Abstract
Background
Fever and hematoma growth are known to be independent predictors of poor outcome after intracerebral hemorrhage (ICH). We sought to assess the distribution of temperature at different stages in relation to hematoma growth and functional outcome at 90 days in a cohort of ICH patients.
Methods
Data of patients registered in the Virtual International Stroke Trials Archive—ICH were analyzed. Temperatures at baseline, 24, 48, 72, and 168 h were assessed in relation to the hematoma growth and functional outcome at 90 days. We calculated the daily linear variation of each subject’s temperature by subtracting 37 °C from the maximal daily recorded temperature (delta-temperature). We used logistic regression and mixed-effects models to identify factors associated with hematoma growth, poor outcome, and temperature elevation after ICH.
Results
303 patients were included in the analysis. The average age was 66 ± 12 years, 200 (66 %) were males, median admission NIHSS was 13 [Interquartile range (IQR), 9–18), median GCS was 15 (IQR, 14–15). Hematoma growth occurred in 22 % and poor functional outcome at 90-days occurred in 41 % of the patients. Cumulative delta-temperature at 72 h was associated with hematoma growth; age, ICH score, hematoma growth, and cumulative delta-temperature at 168 h were associated with poor outcome at 90 days. Factors associated with fever in mixed-models were day after onset of ICH, hypertension, base hematoma volume, intraventricular-hemorrhage, pneumonia, and hematoma growth.
Conclusions
There is a temporal and independent association between fever and hematoma growth. Fever after ICH is associated with poor outcome at 90 days. Future research is needed to study the mechanisms of this phenomenon and if early protocols of temperature modulation would be associated with improved outcomes after ICH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early hematoma growth after spontaneous intracerebral hemorrhage (ICH) is common and associated with neurological deterioration and poor clinical outcome [1, 2]. Prior studies indicate that hematoma growth occurs in up to 38 % of ICH patients when re-scanned within 3 h of onset, and in 16 % of those scanned between 3 and 6 h, even in the absence of coagulopathy [1, 3–5]. The mechanisms that lead to early hematoma growth during the acute stage of ICH remain unclear but many factors such as vascular engorgement related to a reduction in venous outflow, early transient ischemia, activation of pro-inflammatory cytokines, ongoing bleeding, and a local coagulopathic state secondary to increased levels of fibrinolytic proteins and decreased level of coagulation proteins [6–15] have been implicated. In addition to the relative mass effect, the hematoma itself induces early pathophysiologic changes in the surrounding brain tissue such as breakdown of the brain-blood barrier, vasogenic edema, and neuronal and glial cell death due to apoptosis and inflammation [10, 16, 17].
As an adaptive response to injury and inflammation, fever may therefore be seen with the development of the hematoma after ICH [18, 19]. More important to clinical practice, is that fever [20–22] is considered to have detrimental effects after all types of brain injury including ICH [18, 23], because this response is known to increase the metabolism of the brain and can exacerbate neuronal injury, events that occur around the hematoma [7–15, 24, 25]. However, a temporal or independent association between hematoma growth and fever has not been discerned by prior studies that have evaluated the effects of fever on outcomes after ICH [18, 19], while considering the effects of other factors associated with fever. In this study, we sought to assess the distribution of temperature at different stages in relation to hematoma growth and functional outcome at 90 days in a cohort of ICH patients. Specifically, we sought to determine:(a) whether fever was a common occurrence after ICH; (b) whether the presence of fever was associated with hematoma growth; (c) whether fever was associated with poor functional outcome at 90 days; (d) whether the exposure to fever remained significantly associated with hematoma growth and poor functional outcome after adjustment for confounding variables in a multivariate analysis; and (e) to identify independent predictors of fever after ICH. We hypothesized that fever would be temporally associated with hematoma growth and poor outcome after ICH.
Methods
Study Design and Patient Data
A retrospective cohort study using a prospectively compiled and maintained registry from the Virtual International Stroke Archive (VISTA) [26]. Details and methods of the VISTA collaboration have been previously published [26, 27]. In brief, this database includes data from clinical trials that had documented entry criteria and monitoring processes for validation of data. VISTA holds data from 31 trials involving more than 28,000 patients, age range 18–103 years, who experienced an index acute stroke due to either ischemia or ICH [27]. Eligibility for VISTA required the following: (1) documented entry criteria into a trial, with a minimum of 100 patients; (2) local ethics board approval; (3) baseline assessment within 24 h of stroke; (4) baseline assessment of neurologic deficit; (5) confirmation of stroke with imaging; (6) outcome assessment between 1 and 6 months with a validated stroke scale; and (7) data validation through monitoring [27]. Our sample cohort consisted of patients presenting with CT-proven ICH within 6 h of symptom onset. All patients had baseline and 24 h National Institutes of Health Stroke Scale (NIHSS) score [28], Glasgow Coma Scale (GCS) [29], ICH Score [30], follow-up CT scan at 72 h, and 90-days modified Rankin Scores (mRS) [31]. For this analysis, patients were selected from the control arm of the only registered ICH trial: the CHANT trial [32]; all patient data were de-identified by VISTA, and all studies included into the VISTA database required patient consent and local ethics or Institutional Review Board (IRB) approval; this analysis was exempted from full review by the IRB of Thomas Jefferson University Hospital.
Definition of Exposure and Outcome Variables
Our exposure variable was temperature measured in Celsius degrees (T °C), which was recorded daily from either one of the following locations: rectal (3 %), oral (20 %), tympanic (26 %), or axillary (51 %). We defined fever as any daily recorded maximal temperature T ≥37.5 °C at baseline, 24, 48, 72, or 168 h after onset of ICH symptoms. This cutoff was defined a priori and conforms to published results from clinical studies [20, 33] and practice guidelines for initiating antipyretic therapy and diagnostic evaluation of fever or infection causes in the Intensive Care Unit [22]. We also calculated the linear variation of each subject’s temperature by subtracting 37 °C from the maximal daily recorded temperature (delta-temperature); when the maximal daily temperature was below 37.0 °C, a score of 0 was assigned [34, 35]. The analysis of change in volume from baseline to 72 h was adjusted for baseline volume as originally defined by the methods of the source trial [32]. ICH volumes were measured on the baseline and 72 h scan using semi-automated computerized planimetry; scans were read centrally and by blind assessors [32]. Our secondary outcome of interest was a measure of “poor outcome” at 90 days, defined a priori as a mRS [31] of 4–6 [27].
Statistical Analysis
Continuous data are presented as means and standard deviations (SD) or medians and interquartile ranges (IQR) as appropriate based on the distribution of the data; categorical data are reported as number of subjects and proportions. The t test for normally distributed data, the Mann–Whitney-U test for non-parametric data, or the χ2 or Fisher exact tests for proportions were used for determination of predictors and potential confounders of hematoma growth and poor outcome. In the initial bivariate analysis, we assessed the relationship of each of the potential predictors of hematoma growth and 90-days mRS. As our main predictor of interest, the cumulative delta-temperature was not normally distributed, we log-transformed it for all subsequent multivariate analyses and mixed models and to allow for evaluation of linear relationships. Multivariate models were fitted to assess for predictors of hematoma growth and poor outcome by means of logistic regression and with mixed-effects models to assess for predictors of temperature elevation after hospital admission. The multivariate analysis proceeded in two stages. In the first stage, significant factors were identified from the candidate variables. All patient data in Table 1 were considered as candidate variables for the models. In constructing our multivariate models, we included covariates with bivariate p values of <0.2 as seen in Table 2. We also tested for interactions between all independent predictors of outcome in the final models. To determine the relative contributions of the individual co-variates in the model, we compared changes in the likelihood ratio value of the entire model (an estimation of the overall fitness of the model) after individual addition of each significant co-variate. Finally, tests for co-linearity were performed with correlation analysis and the Homer–Lemeshow test was used to assess for the goodness of fit. In the second stage, we evaluated for predictors of temperature elevation with mixed-effects models to identify admission and hospital predictors of fever, with the variable “subject” as a random effect, and the fixed linear effect “ICH day” nested within each subject [34]. An autoregressive pattern (AR1) was tested, assuming decreasing correlation of delta-temperatures within each subject. Statistical analyses were conducted using standard statistical software [SAS Version 9.2 (SAS Institute, Cary, NC) and SPSS Version 19.0 for Macintosh] and significance was judged for p < 0.05 unless indicated.
Results
Description of the Cohort and Distribution of Temperatures
At the time of this analysis, the VISTA—ICH repository contained 603 patients enrolled in one ICH trial: the CHANT trial [32]. The final study cohort consisted of 303 patients participating in the placebo arm of this source trial [32]. Mean age was 66 ± 12 years, of which 66 % were men; median NIHSS on admission was 13 (IQR, 9–18); median GCS was 15 (IQR, 14–15); median ICH score was 1 (IQR, 0–1); median hematoma volume was 15 cc (IQR, 8–33 cc); intraventricular-hemorrhage (IVH) was seen in 34 % of the patients; other baseline characteristics are shown in Table 1. Hematoma growth occurred in 22 % and poor functional outcome at 90 days occurred in 41 % of the patients (Table 1).
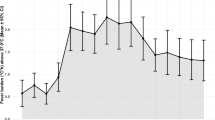
Daily temperatures were measured from rectal (3 %), oral (20 %), tympanic (26 %), or axillary (51 %) routes. The proportion of hyperthermic patients increased over time in relation to hematoma growth and functional outcome (Fig. 1; Table 2). The proportion of fever at 24 (p = 0.009) and 72 h (p = 0.004) was significant in those patients with hematoma growth but this was not seen at baseline, 48, or 168 h (Fig. 1; Table 2). Similarly, the proportion of fever at 24 (p = 0.003), 48 (p = 0.02), and 168 h (p = 0.01) was higher in those patients with poor outcome at 90 days but not at baseline or 72 h, though there was a trend at 72 h (p = 0.06) (Fig. 1; Table 2). The cumulative delta-temperatures were also higher at 72 h (p = 0.08) in those patients with hematoma growth and significantly higher at 168 h in those with poor functional outcome at 90 days (p < 0.001) (Table 2).
Predictors of Hematoma Growth and Poor Outcome
In the bivariate analysis, hematoma growth was associated with: history of atrial fibrillation, hypertension (HTN), international normalized ratio (INR), anticoagulation with warfarin, fever at 24 and 72 h, and cumulative delta-temperature at 72 h (Table 2). Poor outcome was associated with: age, history of HTN and diabetes mellitus, admission NIHSS, GCS, ICH score, INR, white blood cell count, baseline hematoma volume, presence of IVH, fever at 24, 48, 72, and 168 h; cumulative delta-temperature at 168 h; and hematoma growth (Table 2).
In multivariate analysis, history of anticoagulation with warfarin (p = 0.03) and the cumulative delta-temperature at 72 h in log-scale (p = 0.04) was associated with hematoma growth (Table 3). Predictors of poor outcome at 90 days were age (p = 0.03), ICH score (p < 0.001), hematoma growth (p = 0.02), and the total cumulative delta-temperature at 168 h in log-scale (p = 0.04). We did not observe significant interactions between our co-variates.
Predictors of Temperature Elevation After ICH
Independent factors associated with temperature elevation in the mixed-effects analysis were: day after onset of ICH, history of HTN, base hematoma volume ≥15 cc, IVH, pneumonia, and hematoma growth (Table 4). Though we found nine patients exposed to anti-epileptic drugs for seizure prophylaxis, of which 8 (4 %) received phenytoin, our mixed-effect models did not find a significant association between phenytoin exposure and temperature elevation.
Discussion
In this study, we observed that fever was a common occurrence after ICH and independently associated with hematoma growth and poor functional outcome at 90 days. Most importantly, we found that fever was temporally associated with hematoma growth, after adjustment for additional predictors of temperature elevation including: white blood cell count, infectious complications (pneumonia), phenytoin exposure, hematoma volume, and IVH.
Fever is frequently seen after all types of brain injury [18, 20, 36] including ICH, where it can be seen in up to 91 % of the patients [33]. Schwarz et al. [33] reported that the incidence of fever (defined as temperature ≥37.5 °C) was higher during the first 72-h after ICH and that this proportion decreased over time. We observed a smaller incidence of fever in our cohort (Table 2), which may have been explained by the less severe forms of ICH patients enrolled in the source trial [32] as compared to the more severe cohort seen in the Schwarz et al. [33] study, where 50 % of patients had GCS of less than 14 and at least 40 % had ICH volumes >60 cm3. In agreement with Schwarz et al. [33], we also observed that the proportion of fever increased significantly after the first 24 h and trended down later on (Fig. 1). We did not find a significant difference in the proportion of fever at baseline, suggesting that the onset of inflammatory mechanisms associated with the hematoma and its growth, reminiscent of a systemic inflammatory response as seen in other types acute injury (trauma, stroke, sepsis, among others), may be implicated in this phenomenon [10, 16, 17] (Table 2). In support of this observation, is that both hematoma volume and growth remained independently associated with temperature elevation in our mixed-analysis after adjustment for other predictors of fever (Table 4). Because the source trial recorded only maximal daily temperatures and assessed hematoma growth at 72 h, we cannot definitely conclude that the peak temperatures seen after 24 h of onset of ICH in our study were related to the expansion of the hematoma. However, our mixed-analysis included those plausible factors that may have been associated with fever after ICH including: hematoma growth, day after onset of ICH [33], IVH [33], hematoma volume, infections, and drug exposure (Table 4), and provides some evidence of the temporal association of hematoma growth with a febrile response. Similarly, as most of hematoma growth after ICH occurs within the first 6 h [1, 3–5] and less likely beyond 12 h [1, 3–5], and the ascertainment of hematoma growth occurred at 72 h in the source trial [32], we cannot definitely conclude that the proportion of fever observed during the first 24 h (Table 2) was a consequence of hematoma growth. However, it is still possible that fever was a manifestation of hematoma growth [37] and possibly related to several physiopathological mechanisms such as breakdown of the brain-blood barrier, vasogenic edema, and neuronal and glial cell death due to apoptosis and inflammation [10, 16, 17]. We also found a significant association between fever and poor functional outcome at 90 days even when considering the effects of hematoma growth and other predictors of poor functional outcome, supporting the results of other observational studies [18, 19, 33].
As an adaptive response to injury and inflammation, fever would be an expected event after ICH, particularly during the first 72 hrs after onset of symptoms [19, 33]. Fever is known to increase the metabolism of the brain and can exacerbate neuronal injury, events that may occur around the hematoma. Several potential physiopathologic mechanisms that may explain this phenomenon include: excitotoxicity from amino-acid and free radical production, cytoskeletal proteolysis, inhibition of protein kinases, and activation of certain matrix metalloproteinases, which could lead to blood–brain barrier breakdown, hematoma growth, and worsening cerebral edema [7–15, 24, 25]. With newer technologies for temperature modulation available in the intensive care unit, and based on the detrimental effects of fever after brain injury [18], aggressive temperature modulation will play a role in the management of ICH patients to improve functional outcomes. Recent experimental data from animal models of ICH that used bacterial collagenase infusions, suggested that temperature modulation improved recovery and lessened neuronal injury when temperature modulation to hypothermia was initiated after 12 h of onset [38], but this effect was not seen in a different animal model of “whole blood” infusion [39]. A recent study of ICH patients suggested that mild induced hypothermia was associated with less cerebral edema without change in hematoma growth or functional outcome when hypothermia was started after 6 h of onset [40]. To this end, our results are important as they provide support for those initiatives that will study the potential therapeutic implications of early protocols of temperature modulation, which may limit secondary neuronal damage after ICH.
We acknowledge that though association does not necessarily imply causation, our data support prior observations showing that fever after ICH may have adverse effects, not only by its independent association with hematoma growth but also on functional outcomes. Whether ultra-early aggressive therapeutic temperature modulation after ICH improves functional outcomes after ICH by modulation of the neuro-inflammatory response as seen in animal models [38], remains to be answered by future clinical studies aimed at answering this particular question. Novel strategies that go beyond control of glucose, blood pressure, and ICP [41], aimed at reducing hematoma growth and neuro-inflammation could be the new frontier in the management of ICH. Finally, our study demonstrates an association between hematoma growth and fever, independent of infectious adverse events; this could be important in clinical practice, as early onset of fever after ICH may not be an indication of empiric antibiotic regimen in the absence of obvious signs of infection.
Our study has limitations. First, our analysis was observational in nature, which limits the inferences that can be made about etiological or causal relationships. Second, our study is a post hoc analysis of the source trial, which included a heterogeneous population of ICH patients (history of hypertension and exposed to anticoagulants)[32], and was not designed to answer our proposed questions, so our results should be interpreted with caution. Third, we acknowledge our modest sample size and that our exposure variable was measured from different sites allowing for variability or possible underestimation of temperature measurements. Similarly, a single daily measurement of temperature may have not captured the whole burden of fever on our proposed outcomes and most importantly, we did not have temperature data between the baseline and 24 h when hematoma growth after ICH is more likely to occur. However, our results mirror and are in agreement with the findings of earlier observational studies that addressed the effect of fever and functional outcome after ICH [33]. Fourth, our study looked at patient data within 168 h of ICH onset, so the effect of fever on functional outcome after this time may have not been accounted for in our multivariate models. Considering all these shortcomings, our study provides important information about the effects of fever after ICH and its temporal relationship with hematoma growth, implying that this response should not be considered an epi-phenomenon but a possible target for future therapies for the most devastating type of stroke.
In summary, our data confirms the frequent occurrence of fever after ICH. There is a probable temporal association between fever and hematoma growth as early as 24 h. It is also possible that the inflammatory mechanisms implicated in the physiopathology of ICH are responsible for the abnormal early temperature elevation seen in these patients. Future research is needed to study the mechanisms of this phenomenon and if early protocols of temperature modulation would be associated with improved outcomes after ICH.
References
Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5.
Gebel JM Jr, Jauch EC, Brott TG, et al. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636–41.
Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160–6.
Fujii Y, Tanaka R, Takeuchi S, Koike T, Minakawa T, Sasaki O. Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg. 1994;80:51–7.
Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783–7.
Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–62.
Mayer SA. Ultra-early hemostatic therapy for intracerebral hemorrhage. Stroke. 2003;34:224–9.
Mayer SA, Lignelli A, Fink ME, et al. Perilesional blood flow and edema formation in acute intracerebral hemorrhage: a SPECT study. Stroke. 1998;29:1791–8.
Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–32.
Qureshi AI, Ali Z, Suri MF, et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003;31:1482–9.
Gordon CR, Merchant RS, Marmarou A, Rice CD, Marsh JT, Young HF. Effect of murine recombinant interleukin-1 on brain oedema in the rat. Acta Neurochir Suppl (Wien). 1990;51:268–70.
Megyeri P, Abraham CS, Temesvari P, Kovacs J, Vas T, Speer CP. Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood–brain barrier permeability in newborn piglets. Neurosci Lett. 1992;148:137–40.
Marti-Fabregas J, Borrell M, Silva Y, et al. Hemostatic proteins and their association with hematoma growth in patients with acute intracerebral hemorrhage. Stroke. 2010;41:2976–8.
Takahashi H, Urano T, Nagai N, Takada Y, Takada A. Progressive expansion of hypertensive intracerebral hemorrhage by coagulopathy. Am J Hematol. 1998;59:110–4.
Olivot JM, Mlynash M, Kleinman JT, et al. MRI profile of the perihematomal region in acute intracerebral hemorrhage. Stroke. 2010;41:2681–3.
Power C, Henry S, Del Bigio MR, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53:731–42.
Felberg RA, Grotta JC, Shirzadi AL, et al. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Ann Neurol. 2002;51:517–24.
Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39:3029–35.
Rincon F, Patel U, Schorr C, Dellinger RP, Parrillo J, Zanotti-Cavazoni S. Effect of Early hyperthermia on hospital mortality in critically-ill neurological patients: a matched cohort study (NCS abstracts). Neurocrit Care. 2011;15(Suppl 1):181.
Sacho RH, Vail A, Rainey T, King AT, Childs C. The effect of spontaneous alterations in brain temperature on outcome: a prospective observational cohort study in patients with severe traumatic brain injury. J Neurotrauma. 2010;27:2157–64.
Childs C, Wieloch T, Lecky F, Machin G, Harris B, Stocchetti N. Report of a consensus meeting on human brain temperature after severe traumatic brain injury: its measurement and management during pyrexia. Front Neurol. 2010;1:146.
O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36:1330–49.
Badjatia N. Hyperthermia and fever control in brain injury. Crit Care Med. 2009;37:S250–7.
Dietrich WD. The importance of brain temperature in cerebral injury. J Neurotrauma. 1992;9(Suppl 2):S475–85.
Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab. 2007;27:1879–94.
Ali M, Bath PM, Curram J, et al. The virtual international stroke trials archive. Stroke. 2007;38:1905–10.
Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–44.
Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25:2220–6.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4.
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–7.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7.
Lyden PD, Shuaib A, Lees KR, et al. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke. 2007;38:2262–9.
Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354–61.
Fernandez A, Schmidt JM, Claassen J, et al. Fever after subarachnoid hemorrhage: risk factors and impact on outcome. Neurology. 2007;68:1013–9.
Naidech AM, Bendok BR, Bernstein RA, et al. Fever burden and functional recovery after subarachnoid hemorrhage. Neurosurgery 2008;63:212–7; discussion 7–8.
Saini M, Saqqur M, Kamruzzaman A, Lees KR, Shuaib A. Effect of hyperthermia on prognosis after acute ischemic stroke. Stroke. 2009;40:3051–9.
Fang SB, Chang YT, Chuo YH, Tsai ST, Tseng CL. Hyperthermia as an early sign of intracranial hemorrhage in full-term neonates. Pediatr Neonatol. 2008;49:71–6.
MacLellan CL, Girgis J, Colbourne F. Delayed onset of prolonged hypothermia improves outcome after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2004;24:432–40.
MacLellan CL, Silasi G, Poon CC, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–25.
Kollmar R, Staykov D, Dorfler A, Schellinger PD, Schwab S, Bardutzky J. Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke. 2010;41:1684–9.
Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–23.
Acknowledgments
Dr. Rincon has received salary support from the American Heart Association (AHA 12CRP12050342).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted on behalf of the VISTA Collaboration. The members of Collaboration committee are listed in Appendix.
Appendix
Appendix
VISTA-Acute Steering Committee
K.R. Lees (Chair), A. Alexandrov, P.W. Bath, E. Bluhmki, L. Claesson, S.M Davis, G. Donnan, H. C. Diener, M. Fisher, B. Gregson, J. Grotta, W. Hacke, M.G. Hennerici, M. Hommel, M. Kaste, P. Lyden, J. Marler, K. Muir, R. Sacco, A. Shuaib, P. Teal, N.G. Wahlgren, S. Warach, and C. Weimar.
VISTA-ICH Steering Committee
D. F. Hanley (Chair), B. Gregson, K. R. Lees, P. Lyden, K. Muir, T. Steiner, S. Mayer.
Rights and permissions
About this article
Cite this article
Rincon, F., Lyden, P. & Mayer, S.A. Relationship Between Temperature, Hematoma Growth, and Functional Outcome After Intracerebral Hemorrhage. Neurocrit Care 18, 45–53 (2013). https://doi.org/10.1007/s12028-012-9779-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-012-9779-9