee J, Krueger A, Jorres A, Storm C. Mild therapeutic hypothermia alters neuron specific enolase as an outcome predictor after resuscitation: 97 prospective hypothermia patients compared to 133 historical non-hypothermia patients. Crit Care. 2010;14(2):R69.
Fugate JE, Wijdicks EF, Mandrekar J, Claassen DO, Manno EM, White RD, Bell MR, Rabinstein AA. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68(6):907–14.
Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute physiology and chronic health evaluation (apache) IV: Hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34(5):1297–310.
Cummins RO, Chamberlain DA, Abramson NS, Allen M, Baskett P, Becker L, Bossaert L, Delooz H, Dick W, Eisenberg M, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: The utstein style. Task force of the american heart association, the european resuscitation council, the heart and stroke foundation of canada, and the australian resuscitation council. Ann Emerg Med. 1991;20(8):861–74.
Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet. 1998;352(9143):1808–12.
Chiappa KH, Ropper AH. Evoked potentials in clinical medicine (second of two parts). N Engl J Med. 1982;306(20):1205–11.
Chiappa KH, Ropper AH. Evoked potentials in clinical medicine (first of two parts). N Engl J Med. 1982;306(19):1140–50.
Bouwes A, Binnekade JM, Zandstra DF, Koelman JH, van Schaik IN, Hijdra A, Horn J. Somatosensory evoked potentials during mild hypothermia after cardiopulmonary resuscitation. Neurology. 2009;73(18):1457–61.
Leithner C, Ploner CJ, Hasper D, Storm C. Does hypothermia influence the predictive value of bilateral absent n20 after cardiac arrest? Neurology. 2010;74(12):965–9.
Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: A prospective study. Ann Neurol. 2010;67(3):301–7.
Bouwes A, Binnekade JM, Kuiper MA, Bosch FH, Zandstra DF, Toornvliet AC, Biemond HS, Kors BM, Koelman JH, Verbeek MM, Weinstein HC, Hijdra A, Horn J. Prognosis of coma after therapeutic hypothermia: A prospective cohort study. Ann Neurol. 2012;71(2):206–12.
Webb AC, Samuels OB. Reversible brain death after cardiopulmonary arrest and induced hypothermia. Crit Care Med. 2011;39(6):1538–42.
Mader TJ, Nathanson BH, Millay S, Coute RA, Clapp M, McNally B. Out-of-hospital cardiac arrest outcomes stratified by rhythm analysis. Resuscitation. 2012;83(11):1358–62.
Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW. Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non-shockable initial rhythms?: A systematic review and meta-analysis of randomized and non-randomized studies. Resuscitation. 2012;83(2):188–96.
Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL. Part 9: Post-cardiac arrest care: 2010 american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S768–86.
Arrich J, Holzer M, Herkner H, Mullner M (2009) Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev (4):CD004128.
Testori C, Sterz F, Behringer W, Haugk M, Uray T, Zeiner A, Janata A, Arrich J, Holzer M, Losert H. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation. 2011;82(9):1162–7.
Acknowledgments
We thank the staff of the intensive care unit at the Heart Center of the University of Cologne for their great support in the present study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huntgeburth, M., Adler, C., Rosenkranz, S. et al. Changes in Neuron-Specific Enolase are More Suitable Than Its Absolute Serum Levels for the Prediction of Neurologic Outcome in Hypothermia-Treated Patients with Out-of-Hospital Cardiac Arrest. Neurocrit Care 20, 358–366 (2014). https://doi.org/10.1007/s12028-013-9848-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-013-9848-8