Abstract
Background
Seizures are common after intracerebral hemorrhage (ICH) but their impact on outcome is uncertain and prophylactic anti-convulsant use is controversial. We hypothesized that seizures would not increase the risk of in-hospital mortality in a large administrative database.
Methods
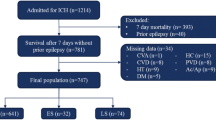
The study population included patients in the 2006 Nationwide Inpatient Sample over the age of 18 with a principal diagnosis of ICH (ICD9 = 431). Subjects with a secondary diagnosis of aneurysm, arterio-venous malformation, brain tumor, or traumatic brain injury were excluded. Seizures were defined by ICD9 codes (345.0x–345.5x, 345.7x–345.9x, 780.39). Logistic regression was used to quantify the relationship between seizures and in-hospital mortality. Pre-specified subgroups included age strata, length of stay, and invasive procedures.
Results
13,033 subjects met all eligibility criteria, of which 1,430 (11.0 %) had a secondary diagnosis of seizure. Subjects with seizure were younger (64 vs. 70 years, p < 0.001), more likely to get craniectomy (2.1 vs. 1.2 %, p = 0.006), ventriculostomy (8.5 vs. 6.0 %, p < 0.001), intubation (32.2 vs. 25.9 %, p < 0.001), and tracheostomy (6.4 vs. 4.2 %, p < 0.001). Seizure patients had lower in-hospital mortality (24.3 vs. 28.0 %, p = 0.003). In a multivariable model incorporating patient and hospital level variables, seizures were associated with reduced odds of in-hospital death (OR = 0.62, 95 % CI 0.52–0.75).
Conclusions
A secondary diagnosis of seizure after ICH was not associated with increased in-hospital death overall or in any of the pre-specified subgroups; however, there may be residual confounding by severity. These findings do not support a need for routine prophylactic anti-epileptic drug use after ICH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early clinical seizures are a frequent complication of intracerebral hemorrhage (ICH) with an incidence that has varied from 4 to 18 % in prospective cohorts.[1–4] There are multiple theoretical reasons why seizures may lead to worsened outcomes following ICH including blood pressure variability, increases in intracranial pressure, and neuronal death due to the combination of hyperexcitability and increased metabolic demand. Despite these theoretical concerns, prospective cohorts of patients with ICH have failed to find an independent association between seizures and poor outcome [2–6]. While reassuring, these studies were limited by small sample size and/or incomplete subgroup analysis.
Though the existing literature has failed to confirm an association between seizures and poor outcome, some practitioners prescribe prophylactic anti-convulsant medications to patients with ICH. There is a conflicting evidence on the impact of prophylaxis although a number of reports have suggested that prophylactic use of anti-epileptic drugs (AED), primarily phenytoin, is associated with worse outcome in this patient population [7–11]. Recent clinical guidelines have discouraged clinicians from prophylactic use of AEDs in patients with ICH, but practice remains highly variable [3, 8, 12].
The Nationwide Inpatient Sample (NIS) is the largest publicly available all-payer database for inpatient care in the United States, containing data from approximately eight million hospital stays each year. Though lacking in detailed clinical information, this administrative dataset provides a large sample size to explore the relationship between seizures and clinical outcome in a wide range of patient subgroups. We evaluated the association of a secondary diagnosis of seizures on in-hospital mortality after ICH overall and in pre-defined subgroups by patient age, length of stay, and surgical procedures.
Methods
Study Population
For this retrospective cohort study data were obtained from the NIS, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality for full calendar year 2006. The NIS is the largest all-payer inpatient database in the United States, representing a 20 % stratified sample of US hospitals. In 2006, the NIS contained data from 38 states.[13].
This analysis was limited to discharged patients who were ≥18 years old with a principal diagnosis of ICH (ICD9 code 431). This ICD9 code is estimated to have a positive predictive value >89 % [14, 15]. Subjects with a secondary ICD9 code of arteriovenous malformation (437.3), malignant brain tumor (191.x), skull fracture (800–801), concussion (850), and traumatic ICH (851–854) were excluded.
The primary exposure of interest was seizure, defined using ICD9 codes. ICD9 codes for epilepsy and convulsions (345.xx and 780.3x) have high validity overall [16]. For this study, we defined seizures using an ICD9 coding algorithm of epilepsy (345.0x–345.5x and 345.7x–345.9x) or other convulsions (780.39). We excluded codes for infantile spasms (345.6x) and febrile convulsions (780.31, 780.32). The primary outcome was in-hospital mortality. Length of stay was investigated as a secondary outcome.
Patient level variables included year of discharge, age, sex, race, primary expected payer (Medicare, Medicaid, private including HMO, self-pay, no charge, other), and median household income, by quartile, in the patient’s ZIP code. Hospital level variables included teaching status, hospital location (urban vs. non-urban), hospital region (Northeast, Midwest, South, West), and volume of ICH cases per year (<25, 25–75, >75). Surgical procedures of interest were defined using ICD9 procedure codes, including craniectomy (01.20–01.29), ventriculostomy (02.2), hematoma drainage (01.39), intubation (96.7x), tracheostomy (31.1, 31.2x), and feeding tube (43.1x, 44.32, 46.32). Multivariable models also incorporated 29 of the Elixhauser medical comorbidities (Supplemental 2) and an all-patient refined diagnosis related group (APR-DRG) based mortality risk indicator [17, 18]. The APR-DRG mortality risk system incorporates diagnoses, procedures, and age to assign each subject a score from 1 to 4, indicating minor, moderate, major, or extreme risk of mortality.
Statistical Analysis
Baseline characteristics for patients with seizures and those without seizures were described using measures of central tendency (means and medians) for continuous variables and proportions for categorical variables. Group differences were evaluated using students t test, Wilcoxon rank sum, and χ2 as appropriate.
Univariate and multivariable logistic regression were used to assess the relationship between seizures and in-hospital mortality. Using logistic regression four different multivariable models were constructed, accounting for the survey design of the NIS and hospital clustering. The first model incorporated demographic and hospital level variables from the univariate analysis which were significant to p < 0.10. The second model incorporated all variables from the first model plus surgical procedures (craniectomy, ventriculostomy, hematoma drainage, intubation, tracheostomy, and feeding tube placement). The third model incorporated all variables from Model 2 plus 29 of the Elixhauser comorbid conditions (Supplement 1). The fourth model incorporated all variables from the third model plus APR-DRG risk of mortality. Models were then repeated using the secondary outcome of length of stay. To account for the non-parametric distribution of length of stay, a generalized linear model with a log link and negative binomial distribution was used. Finally, we evaluated the association of seizures and death in pre-specified subgroups of surgical procedures (craniectomy, ventriculostomy, hematoma drainage, intubation, tracheostomy, and feeding tube placement), age strata (<55, 55–64, 65–74, 75–84, 85+ years), and length of stay (<7 days, 7–14 days, >14 days) using standard logistic regression, which did not account for hospital clustering.
Results
There were 13,033 subjects who met all eligibility criteria, of which 1,430 (11.0 %) had a secondary diagnosis of seizure. Patient and hospital level variables are summarized in Table 1. Of note, subjects with seizure were younger (64 vs. 70 years, p < 0.001), more likely to get craniectomy (2.1 vs. 1.2 %, p = 0.006), ventriculostomy (8.5 vs. 6.0 %, p < 0.001), intubation (32.2 vs. 25.9 %, p < 0.001), and tracheostomy (6.4 vs. 4.2 %, p < 0.001).
Fewer patients with seizures died during the hospitalization than those without seizures (24.3 vs. 28.0 %, p = 0.003; OR 0.82, 95 % CI 0.72–0.95). This finding persisted in all four of the multivariable models (Table 2). The odds of in-hospital death for those with seizures was 0.87 in Model 1 (95 % CI 0.76–1.00), 0.72 in Model 2 (95 % CI 0.61–0.86), 0.71 in Model 3 (95 % CI 0.60–0.85), and 0.62 in Model 4 (0.52–0.75). Seizures did not significantly increase in-hospital death in any of the pre-specified subgroups (Fig. 1).
Seizure patients had a longer median length of stay than those without seizures (6 days vs. 5 days, p < 0.001, unadjusted). This persisted in all four multivariable models (Supplemental Table 2).
Discussion
In this large administrative dataset, a secondary diagnosis of seizure after ICH was not associated with an increased risk of in-hospital death overall or in any of the predefined subgroups of age, length of stay, or surgical procedures despite a slightly longer length of stay.
Many prior studies investigating the relationship between seizures and outcome have pooled ischemic stroke and ICH cases, with ischemic stroke accounting for 60–96 % of cases [2, 5, 6, 19–21]. The studies which have analyzed ICH separately have not identified clinical seizures as an independent risk factor for poor outcome. A population-based cohort study from the Greater Cincinnati/Northern Kentucky Stroke Study which utilized clinical data including NIH stroke scale and Glasgow Coma Scale reported seizures in 7.9 % of ICH cases (n = 546). In a multivariable analysis, seizures were not associated with mortality (OR 0.85, 95 % CI 0.33–2.15) [4]. A prospective hospital based cohort of 522 subjects with spontaneous ICH reported early seizures in 14 %, but failed to detect an association with mortality or functional outcome [22]. Similarly, a prospective, multi-center study from Spain of 266 patients with spontaneous supratentorial ICH found that seizures were not associated with early neurologic deterioration [23]. A prospective study from Italy of 761 patients with non-traumatic, non-aneurysmal ICH detected seizures in 8.1 %. In this study neither immediate seizures (OR 1.08, 95 % CI 0.84–1.40) nor early seizures (OR 1.01, 95 % CI 0.77–1.33) were associated with worse outcome [3]. However, because of the limited size of these prior studies, the confidence intervals were wide and the possibility of harm from seizure could not be excluded. Our analysis adds to this existing literature because of its large sample size, which greatly increases the power to detect an association if one is present. The large sample size also allows for investigation of patient subgroups. Our inability to detect an association between seizures and in-hospital mortality in any of the pre-specified subgroups provides further evidence that clinical seizures are unlikely to be detrimental to patients with ICH.
ICH patients with seizures are most likely placed on an anti-convulsant medication. The absence of an association between seizures and poor outcome would imply that AED use is safe. However, several studies have shown an association between certain types of prophylactic AEDs and poor outcome. In a series of 303 patients with primary ICH enrolled in the placebo arm of a putative neuroprotectant trial, prophylactic AED (mostly phenytoin) was strongly associated with poor outcome (OR 6.8; 95 % CI 2.2–21.2, p = 0.001) after adjustment for age, initial hematoma volume, presence of intraventricular blood, initial Glasgow Coma Score, and prior warfarin use. In a second prospective cohort of 98 ICH patients, the use of phenytoin was associated with more fever and worse modified Rankin Scale at 3 months [8]. Levetiracetam use was not associated with any difference in outcome although fewer patients received this medication and power was limited. In a study of 157 patients with ICH, of which 46 were placed on prophylactic AEDs there was no significant difference in mortality.[9] A retrospective cohort of 85 ICH patients given prophylactic AEDs found that levetiracetam was associated with improved cognition and increased likelihood of being discharged to home compared to phenytoin despite similar hemorrhage severity [11]. A small randomized trial (n = 72) of valproic acid versus placebo found no difference in mortality and the valproic acid treated patients had lower NIHSS post-treatment [10]. A recently published retrospective study found that patients on AEDs were less likely to die than those not on AEDs, but a secondary analysis, designed to reduce confounding by indication, found no association [24].
Interestingly, two prior studies which used continuous EEG monitoring to identify both clinical and subclinical seizures have suggested a potential relationship between electrographic seizures and poor outcome. A study of 63 patients with ICH who underwent continuous EEG monitoring detected electrographic seizures in 28 % [25]. These electrographic seizures were associated with worsening on the NIHSS and an increase in midline shift. In a multivariable model assessing independent predictors of outcome using the Glasgow Outcome Score, seizures approached significance (p < 0.06). Another study of 102 consecutive ICH cases that underwent continuous EEG detected electrographic seizures in 31 % [26]. Electrographic seizures were associated with hemorrhage expansion; though, not significantly associated with poor outcome. The generalizability of these studies is limited as both studies were conducted in relatively small, selected samples of critically ill patients. These studies were also not able to determine causality, and it is possible that expanding hemorrhages or worsening edema cause both electrographic seizures and worse outcome. Nonetheless, the findings in these studies raise the possibility that electrographic seizures after ICH may be detrimental. Larger, prospective studies are needed to definitively determine whether seizures, electrographic or clinical, affect outcome and if AEDs can impact this process to improve outcome. Based on the existing data noted above, any such study should avoid the use of phenytoin.
This study has several important limitations. The NIS does not provide data on clinical variables which are known to predict outcome after ICH, including hemorrhage location, hemorrhage size, and severity [27]. Our finding that seizure patients were less likely to die in the hospital than those without seizures could indicate residual confounding from these important variables. In addition, information bias may be influencing our results. It is possible that subtle seizures are less likely to be diagnosed in patients with severe neurologic injury. Alternatively, patients with extremely severe hemorrhages may die or have care withdrawn before seizures are diagnosed. Finally, seizures may be associated with lobar hemorrhages, which, relative to deep/infratentorial hemorrhages, are less likely to result in a poor outcome [3, 22, 26–28]. All of these scenarios would bias the results to make seizure appear protective. Unfortunately, we are unable to investigate these possibilities in this dataset. The NIS also does not provide data on drug administration so our analysis is unable to account for AED use. In addition, the NIS does not have a measure of functional outcome, so we are unable to assess the impact of seizures on long-term function among survivors. ICD codes to identify seizures have a high validity in administrative datasets; but, their performance when used as a secondary diagnosis after ICH is not known [16]. In addition, ICD9 coding of epilepsy subtypes is less accurate, precluding a detailed analysis of epilepsy subtypes.(Jette 2010) ICD9 identifying generalized status epilepticus reasonably well, with a positive predictive value of 83.9 % (ICD9 345.3) [16]. Previous work in the NIS using data from 1994 to 2002 found that status epilepticus was rare, occurring in 0.3 % of ICH cases, and it was not independently associated with in-hospital mortality. It was associated with pneumonia, mechanical ventilation and length of stay >7 days. Finally, because of the small number of subjects in some subgroups, the subgroup analysis treated the dataset as a large convenience sample and did not account for clustering within hospitals. As a result the reported confidence intervals may be artificially narrow. The point estimates should not be affected and our conclusion that seizures were not significantly associated with increased in-hospital mortality, would not change if the confidence intervals were widened.
Conclusions
In a large administrative database, seizure after ICH did not increase in-hospital mortality overall, or in pre-specified subgroups by patient age, length of stay, and surgical procedures. Larger, prospective studies are needed to definitively determine whether seizures, either clinical or electrographic, impact outcome and, if so, whether AEDs can modulate risk. Until such trials are complete, our finding supports the most recent guideline statement from the AHA that recommends against the routine use of prophylactic AED in patients with ICH, though clinical seizures should be treated appropriately.
References
Berger AR, Lipton RB, Lesse ML, Lantos G, Portenoy RK. Early seizures following intracerebral hemorrhage: implications for therapy. Neurology. 1988;38:1363–5.
Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000;57:1617–22.
Passero S, Rocchi R, Rossi S, Ulivelli M, Vatti G. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002;43:1175–80.
Szaflarski JP, Rackley AY, Kleindorfer DO, et al. Incidence of seizures in the acute phase of stroke: a population-based study. Epilepsia. 2008;49:974–81.
Labovitz DL, Allen Hauser W, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology. 2001;57:200–6.
Kilpatrick CJ, Davis SM, Tress BM, Rossiter SC, Hopper JL, Vandendriesen ML. Epileptic seizures in acute stroke. Arch Neurol. 1990;47:157–60.
Messe SR, Sansing LH, Cucchiara BL, Herman ST, Lyden PD, Kasner SE. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care. 2009;11(1):38–44.
Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke. 2009;40:3810–5.
Reddig RT, Nixdorf KE, Jensen MB. The prophylactic use of an antiepileptic drug in intracerebral hemorrhage. Clin Neurol Neurosurg. 2011;113:895–7.
Gilad R, Boaz M, Dabby R, Sadeh M, Lampl Y. Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment? Epilepsy Res. 2011;95:227–31.
Taylor S, Heinrichs RJ, Janzen JM, Ehtisham A. Levetiracetam is associated with improved cognitive outcome for patients with intracranial hemorrhage. Neurocritical care. 2011;15:80–4.
Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–29.
Overview of the Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project. http://www.hcup-us.ahrq.gov/nisoverview.jsp (2011). Accessed 26 Nov 2011.
Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–81.
Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33:2465–70.
Jette N, Reid AY, Quan H, Hill MD, Wiebe S. How accurate is ICD coding for epilepsy? Epilepsia. 2010;51:62–9.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
Averill RFGN, Muldoon J, Steinbeck BA, Grant TM. A closer look at all-patient refined DRGs. J AHIMA. 2002;73:46–50.
Arboix A, Garcia-Eroles L, Massons JB, Oliveres M, Comes E. Predictive factors of early seizures after acute cerebrovascular disease. Stroke. 1997;28:1590–4.
Burneo JG, Fang J, Saposnik G. Impact of seizures on morbidity and mortality after stroke: a Canadian multi-centre cohort study. Eur J Neurol. 2010;17:52–8.
Reith J, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Seizures in acute stroke: predictors and prognostic significance. The Copenhagen stroke study. Stroke. 1997;28:1585–9.
De Herdt V, Dumont F, Henon H, et al. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology. 2011;77:1794–800.
Leira R, Davalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–7.
Battey TW, Falcone GJ, Ayres AM, et al. Confounding by indication in retrospective studies of intracerebral hemorrhage: antiepileptic treatment and mortality. Neurocrit Care. 2012.
Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441–6.
Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–65.
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–7.
Castellanos M, Leira R, Tejada J, Gil-Peralta A, Davalos A, Castillo J. Predictors of good outcome in medium to large spontaneous supratentorial intracerebral haemorrhages. J Neurol Neurosurg Psychiatry. 2005;76:691–5.
Conflict of interest
Dr. Mullen, Dr. Messé and Dr. Kasner have no relevant disclosures to report.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mullen, M.T., Kasner, S.E. & Messé, S.R. Seizures Do Not Increase In-Hospital Mortality After Intracerebral Hemorrhage in the Nationwide Inpatient Sample. Neurocrit Care 19, 19–24 (2013). https://doi.org/10.1007/s12028-012-9791-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-012-9791-0